Abstract
Impaired mucociliary clearance may increase COPD exacerbation risk. We aimed to compare bronchial ciliary function and epithelial ultrastructure of COPD patients to healthy controls and explore its relationship to exacerbator phenotypes (frequent [FE] and infrequent [IFE] exacerbator). In this cross-sectional study, 16 COPD patients and 12 controls underwent bronchial brushings. Ciliary beat frequency (CBF) and dyskinesia index (DI; % of dyskinetic cilia) were assessed using digital high-speed video microscopy, and epithelial ultrastructure using transmission electron microscopy (TEM). Bronchial epithelium in COPD showed lower CBF and higher DI, compared to controls (median [IQR] CBF: 6.8 (6.1–7.2) Hz vs 8.5 (7.7–8.9) Hz, p<0.001 and DI: 73.8 (60.7–89.8) % vs 14.5 (11.2–16.9) %, p<0.001, respectively). This was true for FE and IFE phenotypes of COPD, which were similar in terms of bronchial CBF or DI. Subgroup analyses demonstrated lower CBF and higher DI in FE and IFE COPD phenotypes compared to controls, irrespective of smoking status. TEM showed more loss of cilia, extrusion of cells, cytoplasmic blebs and dead cells in COPD patients versus controls. Profound dysfunction of bronchial cilia is a feature of COPD irrespective of exacerbation phenotype and smoking status, which is likely to contribute to poor mucus clearance in COPD.
Supplemental data for this article is available online at https://doi.org/10.1080/15412555.2021.1963695 .
Abbreviations
| AFB | = | acid-fast bacillus |
| BAL | = | bronchoalveolar lavage |
| BMI | = | body mass index |
| CAT | = | COPD assessment test |
| CBF | = | ciliary beat frequency |
| CFTR | = | cystic fibrosis transmembrane conductance regulator |
| CIRB | = | Central Institutional Review Board |
| COPD | = | chronic obstructive pulmonary disease |
| DCO | = | transfer factor for carbon-monoxide |
| DI | = | dyskinesia index |
| FE | = | frequent exacerbator |
| FEV1 | = | forced expiratory volume in one second |
| HRCT | = | high-resolution computed tomography |
| ICC | = | intra-class correlation coefficient |
| IFE | = | infrequent exacerbator |
| IQR | = | interquartile range |
| KCO | = | transfer factor for carbon-monoxide corrected for alveolar volume |
| MCC | = | mucociliary clearance |
| mMRC | = | MRC dyspnoea score |
| PC20 | = | provocative concentration causing a 20% fall in FEV1 |
| PCR | = | polymerase chain reaction |
| SGRQ | = | St. George Respiratory Questionnaire |
| TEM | = | transmission electron microscopy |
Introduction
Chronic obstructive pulmonary disease (COPD), characterised by chronic irreversible airflow obstruction and airway inflammation, is a leading cause of morbidity and mortality [Citation1]. Among COPD patients, those with frequent exacerbations, also referred to as the frequent exacerbator (FE) phenotype [Citation2], account for disproportionately high health care costs, and are more likely to suffer from mucus hypersecretion, rapid lung function decline, impaired quality of life, and mortality [Citation3].
COPD exacerbations are commonly triggered by airway infections. Bacteria or viruses can be detected in up to 78% of exacerbations requiring hospitalisation [Citation4]. Microbial colonisation of airways is common in stable state as well [Citation4] and is associated with increased risk of exacerbations [Citation5], and both airways [Citation5–7] and systemic inflammation [Citation7]. These findings suggest impaired airway defence mechanisms against potential pathogenic microorganisms in COPD, associated with on-going disease activity and progression.
The ciliated epithelium that covers the airways surface forms an immunologically active natural barrier to invasion and injury by inhaled organisms and particles. Cilia, tiny hair-like projections from the epithelium, beat at a frequency of 10–14 Hz, propelling mucus. Inhaled pathogens and particulate matter get trapped in the mucus layer and are cleared from the airway by the highly coordinated ciliary beating. This process, known as mucociliary clearance (MCC) [Citation8], is essential in pulmonary defence [Citation9]. Ciliary dysfunction causes impaired MCC leading to increased susceptibility to microbial colonisation, infection and inflammation [Citation9].
Although there is a suggestion that MCC may be more impaired in COPD during exacerbations [Citation10], studies on bronchial ciliary function and epithelial ultrastructure in patients with COPD are very limited and have important methodological limitations. Available data suggest that in COPD, the nasal epithelial ciliary beat frequency (CBF) is lower compared to healthy non-smoking subjects [Citation11–13]. A previous pilot study found comparable CBF in both nasal and bronchial epithelium in COPD patients; however, a control group was not included [Citation13]. Of note, these studies did not take exacerbation frequency and smoking status into account, both of which might affect ciliary function. In this context, we aimed to study the ciliary function and ultrastructure of the bronchial epithelium of patients with COPD and compare that to healthy control smokers. As an additional objective, we also explored the bronchial ciliary function in relation to the COPD exacerbator phenotype and smoking status (current smoker vs. ex-smoker).
Methods
Subjects
COPD patients, aged 40–75 years, diagnosed according to Global Initiative for Chronic Obstructive Lung Disease [Citation1], were recruited from respiratory clinics at the Singapore General Hospital. Exacerbations were defined as acute worsening of COPD symptoms that necessitated antibiotics and/or systemic steroid treatment [Citation2]. Exacerbators’ phenotype was defined as frequent (FE: ≥2 exacerbations in the past year) or infrequent (IFE: ≤1 exacerbation in the past year). Exclusion criteria were FEV1 < 50% of predicted and type-2 respiratory failure (in view of bronchoscopy safety), other respiratory disorders (including asthma and significant bronchiectasis), long-term treatment with macrolides, and co-morbidities that were likely to interfere with the study procedures. Healthy controls (aged 40–75 years, smokers and ex-smokers) were recruited by local advertising. They had no known chronic respiratory or other medical conditions, normal chest X-ray and spirometry, and no bronchial hyperresponsiveness (PC20 methacholine >8mg/ml). Smoking status of subjects was defined as current smokers or ex-smokers (quit smoking ≥1 year) with a smoking history of ≥10 pack-years. All subjects were recruited between July 2014 and May 2016. The SingHealth Central Institutional Review Board approved the study (CIRB: 2013/935/C) and written informed consent was obtained from all subjects.
Measurements
This was a cross-sectional observational study, including three visits within a maximum window of 8 weeks. Clinical assessment, lung function tests and radiology investigations were done at the first two visits; and bronchoscopy at the third visit. COPD patients completed the St. George’s Respiratory Questionnaire [Citation14], modified Medical Research Council dyspnoea scale [Citation15] and the COPD assessment test [Citation16]. All subjects underwent spirometry (after 400 μg of inhaled salbutamol in COPD patients), and COPD patients underwent measurement of diffusion capacity and a high-resolution computed tomography (HRCT) of the chest. Controls had a chest X-ray and methacholine challenge test using standard techniques [Citation17]. All subjects underwent elective flexible bronchoscopy conducted according to British Thoracic Society guidelines [Citation18], scheduled such that subjects were free from intercurrent respiratory infections and/or exacerbations for ≥6 weeks. Subjects were asked to refrain from smoking on the day of the bronchoscopy and from alcohol use from the day before the bronchoscopy. Bronchoalveolar lavage (BAL) was obtained from the middle lobe [Citation18]. Pooled BAL was used for microbiological investigations (gram stain, aerobic culture, fungal smear, fungal culture, respiratory virus multiplex polymerase chain reaction (PCR; Seegene, Anyplex II RV16), AFB smear, mycobacterial culture, Mycoplasma pneumoniae and Chlamydophila pneumoniae PCR). Strips of bronchial epithelium were obtained by brushing the bronchus intermedius using disposable cytology brushes (Olympus BC-202D-1210) [Citation19].
Ciliary function assessment
Assessment of CBF and beat pattern were done blinded to the subject phenotype, using a high-speed digital video microscopy system (IDT Motion Xtra NR4-S3, IDT Inc (USA) and Leica DM2500 microscope with heated stage [37 °C]), as described previously [Citation19]. Seven to ten ciliated epithelial strips per sample were analysed within 2 h of collection. Briefly, live images of ciliated epithelial strips were captured at 500 frames per second. Videos were played back and analysed in slow motion, frame by frame. Groups of beating cilia were identified and the number of frames required to complete 10 beat cycles was calculated and converted to CBF (CBF = [500/number frames for 10 beats] × 10; Hz). Beat pattern was classified as normal or dyskinetic. Dyskinesia included reduced beat amplitude, stiff beat pattern, failure to bend along the length of the ciliary shaft, a flickering or twitching motion and static cilia. Beat pattern was quantified by estimating the dyskinesia index (DI), defined as the percentage of dyskinetic cilia within each sample (number of dyskinetic readings/total number of readings for the sample ×100). Immotility index was defined as the percentage of immotile cilia within the sample (number of immotile readings/total number of readings for the sample ×100). Images were analysed blinded to the subject phenotype by observer 1 (B.T.), re-analysed by observer 2 (Q.H.) and on a second occasion by the original observer (B.T.).
Ultrastructure assessment
Ultrastructure of bronchial ciliated epithelial cells was assessed by transmission electron microscopy (TEM), blinded to the subject phenotype, at the Centre for PCD Diagnosis and Research (University of Leicester, UK), as described previously [Citation20]. Proportions of different cell types (ciliated cells, unciliated cells, mucus cells and dead cells) and cells with ultrastructural defects (loss of cilia, cellular projections [extrusion of cells from the epithelial edge], cytoplasmic blebbing and mitochondrial damage [swelling and disruption of mitochondrial cristae]) among all cells examined were calculated. Damage to individual cilium was evaluated and the percentage of cilia with microtubular or dynein arm defects was calculated. Intracellular ciliary orientation, defined as the standard deviation (SD) of the angles of lines through the central pair of microtubules of cilia originating from a single ciliated cell, was determined [Citation20].
Statistical analysis
Sample size was calculated on the basis of CBF being the primary outcome measure. A 16% increase in CBF correlates with a 56% increase in the tracheal surface liquid velocity on freshly excised sheep trachea [Citation21]. We assumed that an absolute mean difference in CBF of 2 Hz has potential biological significance [Citation20]. To detect a mean difference in CBF of 2 Hz (with a SD of 1 Hz) between two groups at the 5% significance level with 80% power, a sample size of 6 in each group would be required. Continuous variables were summarised as either mean ± SD or median (interquartile range [IQR]). Comparisons between two groups were performed using the Mann–Whitney U test and ≥3 groups were compared using the Kruskal–Wallis test with post hoc analysis using the Dunn method. p Values of <0.05 were regarded statistically significant. GraphPad Prism 5 (GraphPad software Inc, CA) and SAS/STAT software (SAS Institute Inc., NC) were used. Agreement between the observers on measures of ciliary function (CBF and DI) was assessed by interclass correlation (ICC).
Results
Sixteen COPD patients (7 FE and 9 IFE; 11 current and 5 ex-smokers) and 12 healthy subjects (6 current and 6 ex-smokers) were studied. Demographic and clinical details of the main groups and the subgroups (FE and IFE) are summarised in and Table E1 (Supporting Information Appendix), respectively. COPD patients were older and had smoked more pack-years than controls. HRCT of one COPD patient showed mild localised bronchiectasis in addition to emphysema. Of note, the proportion of patients with chronic bronchitis was 55.6% in the COPD IFE group compared to 0% in the FE group (p < 0.05).
Table 1. Patient characteristics.
Ciliary function
Bronchial ciliary function data are presented in and . Patients with COPD had significantly lower CBF and higher DI compared to healthy controls, irrespective of the exacerbator phenotype (Table E2, Fig E1, Supporting Information Appendix). The immotility index of IFE was significantly higher compared to controls. However, between FE and IFE phenotypes, there was no significant difference in bronchial CBF, DI or immobility index. Subgroup analyses showed lower CBF and higher DI in both FE and IFE COPD phenotypes, irrespective of smoking status (current or ex-smokers) compared to controls (Table E3, Supporting Information Appendix).
Figure 1. Legend: Bronchial ciliary function in COPD and controls. (A) CBF: ciliary beat frequency, (B) dyskinesia index, (C) immotility index. ***p < 0.001.

Table 2. Ciliary function in COPD and controls.
In the COPD group, BAL microbiology was positive in three patients (Haemophilus influenzae [n = 1], respiratory syncytial virus [n = 1] and Coronavirus OC43 [n = 1]). Re-analysis of the data excluding these patients and one COPD FE patient with mild bronchiectasis still showed significant ciliary dysfunction in both the COPD subgroups (FE and IFE) compared to controls (Supporting Information Table E4).
Inter-observer agreement was excellent for measurement of CBF (ICC: 0.985) and DI (ICC: 0.995). Repeatability (intra-observer agreement) was also excellent (ICC: 0.969 for CBF and 0.996 for DI).
Epithelial ultrastructure
Bronchial epithelium obtained from two control subjects and five patients with COPD (2 FE and 3 IFE) were insufficient for TEM assessment. Results are summarised in and representative TEM images are shown in . Both FE and IFE COPD subgroups showed significantly more ultrastructural abnormalities compared to controls (Supporting Information Table E5), characterised by loss of cilia, extrusion of cells and cytoplasmic blebs, and a trend towards a higher proportion of dead cells (p = 0.06). The number of subjects was too small to analyse the TEM results in relation to smoking status.
Figure 2. Legend: transmission electron microscopy (TEM) images of ciliated respiratory epithelium from a healthy ex-smoking control subject (A) showing normal mitochondria (arrow), and a patient with COPD (B–F) showing significant loss of cilia (B), abnormal mitochondria (C), projecting cell (D), cytoplasmic bleb (E) and dead cell (F). Internal scale bar = 2 µm.
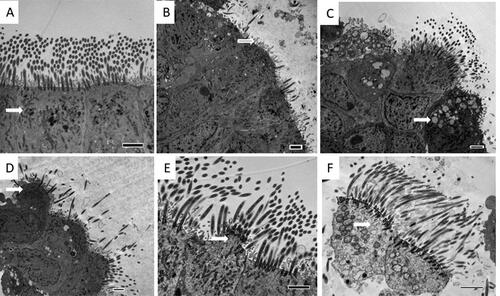
Table 3. Ultrastructure of bronchial epithelium in COPD and controls.
Discussion
We demonstrated that dysfunctional cilia in the bronchial epithelium, characterised by reduced CBF and markedly increased dyskinesia, is a feature of COPD. Moreover, epithelium of COPD patients showed significantly higher ultrastructural abnormalities compared to healthy controls. These changes were similar in FE and IFE, as well as current and ex-smokers with COPD. Together, these changes demonstrate profound bronchial ciliary dysfunction in COPD, irrespective of exacerbation phenotype, that persists even after smoking cessation. These findings suggest a role for impaired ciliary function in the pathophysiology of COPD but may not fully explain the susceptibility to frequent exacerbations.
To our knowledge, this is the first study of bronchial ciliary function and epithelial ultrastructure; and their relation to exacerbation phenotype, in a well-characterised group of COPD patients. It has been reported that MCC is more impaired in COPD exacerbators than non-exacerbators [Citation10]; however, the underlying mechanisms, including ciliary function and structure, were not investigated. Our results extend these previous findings, by showing similar degrees of profound ciliary dysfunction and impaired epithelial ultrastructure in both FE and IFE COPD phenotypes. Studies on ciliary function in COPD are very limited and suggest that the nasal epithelial CBF is lower compared to healthy non-smoking subjects [Citation11,Citation12]. The only study to date that assessed bronchial CBF involved 17 active smokers with moderate COPD, which reported low CBF in both nasal and bronchial epithelium [Citation13]. However, this was an uncontrolled study and all patients were active smokers [Citation22]. Moreover, the CBF was measured at 25 °C rather than the recommended 37 °C, which may have underestimated the CBF [Citation23]. Hence these results should be interpreted with caution. Our study is unique in that we performed a controlled study including healthy current and ex-smokers for comparison; and assessed the ciliary function by studying both CBF and beat pattern. This was possible by using a digital high-speed video microscopy system that allowed the motion of cilia to be recorded in different planes including sideways view, view from above and view with the cilia beating towards the observer. Using this method, it has been shown that cilia in certain situations may have a relatively normal beat frequency but an abnormal beat pattern [Citation24], therefore normal CBF alone may not necessarily imply normal ciliary function.
Studies investigating the bronchial epithelial ultrastructure in COPD patients are limited [Citation25–27]. Smoking related destruction of epithelial cells, loss of cilia, cilia with abnormal microtubular configuration and irregular orientation have been reported [Citation26]. Bronchial epithelium in smokers with chronic bronchitis shows increased intercellular space in the superficial zone of the epithelium and more abnormal cilia, compared to asymptomatic smokers [Citation27]. Furthermore, abnormalities in epithelial mitochondrial structure in ex-smoker patients with severe COPD have been reported [Citation25]. Our findings of ultrastructural abnormalities in COPD showing loss of cilia, extrusion of cells, cytoplasmic blebs and increased proportion of dead cells, extend these previous published data and demonstrate more pronounced ultrastructural changes specific to COPD. Additionally, our data show that these profound ultrastructural changes in COPD are seen irrespective of the exacerbation phenotype. The ultrastructural and ciliary function abnormalities seen in COPD are somewhat similar to those previously reported in severe asthma [Citation20], although the significant ciliary disorientation found in severe asthma was not observed in COPD patients.
Our study has some limitations. In view of safety related to bronchoscopies, we only included patients with less severe airflow obstruction, and therefore our findings may not be extrapolated to more severe COPD patients who are more likely to have frequent exacerbations. Epithelial samples were taken from central airways, and it remains to be determined if similar degrees of ciliary dysfunction are present in peripheral airways. Furthermore, COPD patients were significantly older than controls, and one could argue this may partly explain the differences found in CBF [Citation28]. However, CBF of healthy adults aged 60–80 years is reported as 12–13 Hz (range 9.5–16 Hz) [Citation28], which is much higher than that we observed in our COPD cohort, hence the difference in age alone is unlikely to explain their profound ciliary dysfunction. Given small sample size of FE and IFE groups, it may not be possible to draw definitive conclusions about the relationship between dysfunctional cilia and FE phenotype. We did not study the effect of dysfunctional cilia on MCC. This is a single centre study and given the cross-sectional design, the within subject variability in ciliary function over time could not be ascertained. Future studies at other centres may help validate our findings including measures of ciliary beat patterns abnormalities (DI and immotility index) that we described. Despite these limitations, our methodology for assessing ciliary function is highly repeatable and based on available data [Citation21,Citation29], the profound ciliary dysfunction and epithelial abnormalities observed in COPD are undoubtedly of clinical relevance.
Impaired MCC in COPD patients is multifactorial in causation, and our finding of profound ciliary dysfunction is likely to play a significant role in this. Other possible contributory mechanisms include effects of chronic cigarette smoke exposure, such as acquired cystic fibrosis transmembrane conductance regulator (CFTR) dysfunction that leads to qualitative and quantitative alterations in periciliary fluid and airway surface liquid [Citation30–32], increased MUC5AC expression, mucus hypersecretion and transport [Citation33,Citation34]; defective ciliogenesis and shortening of cilia [Citation35–37], and ciliophagy – increased turnover of ciliary proteins by autophagy [Citation38,Citation39]. However, our finding of worse ciliary function in COPD smokers compared to healthy smokers suggests that in addition to the effects of chronic smoke exposure, other mechanisms such as effects of chronic inflammation, altered ciliary axonemal ultrastructure, alterations in airway microbiome, abnormal injury repair mechanisms and airway remodelling in COPD may also play a role. COPD exacerbations are frequently triggered by infections and a growing body of evidence suggests defective epithelial innate immune responses in COPD [Citation40].
Improving MCC may reduce symptoms, exacerbation frequency and disease progression in COPD patients. Ciliary dysfunction and epithelial abnormalities demonstrated in COPD may be amenable to treatment. For instance, β2-agonists and roflumilast may increase CBF [Citation41,Citation42]. However, increasing the beat frequency of cilia may not improve MCC if the cilia have a dyskinetic beat pattern. It has also been shown that cigarette smoke induced ciliophagy may be mediated by cytosolic deacetylase HDAC6, and inhibitors of HDAC6 (such as tubastatin A) may offer protection against smoke-induced reduction in MCC [Citation38]. Finally, acquired CFTR dysfunction and defective innate immune responses in COPD offer other potential therapeutic targets for improving MCC and reducing exacerbation frequency.
Conclusions
Our study shows that profound bronchial epithelial ciliary dysfunction and ultrastructural abnormalities are features of COPD, irrespective of exacerbator phenotype and smoking status. These may contribute to impaired MCC and increased susceptibility to respiratory infections in COPD patients. The mechanisms underlying ciliary dysfunction in COPD patients and its clinical implications remain to be determined.
Authors contributions
B. Thomas and T.S. Lapperre, both guarantor of the paper, were involved in the conception, design and conduct of the study and preparation of the manuscript. M.S. Koh, S.Y. Low, T.H. Ong, C.M. Loo and Teoh O.H were involved in the design, conduction of the study and critically reviewed and revised the manuscript. C. O’Callaghan, A. Rutman and R.A. Hirst were involved in the design of the study, conduction of TEM studies, and critically reviewed and revised the manuscript. J Connolly and J.C. Allen Jr were involved in the design and reviewed the manuscript. L.L.E. Oon was responsible for the microbiology investigations and reviewed the manuscript. W.T. Lim was involved in the design of the study, and the recruitment and measurements in healthy control subjects, and reviewed the manuscript. Q. He performed ciliary function studies.
Acknowledgments
The authors thank the subjects for their participation in this study. They also thank the following Research nurses and coordinators for their assistance in conducting the study: Chwee Bee Yap, Nicole Yu Chia Shuang and Karen Tan (Dept. of Respiratory and Critical Care Medicine, Singapore General Hospital, Singapore); Michellore Aguilar and Robyn Yip Yeok (SingHealth Investigational Medicine Unit, Singapore). Finally, they thank Roy Sudipto (Institute of Cell and Molecular Biology, A*STAR) for helping with the TEM samples, and Wan Loo Tan and staff of the Molecular Laboratory (Department of Molecular Pathology) for their help in the molecular microbiology tests.
Declaration of interest
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Global initiative for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2021. https://goldcopd.org
- Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138.
- Wedzicha J. A, Singh R, Mackay AJ. Acute COPD exacerbations. Clin Chest Med. 2014;35(1):157–163. DOI:https://doi.org/10.1016/j.ccm.2013.11.001
- Beasley V, Joshi PV, Singanayagam A, et al. Lung microbiology and exacerbations in COPD. Int J COPD. 2012;7:555–569.
- Patel IS, Seemungal TAR, Wilks M, et al. Relationship between bacterial colonisation and the frequency, character, and severity of COPD exacerbations. Thorax. 2002;57(9):759–764. DOI:https://doi.org/10.1136/thorax.57.9.759
- Soler N, Ewig S, Torres A, et al. Airway inflammation and bronchial microbial patterns in patients with stable chronic obstructive pulmonary disease. Eur Respir J. 1999;14(5):1015–1022. DOI:https://doi.org/10.1183/09031936.99.14510159
- Marin A, Garcia-Aymerich J, Sauleda J, et al. Effect of bronchial colonisation on airway and systemic inflammation in stable COPD. COPD. 2012;9(2):121–130. DOI:https://doi.org/10.3109/15412555.2011.636407
- Wanner A, Salathé M, O’Riordan TG. Mucociliary clearance in the airways. Am J Respir Crit Care Med. 1996;154(6 Pt 1):1868–1902. DOI:https://doi.org/10.1164/ajrccm.154.6.8970383
- Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest. 2002;109(5):571–577. DOI:https://doi.org/10.1172/JCI0215217
- Siddiqi A, Berim I, Nabi H, et al. Association of impaired mucociliary clearance with occurrence of exacerbations in COPD. Proceedings of the International Conference on American Thoracic Society A5350. 2009 May 15–20, San Diego, CA. DOI:https://doi.org/10.1164/ajrccm-conference.2009.179.1_MeetingAbstracts.A5350
- Koblizek V, Tomsova M, Cermakova E, et al. Impairment of nasal mucociliary clearance in former smokers with stable chronic obstructive pulmonary disease relates to the presence of a chronic bronchitis phenotype. Rhinology. 2011;49(4):397–406. DOI:https://doi.org/10.4193/Rhino11.051
- Yaghi A, Zaman A, Cox G, et al. Ciliary beating is depressed in nasal cilia from chronic obstructive pulmonary disease subjects. Respir Med. 2012;106(8):1139–1147. DOI:https://doi.org/10.1016/j.rmed.2012.04.001
- Koblízek V, Dobesová T, Salajka F, et al. [Examination of function and structure of respiratory cilia of adult patients suffering from chronic obstructive pulmonary disease (COPD)–comparison of nasal and bronchial mucosa (pilot of CILIARY STUDY)]. Vnitr Lek. 2009;55(11):1035–1042.
- Jones PW, Quirk FH, Baveystock CM. The St george’s respiratory questionnaire. Respir Med. 1991;85(Suppl B):25–31. DOI:https://doi.org/10.1016/S0954-6111(06)80166-6
- Bestall JC, Paul E. A, Garrod R, et al. Usefulness of the medical research council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–586. DOI:https://doi.org/10.1136/thx.54.7.581
- COPD Assessment Test (CAT) [Internet]. [cited 2015 Apr 14]. Available from: http://www.catestonline.org/.
- Crapo RO, Casaburi R, Coates A. L, et al. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS board of directors, July 1999. Am J Respir Crit Care Med. 2000;161(1):309–329. DOI:https://doi.org/10.1164/ajrccm.161.1.ats11-99
- Rand IA, Du Blaikley J, Booton R, et al. British thoracic society guideline for diagnostic flexible bronchoscopy in adults: accredited by NICE. Thorax. 2013;68(Suppl 1):i1–i44. DOI:https://doi.org/10.1136/thoraxjnl-2013-203618
- Thomas B, Rutman A, O’Callaghan C. Disrupted ciliated epithelium shows slower ciliary beat frequency and increased dyskinesia. Eur Respir J. 2009;34(2):401–404. DOI:https://doi.org/10.1183/09031936.00153308
- Thomas B, Rutman A, Hirst RA, et al. Ciliary dysfunction and ultrastructural abnormalities are features of severe asthma. J Allergy Clin Immunol. 2010;126(4):722–729.e2. DOI:https://doi.org/10.1016/j.jaci.2010.05.046
- Seybold ZV, Mariassy AT, Stroh D, et al. Mucociliary interaction in vitro: effects of physiological and inflammatory stimuli. J Appl Physiol (1985). 1990;68(4):1421–1426. DOI:https://doi.org/10.1152/jappl.1990.68.4.1421
- Agius AM, Smallman LA, Pahor AL. Age, smoking and nasal ciliary beat frequency. Clin Otolaryngol Allied Sci. 1998;23(3):227–230. DOI:https://doi.org/10.1046/j.1365-2273.1998.00141.x
- Barbato A, Frischer T, Kuehni CE, et al. Primary ciliary dyskinesia: a consensus statement on diagnostic and treatment approaches in children. Eur Respir J. 2009;34(6):1264–1276. DOI:https://doi.org/10.1183/09031936.00176608
- Chilvers MA, McKean M, Rutman A, et al. The effects of coronavirus on human nasal ciliated respiratory epithelium. Eur Respir J. 2001;18(6):965–970. DOI:https://doi.org/10.1183/09031936.01.00093001
- Hoffmann RF, Zarrintan S, Brandenburg SM, et al. Prolonged cigarette smoke exposure alters mitochondrial structure and function in airway epithelial cells. Respir Res. 2013;14(1):97. DOI:https://doi.org/10.1186/1465-9921-14-97
- Kaidoglou K, Aivazis V, Alvanou A, et al. Ultrastructural study of bronchial epithelium in chronic respiratory diseases. Histol Histopathol. 1991;6(2):229–233.
- Trevisani L, Sartori S, Bovolenta MR, et al. Structural characterization of the bronchial epithelium of subjects with chronic bronchitis and in asymptomatic smokers. Respiration. 1992;59(3):136–144. DOI:https://doi.org/10.1159/000196044
- Ho JC, Chan KN, Hu WH, et al. The effect of aging on nasal mucociliary clearance, beat frequency, and ultrastructure of respiratory cilia. Am J Respir Crit Care Med. 2001;163(4):983–988. DOI:https://doi.org/10.1164/ajrccm.163.4.9909121
- Yates GT, Wu TY, Johnson RE, et al. A theoretical and experimental study on tracheal muco-ciliary transport. Biorheology. 1980;17(1-2):151–162. DOI:https://doi.org/10.3233/bir-1980-171-216
- Cantin AM, Hanrahan JW, Bilodeau G, et al. Cystic fibrosis transmembrane conductance regulator function is suppressed in cigarette smokers. Am J Respir Crit Care Med. 2006;173(10):1139–1144. DOI:https://doi.org/10.1164/rccm.200508-1330OC
- Dransfield MT, Wilhelm AM, Flanagan B, et al. Acquired cystic fibrosis transmembrane conductance regulator dysfunction in the lower airways in COPD. Chest. 2013;144(2):498–506. DOI:https://doi.org/10.1378/chest.13-0274
- Clunes LA, Davies CM, Coakley RD, et al. Cigarette smoke exposure induces CFTR internalization and insolubility, leading to airway surface liquid dehydration. FASEB J. 2012;26(2):533–545. DOI:https://doi.org/10.1096/fj.11-192377
- Kreindler JL, Jackson AD, Kemp PA, et al. Inhibition of chloride secretion in human bronchial epithelial cells by cigarette smoke extract. Am J Physiol Lung Cell Mol Physiol. 2005;288(5):L894–L902. DOI:https://doi.org/10.1152/ajplung.00376.2004
- Iravani J, As van A. Mucus transport in the tracheobronchial tree of normal and bronchitic rats. J Pathol. 1972;106(2):81–93. DOI:https://doi.org/10.1002/path.1711060204
- Tamashiro E, Xiong G, Anselmo-Lima WT, et al. Cigarette smoke exposure impairs respiratory epithelial ciliogenesis. Am J Rhinol Allergy. 2009;23(2):117–122. DOI:https://doi.org/10.2500/ajra.2009.23.3280
- Leopold PL, O’Mahony MJ, Lian XJ, et al. Smoking is associated with shortened airway cilia. PLoS One. 2009;4(12):e8157. DOI:https://doi.org/10.1371/journal.pone.0008157
- Hessel J, Heldrich J, Fuller J, et al. Intraflagellar transport gene expression associated with short cilia in smoking and COPD. PLoS One. 2014;9(1):e85453. DOI:https://doi.org/10.1371/journal.pone.0085453
- Lam HC, Cloonan SM, Bhashyam AR, et al. Histone deacetylase 6-mediated selective autophagy regulates COPD-associated cilia dysfunction. J Clin Invest. 2013;123(12):5212–5230. DOI:https://doi.org/10.1172/JCI69636
- Cloonan SM, Lam HC, Ryter SW, et al. "Ciliophagy": the consumption of cilia components by autophagy. Autophagy. 2014;10(3):532–534. DOI:https://doi.org/10.4161/auto.27641
- Hiemstra PS, McCray PB, Bals R. The innate immune function of airway epithelial cells in inflammatory lung disease. Eur Respir J. 2015;45(4):1150–1162. DOI:https://doi.org/10.1183/09031936.00141514
- Hasani A, Toms N, O’Connor J, et al. Effect of salmeterol xinafoate on lung mucociliary clearance in patients with asthma. Respir Med. 2003;97(6):667–671. DOI:https://doi.org/10.1053/rmed.2003.1498
- Schmid A, Baumlin N, Ivonnet P, et al. Roflumilast partially reverses smoke-induced mucociliary dysfunction. Respir Res. 2015;16(1):135. DOI:https://doi.org/10.1186/s12931-015-0294-3
