Abstract
Integrin α6β4–mediated adhesion interactions play key roles in keratinocyte and epithelial tumor cell biology. In order to evaluate how α6β4 adhesion interactions contribute to these important cellular processes, the authors generated soluble versions of the integrin by recombinant expression of the subunit ectodomains fused to a human immunoglobulin G (IgG) Fc constant domain. Coexpression of the appropriate subunits enabled dimerization, secretion and purification of stable Fc-containing α6β4 heterodimers. The soluble proteins exhibited the same metal ion and ligand dependency in their binding characteristics as intact α6β4. Using these reagents in combination with anti-β4 antibodies, the authors identified two distinct functional epitopes on the β4 subunit. They demonstrated the involvement of one epitope in adhesion interactions and the other in regulating adhesion-independent growth in α6β4-expressing tumor cell lines. The availability of these soluble integrin reagents and the data provided herein help to further delineate the structure-function relationships regulating α6β4 signaling biology.
INTRODUCTION
Integrins are heterodimeric cell surface adhesion receptors mediating cell-cell and cell-matrix interactions in a wide variety of cell types. Integrins each contain a noncovalently associated α and β subunit, and each αβ combination possesses specific binding and signaling properties (Hynes Citation2002). Signaling events leading to a wide variety of cellular processes including migration, proliferation, and survival, can be regulated via integrin interaction with both the extracellular matrix environment and the intracellular cytoskeletal compartment (Giancotti and Ruoslahti Citation1999; Watt Citation2002). Integrin α6β4 functions as an adhesion receptor for laminins, and is expressed predominantly on the basolateral surface of most epithelial cell types (Kennel et al. Citation1992; Lee et al. Citation1992; Natali et al. Citation1992; Niessen et al. Citation1994; Sonnenberg et al. Citation1990). In normal tissues, α6β4 plays a role in the maintenance of epithelial integrity, particularly in the epidermis where as a component of the hemidesmosome protein complex it serves to anchor basal keratinocytes to the underlying basement membrane (Wilhelmsen et al. Citation2006). Signaling events mediated by α6β4 are involved in driving keratinocyte proliferation in response to wounding (Rabinovitz et al. Citation1999, Citation2004). There is now accumulating evidence that α6β4 signaling events can also contribute to tumorigenesis, and that cooperative signaling between growth factor receptors and α6β4 can enhance mitogenic and survival signaling in cancer cells (Folgiero et al. Citation2007; Gambaletta et al. Citation2000; Guo et al. Citation2006; Yoon et al. Citation2006). Overexpression of β4 has been described recently in pancreatic adenocarcinomas, where up-regulation was an early event in the malignant process (Cruz-Monserrate et al. Citation2007). Interestingly, co-expression of α6β4 and laminin-5 in breast carcinoma was found to correlate with poor prognosis, further indicative of an important biological role for α6β4 adhesion interactions in the tumorigenic process (Tagliabue et al. Citation1998).
The α6β4 integrin differs from other integrin family members due to the unusually large size of the β4 subunit. The large (>1000 amino acid) intracellular domain of β4 comprises repeating structural units involved in hemidesmosome assembly, plus a C-terminal domain containing several serine and tyrosine residues, phosphorylation of which is required for recruitment of signaling adaptor molecules (Hogervorst et al. Citation1990; Spinardi et al. Citation1993; Suzuki and Naitoh Citation1990). The uniqueness of this intracellular portion suggests that signal transduction through α6β4 may differ from that of other integrins. This hypothesis is supported by the observation that β4 can directly recruit signaling effector molecules ShcFootnote1 and IRS2, in contrast to other β integrins that typically signal through FAK or ILK (Grashoff et al. Citation2004; Guo and Giancotti Citation2004; Mainiero et al. Citation1995; Shaw Citation2001).
There is significant interest in understanding the structural and functional characteristics of integrin binding, given the key roles these adhesion interactions can play in cancer and other cellular processes. To this end, several groups have succeeded in producing soluble variants of integrins for structural studies, either by removing the transmembrane or cytoplasmic regions of the proteins (Clark et al. Citation2000; Denda et al. Citation1998; Eble et al. Citation1998; Mehta et al. Citation1998; Peterson et al. Citation1998), or generating soluble ectodomain Fc-fusion proteins (Stephens et al. Citation2000). We chose to pursue a similar approach for integrin α6β4, and describe here the production and characterization of soluble Fc-containing heterodimeric forms of the integrin. We demonstrate the adhesive behavior exhibited by these molecules is comparable to that of intact α6β4, thereby confirming these soluble proteins are new tools for exploring α6β4 biology. Using these new reagents in combination with α6β4-specific antibodies, we highlight the involvement of different conformational domains of the β4 subunit in modulating adhesive interactions. These observations provide useful insight into the biology of α6β4 in normal and cancerous environments.
METHODS
Reagents and Cell Lines
Human placental collagen, antibodies GoH3 (anti-α6) and 450-9D (anti-β4) were purchased from BD Pharmingen (San Jose, CA). Mab 13 (anti-β1) was purified from hybridoma supernatant by Protein A affinity chromatography as described previously (Akiyama et al. Citation1989). Purified rat laminin-5, human fibronectin, antibodies MAB1974 (anti-β3), 4F10 (anti-α6), 3E1, ASC-3, and ASC-8 (all anti-β4) were from Chemicon International (Temecula, CA). HRP-conjugated secondary antibodies were from BioRad (Hercules, CA) and Jackson ImmunoResearch (West Grove, PA). Anti-human IgG and goat anti-mouse IgG-PE–conjugated antibodies were from Southern Biotech (Birmingham, AL) and Jackson ImmunoResearch. Collagen and BSA were from Sigma-Aldrich (St. Louis, MO). Normal goat serum was from Invitrogen (Carlsbad, CA). LTβR-Fc was generated as described previously (Browning et al. Citation1997). Sulfo-NHS-LC-Biotin was obtained from Pierce (Rockford, IL).
CHO cell line DG44i was derived for growth without insulin from parental DG44 cells, a CHO suspension cell line (Urlaub and Chasin Citation1980). Cells were cultured in MEM alpha + medium (Invitrogen) supplemented with 10% FBS (Invitrogen). K562-α6 transfectant cell line was a gift from Dr. A. Sonnenberg (Netherlands Cancer Institute, Amsterdam). All other tumor cell lines were obtained from ATCC (Manassas, VA) and grown according to the supplier's recommendations.
Purification of α6β1
K562-α6 transfectant cells were lysed in 1% Nonidet P-40 (Sigma-Aldrich), 25 mM Tris-HCl, pH 7.4 buffer containing 1 mM CaCl2, 1 mM MgCl2, 1 mM PMSF. Cell lysate was incubated for 2 h at 4°C with Sepharose 4B conjugated with Mab 13 at 5 mg/ml resin. The resin was collected in a column, washed with lysis buffer followed by washes with 0.1% Triton X-100, 25 mM Tris-HCl, pH 7.4 buffer containing 1 mM CaCl2, 1 mM MgCl2. α6β1 was eluted with 10 mM CH3COONa, pH 3.2, 0.1% Triton X-100, 1 mM CaCl2, 1 mM MgCl2 and immediately neutralized with HEPES buffer, pH 8.0. Peak fractions were pooled and stored at −70°C.
Cloning and Expression of α6β4 Constructs
The extracellular domains of human integrin α6 and β4 subunits were cloned from human skin poly A+ RNA (BD Pharmingen). The RNA was subjected to reverse transcription with the Superscript first strand synthesis system (Invitrogen) using specific α6 or β4 primers, and the resulting PCR products were cloned into CMV-IE promoter driven proprietary mammalian expression vectors, pV90 or pV100. To generate Fc-fusion constructs, a 3′ SalI site was introduced into each extracellular fragment using PCR techniques. The resulting NotI-SalI PCR fragment was subsequently cloned into pV90 or pV100 with a SalI fragment encoding the Fc portion of a human IgG1 in a three-way ligation.
To generate stable cell lines expressing the soluble integrin constructs, CHO DG44i cells were transfected with equimolar amounts of plasmids α6-Fc pV100 and β4 pV90 to generate α6-Fcβ4, or plasmids β4-Fc pV100 and α6 pV90 to generate α6β4-Fc. All transfections used the FuGENE transfection reagent (Roche, Indianapolis, IN) according to the manufacturer's recommended protocol. Cells were expanded 48 h post-transfection into MEM alpha − medium supplemented with 10% dialyzed FBS and 400 µg/ml of geneticin (Invitrogen). Two to three weeks after selection, the cells were stained with anti-human IgG-PE–conjugated antibody and positive staining cells sorted by flow cytometry using a FACS Vantage SE cell sorter (Beckton Dickinson). The positive staining cell pool was further analyzed for β4 expression using mouse anti-β4 antibody 450-9D, followed by a goat anti-mouse IgG-PE–conjugated antibody. The double-positive population was single cell sorted into a 96-well plate and cultured for 14 days, after which time expression of secreted α6-Fcβ4 and α6β4-Fc proteins were analyzed by ELISA. Wells expressing the highest level of secreted protein were subsequently scaled up to 1 L cultures.
Purification and Characterization of Soluble α6β4 Proteins
All buffers and columns used during purification were pyrogen free. CHO cell supernatants harvested from cells stably expressing either the α6-Fcβ4 or α6β4-Fc integrin constructs were filtered through a 0.2 µm membrane filter and loaded onto a Protein A Sepharose 4 Fast Flow column (Amersham Biosciences, Pittsburgh, PA) equilibrated and washed with PBS. Bound proteins were eluted using 100 mM glycine pH 2.8 buffer, neutralized by addition of 1 M Tris-HCl pH 8.9, and dialyzed against PBS. Protein concentration was determined by absorbance at 280 nm using an extinction coefficient of 0.93. To isolate soluble β4 protein, α6-Fcβ4 was treated with 3 M urea in PBS for 60 min at room temperature. The protein mixture was loaded onto a Protein A column equilibrated using PBS containing 3 M urea. Flow through fractions containing free unbound β4 were collected and dialyzed into PBS.
Protein purity was evaluated by SDS-PAGE under both reducing and nonreducing conditions. Both α6-Fcβ4 and α6β4-Fc proteins were analyzed by size exclusion chromatograpy using a BioSep-SEC-S 3000 column (Phenomenex, Torrance, CA) run using a Waters Alliance HPLC (Waters, Milford, MA) in PBS containing 25 mM NaH2PO4 pH 7.2, 150 mM NaCl, 0.04% NaN3. The chromatography system was coupled to a refractive index detector (Waters) and light scattering detector (PD2000; Precision Detectors, Bellingham, MA). The weight average molar masses were determined using the Precision Detectors Software.
Membrane bound samples of α6-Fcβ4 and α6β4-Fc were subject to N-terminal sequencing by Edman degradation using an Applied Biosystems Procise 494HT sequencer (Applied Biosystems, Foster City, CA), run in the pulsed liquid mode. The resulting PTH amino acids were separated using an ABI 140C Microgradient System with a PTH C18 Column (Applied Biosystems) and analyzed using an ABI 785A programmable absorbance detector with ABI 610A data analysis software (Applied Biosystems).
α6-Fcβ4 and α6β4-Fc were deglycosylated by incubation with PNGase F (Roche) for 48 h at 37°C, then reduced in 40 mM DTT and 4 M urea. Samples were analyzed on a ZMD mass spectrometer and the molecular masses were generated by deconvolution using the MaxEnt 1 software (Waters). Identification of α6-Fcβ4 and α6β4-Fc proteins was also determined by Western blotting using anti-α6 monoclonal antibody, GoH3, or anti-β4 monoclonal antibodies 3E1 and 450-9D.
Direct Binding and Competition ELISAs
Ninety-six-well microtiter plates (Costar, Cambridge, MA) were coated overnight at 4°C with 1 µg/ml of purified α6-Fcβ4, α6β4-Fc, or control proteins (α6β1 or LTβR-Fc) in 50 mM NaHCO3 buffer, pH 9.2, containing 10 µg/ml BSA. Plates were blocked for 1 h at room temperature with blocking buffer (50 mM Tris-HCl pH 7.5, 150 mM NaCl, and 2% nonfat dry milk) then washed with TBS binding buffer (25 mM Tris-HCl pH 7.5, 150 mM NaCl, 0.1% BSA, 2 mM glucose, 10 mM HEPES) containing 10 mM MgCl2. Primary α6- or β4-specific antibodies were diluted to a final concentration of 1 µg/ml in TBS binding buffer containing 10 mM MgCl2 and allowed to adhere to the coated microtiter plates for 1 h at room temperature. After washing, the plates were incubated with HRP-conjugated goat anti-mouse IgG antibody for 1 h at room temperature. HRP activity was measured using HRP substrate (Sigma-Aldrich) stopped by addition of 1 N H2SO4 and detected by absorbance measurement at 450 nm using a Spectramax-384 plate reader (Molecular Devices, Sunnyvale, CA).
For antibody competition assays, ASC-3 and ASC-8 were biotinylated using the EZ-Link sulfo-NHS-LC-Biotin Biotinylation Kit (Pierce). Saturating concentrations of unlabeled antibodies (10 µg/ml of ASC-3 or 5 µg/ml of ASC-8) were preincubated on α6-Fcβ4–coated plates for 1 h at room temperature, followed by an additional 1 h incubation in the presence of biotinylated antibodies (3 µg/ml of ASC-3 or 0.1 µg/ml of ASC-8). Avidin-HRP was used to detect bound biotinylated antibodies.
For β4 soluble protein competition assays, 96-well microtiter plates were coated overnight at 4°C with 0.1 µg/ml of purified α6-Fcβ4 protein. The plates were blocked, washed, and then incubated with 0.1 µg/ml of ASC-3 or ASC-8 antibodies in the presence of increasing amounts of β4 protein for 1 h. Antibody binding was detected using HRP-conjugated goat anti-mouse IgG antibody as described above.
Extracellular Matrix Adhesion Assays
Ninety-six-well plates were coated with 1 µg/ml of rat laminin-5, collagen, or fibronectin in PBS with 10 µg/ml BSA, as described above. After blocking and washing, 2 µg/ml α6-Fcβ4 or α6β4-Fc diluted in TBS binding buffer was allowed to bind to the plate in the presence or absence of divalent cations (10 mM MgCl2 or 1 mM MnCl2) or 10 mM EDTA for 1 h. Bound α6β4 proteins were detected using HRP-conjugated donkey anti-human IgG antibody (BioRad) as described above. The absorbance measured in TBS wells without α6-Fcβ4 was less than 5% relative to maximum absorbance values, and was routinely subtracted from the sample values. To assess the effects of anti-β4 antibodies on the integrin-matrix interaction, 10 µg/ml of ASC-8 or ASC-3 in TBS binding buffer containing 10 mM MgCl2 were preincubated with 2 µg/ml of α6-Fcβ4 or α6β4-Fc for 30 min prior to addition to the laminin-5–coated plates. The remaining steps were as described above.
For cellular adhesion assays, colorectal tumor cell line SW480 was starved for 24 h in serum-free medium. Cells were harvested using cell dissociation buffer (Invitrogen) and resuspended at 1×106 cells/ml in Leibovitz L-15 medium (Invitrogen). For antibody treatments, 10 µg/ml of ASC-3 or ASC-8 were added to the resuspended cells, and incubated for 1 h on ice. Cells were then plated on 96-well plates precoated with 10 µg/ml laminin-5, fibronectin, or collagen, at 1×105 cells per well and incubated for 1 h at 4°C. After washing the plates with Leibovitz L-15 medium, adherent cells were quantified using Cell Titer Glo cell viability assay (Promega, Madison, WI) and luminescence reading on a SpectraMax M5 plate reader.
Flow Cytometry
Cells were dissociated with cell dissociation buffer, washed and resuspended in FACS buffer (PBS containing 10% normal goat serum, 0.2% BSA, 0.1% NaN3) at a concentration of 5×106 cells/ml. Antibodies were diluted to the desired concentrations in FACS buffer and incubated with cells (2.5×105 cells/well) in 96-well round bottom plates for 1 h on ice. Cells were then washed with FACS buffer and incubated with a PE-conjugated goat anti-mouse antibody at a 1:200 dilution for 1 h on ice. Cells were washed again and resuspended in FACS buffer containing 1 µg/ml propidium iodide (Molecular Probes, Eugene, OR). Viable cells were analyzed using a FACSArray bioanalyzer (BD Biosciences), and mean fluorescence intensity was graphed using GraphPad PRISM (GraphPad Software, San Diego, CA).
Surface Plasmon Resonance
Real-time antibody-integrin interactions were measured using a Biacore 3000 instrument (Biacore, Piscataway, NJ). Anti-human IgG1 isotype-specific monoclonal antibody clone 2C11 (AbD Serotec, Raleigh, NC) was covalently coupled to a CM5 chip (Biacore) at high density and used to capture approximately 141 RU of α6-Fcβ4. Multiple concentrations of ASC-3 or ASC-8 diluted in HBS-P buffer (0.01 M HEPES pH 7.4, 0.15 M NaCl, 0.005% [v:v] surfactant P-20, 10 mM MgCl2) were then passed over the chip, and the surfaces regenerated with a 40 s pulse of 10 mM glycine, pH 2.0. The experiments were conducted at 25°C, with a flow rate of 10 µl/min. Control experiments demonstrated that the conditions were not diffusion (mass-transport) limiting. Data sets were double referenced by subtracting a concurrently run control (capture antibody only) reference cell followed by subtraction of the similarly run buffer blank. To derive apparent rate constants, the datasets were globally fit with a 1:1 (Langmuir) binding model using BIAevaluation 4.1 software. The results of three independent experiments (run in duplicate) were averaged to obtain the apparent affinity values±one standard deviation.
Anchorage-Independent Growth
The inhibition of anchorage-independent growth was determined by monitoring colony formation in soft agar. A bottom layer of 1 ml medium (RPMI for MDA-MB-231; Leibovitz L-15 for SW620) containing 0.6% nobel agar (BD-Difco, Sparks, MD), 10% FBS and 10 µg/ml antibody was prepared in 12-well ultra-low-cluster cell culture plates. After the bottom layer solidified, cells (MDA-MB-231: 15,000 cells/well; SW620: 10,000 cells/well) were added to a top agar layer (1 ml) containing 0.3% nobel agar, 10% FBS, and 10 µg/ml antibody and duplicate samples added to the solidified bottom agar layers. Cells were incubated in 5% CO2 (MDA-MB-231) or 0% CO2 (SW620) incubators at 37°C and supplemented twice weekly with additional antibody-containing medium. Colonies measuring >150 µm were counted on a Nikon Eclipse TE2000-U system after 21 to 27 days.
RESULTS
Construction, Production, and Purification of Soluble α6β4 Proteins
To produce soluble α6β4 integrin, the ectodomains of both α6 and β4 subunits were coexpressed recombinantly in CHO cells. The proteins were engineered to contain a human IgG Fc domain C-terminal to either the α6 or β4 ectodomain to facilitate expression and purification. CHO cells were cotransfected with the appropriate plasmids to generate both α6-Fcβ4– and α6β4-Fc–expressing cells, which resulted in detectable levels of α6 and β4 integrins in the medium in each case. For both sets of integrin constructs, as much as 100 mg of protein per liter of culture medium were recovered. A classical Protein A Sepharose method was used for purification of the soluble integrin proteins from the CHO cell supernatants. Representative coomassie stained SDS-PAGE gels of the purified material recovered in the elution fractions are shown in A. Two major protein bands of the apparent sizes of an α6-Fc dimer (272 kDa) and a β4 subunit (77 kDa) were observed in the analysis of the α6-Fcβ4 under nonreducing conditions. Similarly, two protein bands of the apparent sizes of a β4-Fc dimer (204 kDa) and an α6 subunit (110 kDa) were observed upon analysis of the α6β4-Fc fractions.
Figure 1. Purification of soluble α6β4 proteins. (A) α6-Fcβ4 (top panel) and α6β4-Fc (bottom panel) proteins were purified from α6-Fcβ4– or α6β4-Fc–transfected CHO cell supernatants respectively by protein A chromatography. Protein purity in column fractions in the load (L), flow through (FT), wash (W), and elution fractions (1, 2, 3) was assessed by SDS-PAGE under nonreducing conditions. α6-Fc, β4, β4-Fc, and α6 bands at predicted sizes of 272, 77, 204, 110 kDa, respectively, are indicated. (B) The identity of the purified α6-Fcβ4 (top panel) or α6β4-Fc (bottom panel) material was confirmed by Western blotting under nonreducing conditions with antibodies to α6 and β4. (C) SEC and light scattering (top right inset; RI = refractive index, MW = molecular weight) analysis shows soluble α6-Fcβ4 migrating at the approximate size of a tetramer (449.4 kDa). Predicted size of two α6-Fc subunits plus two β4 subunits is 426 kDa. Inset (top left) is a cartoon of the α6-Fcβ4 tetramer.
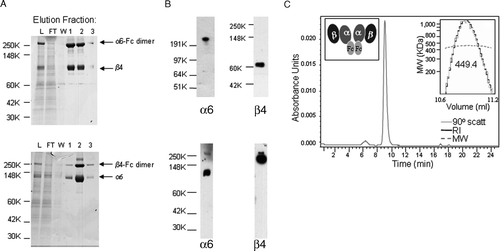
The identity of the α6-Fcβ4 and α6β4-Fc protein bands was confirmed by Western blotting. As seen in B for α6-Fcβ4 (top panels), anti-α6 monoclonal antibody GoH3 recognized the 272 kDa band, whereas anti-β4 monoclonal antibody 450-9D recognized the 77 kDa band. In the case of α6β4-Fc (bottom panels), GoH3 recognized the 110 kDa band, whereas 450-9D recognized the 204 kDa band. An anti-human IgG antibody also recognized the 272 kDa and 204 kDa bands, verifying that each contained the Fc moiety (data not shown).
Size exclusion chromatrography using a G3000SW column and gel filtration followed by light scattering analysis indicated that purified α6-Fcβ4 protein ran as a single peak (C), demonstrating that the soluble protein preparation contained only one protein species. The soluble protein chromatographed with an apparent molecular weight of 449 kDa. Based on the amino acid sequence, the predicted size of two α6-Fc subunits plus two β4 subunits is 426 kDa, suggesting that the soluble protein was a tetramer in solution. Similar analysis of the α6β4-Fc protein showed this also chromatographed as a single peak with an apparent molecular weight of 449 kDa and existed as a tetramer species (data not shown).
N-terminal sequencing analysis also confirmed the identity of both the α6-Fcβ4 and α6β4-Fc proteins. The N-terminal amino acid sequence obtained for α6 from both constructs was FNLDTREDNV, whereas that for β4 from both constructs was NRCKKAPVKS, in agreement with the predicted sequences. Taken together, these data confirm that two soluble Fc-containing versions of α6β4 integrin have been successfully generated and dimerized appropriately through the addition of the Fc to the molecules.
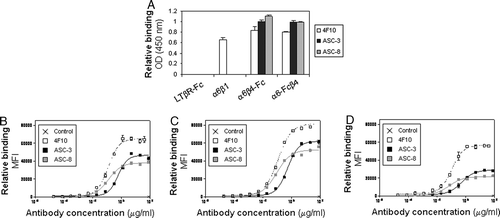
The purified proteins were further characterized by ELISA with additional α6 and β4 antibodies (A). The α6-specific antibody 4F10 bound equally well to α6β1, α6-Fcβ4, and α6β4-Fc, whereas the β4-specific antibodies, ASC-3 and ASC-8, recognized only the α6β4 fusion constructs and not α6β1. None of the antibodies bound to a control Fc-fusion protein. These antibodies showed similar binding characteristics on a panel of tumor cell lines expressing endogenous α6β4 (B to D), suggesting that the purified proteins exist in a physiologically relevant conformation with epitope availabilities comparable to that of native α6β4.
Figure 2. α6β4 antibodies recognize both soluble and endogenous proteins. (A) Plates were coated with the indicated proteins then incubated with α6- (4F10) or β4- (ASC-3 and ASC-8) specific antibodies. Antibody binding was detected using an HRP-conjugated anti-mouse antibody as described. (B–D) Binding characteristics of β4- or α6-specific antibodies were determined by flow cytometry on colorectal tumor cell lines SW620 (B) and SW480 (C), and breast tumor cell line MDA-MB-231 (D).
Ligand-Binding Characteristics of the Soluble α6β4 Integrins Are Comparable to Those of Intact α6β4
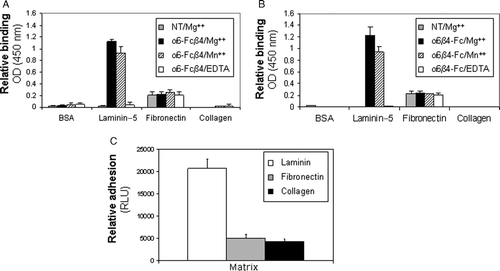
Integrin engagement with the extracellular matrix is known to be cation-dependent and ligand specific (Dransfield et al. Citation1992; Staatz et al. Citation1989, Citation1990). To determine whether the soluble integrins retained these intrinsic properties of native integrins, we investigated the binding characteristics of the Fc-fusion proteins to different extracellular matrix proteins in the presence and absence of divalent cations. (AgB) shows cation-dependent binding of both α6-Fcβ4 and α6β4-Fc to laminin-5, the preferred ligand for α6β4. Both α6β4 proteins bound to laminin-5–coated plates in the presence of Mg2 + or Mn2 + . Binding to laminin-5 was completely abolished by the addition of EDTA, a cation chelator. No binding was observed on BSA- or collagen-coated plates under any conditions, demonstrating ligand specificity of the assay. A small amount of binding was detected on fibronectin-coated plates; however, this was unaffected by cation presence and occurred in the absence of α6β4 integrin, and was therefore considered nonspecific background. Colorectal tumor cell line SW480 previously shown to express α6β4 endogenously (C) exhibited similar adhesive behavior as the soluble protein constructs (C). The collective results confirm that the soluble integrins retain the ligand- and metal ion-dependent binding characteristics of their physiological counterparts.
Figure 3. Ligand binding specificity of soluble α6β4 proteins is comparable to that of endogeous α6β4. (A, B) Purified α6-Fcβ4 (A) or α6β4-Fc (B) were incubated on plates coated with the indicated extracellular matrix proteins in the presence of 10 mM MgCl2 (grey and black bars), 1 mM MnCl2 (dashed lines), or 10 mM EDTA (white bars). After washing, bound α6-Fcβ4 or α6β4-Fc was detected using an HRP-conjugated anti-human antibody. (C) Colorectal tumor cells (SW480) were starved for 24 h in serum-free medium. Cells were held in suspension for 1 h on ice prior to plating on 96-well plates coated with the indicated matrices for 1 h at 4°C. Relative adhesion was measured using a luciferase-based luminescent viability assay.
Soluble α6β4 Integrins as Tools to Evaluate Functional Epitopes on α6β4
The β4-specific antibody ASC-8 has previously been shown to block adhesion to laminin in cells expressing endogenous α6β4 (Russell et al. Citation2003). We therefore sought to determine if this same effect was observed with the soluble integrin constructs. Preincubation of ASC-8 with either α6-Fcβ4 or α6β4-Fc prior to addition of the proteins to laminin-5–coated plates resulted in complete blockade of soluble α6β4-laminin interactions (A). Interestingly, preincubation with another β4-specific antibody ASC-3 had no inhibitory effect (A). Similarly, ASC-8 blocked SW480 cell adhesion to laminin-5 matrix, whereas ASC-3 had no effect on cellular adhesion (B). A β3 integrin–specific antibody or an isotype control had no effect on either soluble protein or cell adhesion. These observations further confirm the relevance of the soluble integrin constructs and together with published data using these antibodies, suggest that the epitope recognized by ASC-8 plays a critical role in mediating the adhesive interactions of the β4 subunit with laminin-5, whereas the epitope recognized by ASC-3 seems to be irrelevant for adhesion. These putative epitope differences were investigated further in a competition ELISA, where biotinylated antibodies were allowed to bind to α6-Fcβ4–coated plates in the presence of excess amounts of unlabeled antibody. As shown in A, ASC-3 and ASC-8 did not compete with each other for binding, further confirming each recognized a different epitope on the protein.
Figure 4. Effect of β4-specific antibodies on α6β4-laminin adhesion. (A) Purified α6-Fcβ4 or α6β4-Fc proteins were left untreated (NT; white bars), preincubated with a β3-specific control antibody (Anti-β3; dashed bars) or β4-specific antibodies (ASC-3; black bars, ASC-8; grey bars) prior to plating on laminin-5–coated ELISA plates. Bound α6β4 proteins were detected using an HRP-conjugated anti-human antibody. (B) Colorectal tumor cells (SW480) were starved for 24 h in serum-free medium. Cells were left untreated (NT; white bars), treated with a β3-specific control antibody (Anti-β3; dashed bars) or with β4-specific antibodies (ASC-3; black bars, ASC-8; grey bars) and held in suspension for 1 h on ice prior to plating on laminin-5–coated plates for 1 h at 4°C. Relative adhesion was measured using a luciferase-based luminescent viability assay.
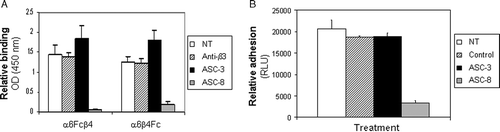
Figure 5. β4 antibodies recognize different epitopes on α6β4. (A) Biotinylated ASC-3 or ASC-8 were allowed to bind to α6-Fcβ4–coated plates preincubated with saturating concentrations of unlabeled antibodies for 1 h, then detected using avidin-HRP. (B) Purified β4 was obtained from α6-Fcβ4 as described and analyzed by SDS-PAGE under nonreducing conditions (lane 1: α6Fc-β4; lane 2: purified β4 protein). α6-Fc and β4 bands at predicted sizes of 272 and 77 kDa, respectively are indicated. (C) ASC-3 or ASC-8 (0.1 µg/ml) were incubated on α6-Fcβ4 (0.1 µg/ml)–coated plates in the presence of increasing amounts of β4 protein. Bound antibodies were detected using an HRP-conjugated anti-mouse antibody as described.
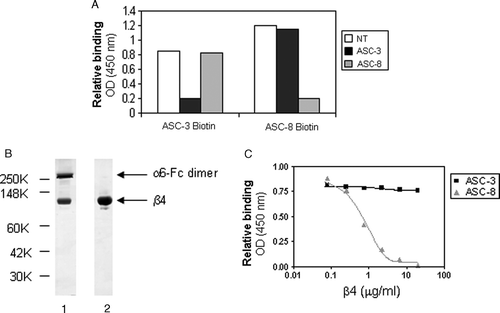
We next sought to determine whether the epitopes recognized by ASC-3 and ASC-8 on the β4 subunit were linear or conformational in nature and developed a method to generate soluble β4 integrin independent from the α6 binding partner to ascertain this. The soluble β4 subunit protein was separated from α6-Fcβ4 using mild urea denaturation followed by removal of α6-Fc by Protein A affinity chromatography. A nonreducing SDS-PAGE coomassie-stained gel of the purified material is shown in B. The β4 subunit was detected in the flow-through from the column, and was soluble and stable after refolding in PBS (data not shown). C depicts an ELISA where the purified β4 material was preincubated with either ASC-3 or ASC-8 prior to addition to α6-Fcβ4–coated plates. Increasing concentrations of β4 decreased the binding of ASC-8 to α6-Fcβ4, but had no effect on ASC-3 binding. This observation demonstrated that ASC-8 and ASC-3 recognize different epitopes on β4, and suggests that the ASC-8 epitope is contained completely within the β4 subunit while that of ASC-3 is conformational, requiring the correct association of the α6 and β4 subunits.
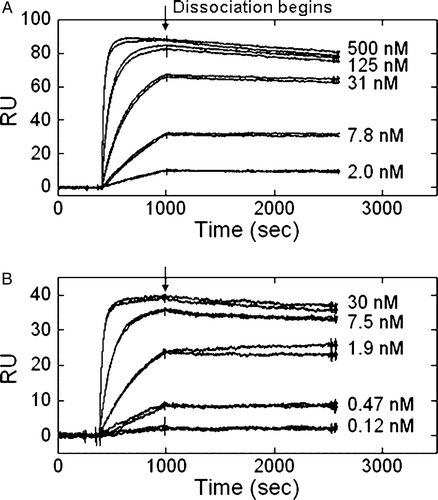
Flow cytometry analysis of antibody binding to tumor cell lines (B to D) indicated a five- to eight-fold lower EC50 value for ASC-8 compared to ASC-3 (data not shown). We sought to explore these potential affinity differences further using surface plasmon resonance to measure the apparent KD values for binding as shown in . A range of concentrations of the antibodies were allowed to flow over an anti-human IgG1 antibody-coated chip saturated with α6-Fcβ4 protein at 25°C. The sensorgrams showed that both ASC-3 and ASC-8 had fast association rates but slow dissociation rates. Calculated KD values were 0.6 nM for ASC-3 and 0.03 nM for ASC-8, demonstrating that ASC-3 had a 10-fold weaker affinity for α6-Fcβ4 than ASC-8. The on- and off-rates measured for ASC-3 were 9.8×104 M−1 s−1 and 5.6×10−5 s−1, respectively, whereas those for ASC-8 were 1.0×106 M−1 s−1 and 3.5×10−5 s−1, respectively. These data indicated the difference in affinity between the two antibodies was in their on-rates, with that of ASC-8 being 10-fold faster.
The Domains Defined by ASC-3 & ASC-8 Can Modulate Cellular Functions of α6β4
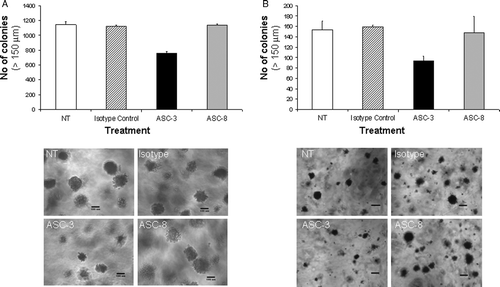
Given the above data depicting differences in functional properties of ASC-3 and ASC-8 using the soluble integrin constructs, we next asked whether these observations were recapitulated by endogenous α6β4 in cellular assays. We chose to explore the effects of ASC-3 and ASC-8 on anchorage-independent growth on tumor cell lines expressing endogenous protein based on the proposed involvement of α6β4 signaling in tumor progression (Cruz-Monserrate et al. Citation2007; Folgiero et al. Citation2007). shows the results of these assays using SW620 (colorectal) and MDA-MB-231 (breast) cancer cell lines. ASC-3 inhibited colony formation by 33% in SW620 and 40% in MDA-MB231. Furthermore, colony size in the ASC-3–treated group was smaller, with the majority of the colonies less than 150 µm in diameter. In contrast, ASC-8 had no effect on colony size or formation in either cell line. These data confirm that ASC-3 and ASC-8 exert different functional effects on endogenous α6β4 as was observed on recombinant soluble α6β4. Interestingly, the laminin-blocking effect exhibited by ASC-8 does not appear to play an important role in adhesion-independent growth.
Figure 7. Effects of β4-specific antibodies on anchorage-independent growth. (A) Colon (SW620) or (B) breast (MDA-MB-231) cancer cells were grown in 0.3% soft agar containing ASC-3 or ASC-8 (10 µg/ml). Plates were supplemented twice weekly with antibody-containing medium. Anchorage-independent growth was scored by manual counting of colonies >150 µm 21 to 27 days after plating. Error bars represent variation between duplicates. Pictures of colonies are representative examples. Size bar represents 199 µm.
DISCUSSION
Characterization of integrin binding interactions is often challenging given the complexities involved in purifying large quantities of these transmembrane proteins, coupled with the typically low protein yields obtained from expression of truncated molecules. This paper describes for the first time the generation of soluble active versions of integrin α6β4. Although our initial attempts to obtain soluble untagged proteins in significant quantities were unsuccessful, using methodology previously described for α4β1 (Stephens et al. Citation2000), we were rapidly able to generate high yields of soluble heterodimeric proteins by genetically fusing the Fc domain of human IgG1 C-terminal to either the α6 or β4 extracellular domain, thereby creating both α6β4-Fc and α6-Fcβ4 proteins. Our experience supports a role for the Fc moiety in facilitating dimerization, as suggested previously for α4β1-Fc (Stephens et al. Citation2000). Furthermore, subunit cytoplasmic and transmembrane regions are known to play a role in heterodimerization and surface expression of many integrins, including α6β4 (Briesewitz et al. Citation1993; De Melker and Sonnenberg Citation1996; Lu and Springer Citation1997), suggesting addition of the Fc in this case was necessary for successful production of the α6β4 ectodomain soluble proteins.
Both α6-Fcβ4 and α6β4-Fc proteins exhibited similar behavior patterns as endogenously expressed α6β4 in terms of ligand and ion dependency of adhesion interactions, as well as recognition by α6- or β4-specific antibodies. Collectively these observations confirm that the soluble α6β4 proteins exist in a physiologically relevant conformation and that the addition of the Fc moiety on either the α or β subunit does not alter the conformation and physiological function of the soluble integrin. Furthermore, the observed cation binding effects are similar to what we have described previously for interactions of α4β1 with the VCAM ligand, suggesting that α6β4-ligand interactions are regulated by divalent cations in a manner analogous to that of β1 integrins (Chen et al. Citation1999).
Monoclonal antibodies that recognize and block the function of integrins have become valuable tools to understand integrin biology. For example, integrin clustering induced by antibody ligation can result in conformational changes in integrin structure, leading to alterations in binding interactions and signaling events (Juliano and Haskill Citation1993; Schaapveld et al. Citation1998). With respect to α6β4, binding of certain β4-specific antibodies can inhibit adhesion to laminin, whereas others can induce Ras or PI3K signaling pathways (Chung et al. Citation2002; Mainiero et al. Citation1995; Russell et al. Citation2003). Here we show that the β4-specific antibodies ASC-3 and ASC-8 demonstrate similar recognition but different effects on soluble α6β4-laminin interactions, with the latter completely blocking adhesion and the former having no inhibitory effect. As the antibodies did not compete for binding to soluble α6β4, these combined data suggest that ASC-3 and ASC-8 recognize different epitopes on the protein. Taking advantage of the soluble α6β4 Fc proteins, we were able to purify the β4 subunit as a separate and stable entity, using it to further delineate epitope differences. The observed structural differences described here could not be identified in our cell based binding assays, due to the heterodimeric nature of the endogenously expressed molecule.
In efforts to explore whether the epitope differences between these two antibodies translated into functional effects on α6β4 activity in a physiological setting, we examined their effects in cellular assays in tumor cell lines. The ability to grow in an anchorage-independent manner is a common hallmark of cancer cells, and previous studies have suggested that α6β4 adhesion interactions contribute to this process (Lipscomb et al. Citation2005). We show a clear functional difference between the two β4 antibodies in this context, with ASC-3 having a pronounced inhibitory effect. As ASC-3 was previously shown to block tumor growth in vivo in a squamous carcinoma model (Dajee et al. Citation2003), these combined data suggest that anchorage-independent survival of some tumor cell types is in part α6β4-dependent. Furthermore, as cooperative signaling between α6β4 and receptor tyrosine kinases has been implicated previously in sustaining anchorage-independent growth (Bertotti Citation2005, Citation2006), it is possible that ASC-3 blocks a physical interaction between α6β4 and the requisite growth factor receptor regulating this process. Studies are currently underway to elucidate such interactions.
In contrast to the effects of ASC-3, ASC-8 had no effect on the adhesion-independent growth of the cells tested. As we clearly demonstrate the adhesion blocking effects of ASC-8 elsewhere in this paper, it seems plausible that different domains of the β4 integrin play different functional roles in mediating survival and adhesion. Indeed, previous studies showed that a truncated version of β4 lacking the ectodomain was equally capable of association with ErbB2 and activation of PI3K signaling as its full length counterpart in transfected NIH3T3 cells, whereas deletion of an intracellular domain of the protein abrogated these effects (Gambaletta et al. Citation2000). Similarly, a chimeric β4 comprising the β4 transmembrane and intracellular domain fused to a heterologous ectodomain showed normal clustering behavior but failed to induce Erk signaling in β4-null cancer cells (Merdek et al. Citation2007). It is possible to thus envision how different domains of α6β4 modulate different aspects of its biology, and that antibody blockade of specific domains could selectively alter specific α6β4 functions. The different kinetic parameters we observed for antibody binding may be indicative of epitope accessibility on the integrin, which could also contribute to the observed biological effects. Ongoing epitope mapping studies should provide additional insight into this. In summary, the availability of the soluble integrin tools described here will make it possible for us to further explore the nature of these functional epitopes, ultimately gaining a deeper understanding of α6β4 structural biology and how it relates to the physiological function of the integrin.
Acknowledgements
The authors wish to thank Y. Ren and E. Sewell for technical assistance, and Drs. P. Weinreb, M. Reff, D. Aivazian, and A. MacLaren for scientific discussion and critical review of the manuscript.
Notes
1Abbreviations: BSA, bovine serum albumin; CHO, chinese hamster ovary; CMV, cytomegalovirus; DTT, dithiothreitol; ELISA, enzyme-linked immunosorbent assay; Erk, extracellular signal-related kinase; FACS, fluorescence-activated cell sorting; FAK, focal adhesion kinase; FBS, fetal bovine serum; HPLC, high-performance liquid chromatography; HRP, horseradish peroxidase; Ig, immunoglobulin; IE, intermediate-early; ILK, integrin-linked kinase; IRS2, insulin receptor substrate 2; LTβR, lymphotoxin beta receptor; MEM, minimum essential medium; PBS, phosphate-buffered saline; PCR, polymerase chain reaction; PE, phycoerythrin; PI3K, phosphoinositide 3-kinase; PMSF, phenyl methane sulfonyl fluoride; PNGase F, peptide-N-glycosidase F; PTH, phenylthionhydantoin; RU, resonance units; SDS-PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis; SEC, size exclusion chromatography; Shc, Src homology domain containing; TBS, Tris-buffered saline; VCAM, vascular cell adhesion molecule.
References
- Akiyama SK, Yamada SS, Chen WT, Yamada KM. Analysis of fibronectin receptor function with monoclonal antibodies: Roles in cell adhesion, migration, matrix assembly, and cytoskeletal organization. J Cell Biol. 1989; 109: 863–875
- Bertotti A, Comiglio P, Trusolino L. Beta4 integrin is a transforming molecule that unleashes Met tyrosine kinase tumorigenesis. Cancer Res 2005; 65: 10674–10679
- Bertotti A, Comiglio P, Trusolino L. Beta4 integrin activates a Shp2-Src signaling pathway that sustains HGF-induced anchorage-independent growth. J Cell Biol 2006; 175: 993–1003
- Briesewitz R, Kern A, Marcantonio EE. Ligand-dependent and -independent integrin focal contact localization: The role of the alpha chain cytoplasmic domain. Mol Biol Cell 1993; 4: 593–604
- Browning JL, Sizing ID, Lawton P, Bourdon PR, Rennert PD, Majeau GR, Ambrose CM, Hession C, Miatkowski K, Griffiths DA, Ngam-ek A, Meier W, Benjamin CD, Hochman PS. Characterization of lymphotoxin-alpha beta complexes on the surface of mouse lymphocytes. J Immunol 1997; 159: 3288–3298
- Chen LL, Whitty A, Lobb RR, Adams SP, Pepinsky RB. Multiple activation states of integrin alpha4beta1 detected through their different affinities for a small molecule ligand. J Biol Chem 1999; 274: 13167–13175
- Chung J, Bachelder RE, Lipscomb EA, Shaw LM, Mercurio AM. Integrin (alpha6beta4) regulation of eIF-4E activity and VEGF translation: A survival mechanism for carcinoma cells. J Cell Biol 2002; 158: 165–174
- Clark K, Newham P, Burrows L, Askari JA, Humphries MJ. Production of recombinant soluble human integrin alpha4beta1. FEBS Lett 2000; 471: 182–186
- Cruz-Monserrate Z, Qiu S, Evers BM, O'Connor KL. Upregulation and redistribution of integrin alpha6beta4 expression occurs at an early stage in pancreatic adenocarcinoma progression. Mod Pathol 2007; 20: 656–667
- Dajee M, Lazarov M, Zhang JY, Cai T, Green CL, Russell AJ, Marinkovich MP, Tao S, Lin Q, Kubo Y, Khavari PA. NF-kappaB blockade and oncogenic Ras trigger invasive human epidermal neoplasia. Nature 2003; 421: 639–643
- De Melker AA, Sonnenberg A. The role of the cytoplasmic domain of alpha6 integrin in the assembly and function of alpha6beta1 and alpha6beta4. Eur J Biochem 1996; 241: 254–264
- Denda S, Muller U, Crossin KL, Erickson HP, Reichardt LF. Utilization of a soluble integrin-alkaline phosphatase chimera to characterize integrin alpha8beta1 receptor interactions with tenascin: Murine alpha8beta1 binds to the RGD site in tenascin-C fragments, but not to native tenascin-C. Biochemistry 1998; 37: 5464–5474
- Dransfield I, Cabanas C, Craig A, Hogg N. Divalent cation regulation of the function of the leukocyte integrin LFA-1. J Cell Biol 1992; 116: 219–226
- Eble JA, Wucherpfennig KW, Gauthier L, Dersch P, Krukonis E, Isberg RR, Hemler ME. Recombinant soluble human alpha3beta1 integrin: Purification, processing, regulation, and specific binding to laminin-5 and invasin in a mutually exclusive manner. Biochemistry 1998; 37: 10945–10955
- Folgiero V, Bachelder RE, Bon G, Sacchi A, Falcioni R, Mercurio AM. The alpha6beta4 integrin can regulate ErbB-3 expression: Implications for alpha6beta4 signaling and function. Cancer Res 2007; 67: 1645–1652
- Gambaletta D, Marchetti A, Benedetti L, Mercurio AM, Sacchi A, Falcioni R. Cooperative signaling between alpha6beta4 integrin and ErbB-2 receptor is required to promote phosphatidylinositol 3-kinase-dependent invasion. J Biol Chem 2000; 275: 10604–10610
- Giancotti FG, Ruoslahti E. Integrin signaling. Science 1999; 285: 1028–1032
- Grashoff C, Thievessen I, Lorenz K, Ussar S, Fassler R. Integrin-linked kinase: Integrin's mysterious partner. Curr Opin Cell Biol 2004; 16: 565–571
- Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol 2004; 5: 816–826
- Guo W, Pylayeva Y, Pepe A, Yoshioka T, Muller WJ, Inghirami G, Giancotti FG. Beta4 integrin amplifies ErbB2 signaling to promote mammary tumorigenesis. Cell 2006; 126: 489–502
- Hogervorst F, Kuikman I, Von dem Borne AE, Sonnenberg A. Cloning and sequence analysis of beta4 cDNA: An integrin subunit that contains a unique 118 kd cytoplasmic domain. EMBO J 1990; 9: 765–770
- Hynes RO. Integrins: Bidirectional, allosteric signaling machines. Cell 2002; 110: 673–687
- Juliano RL, Haskill S. Signal transduction from the extracellular matrix. J Cell Biol 1993; 120: 577–585
- Kennel SJ, Godfrey V, Chang LY, Lankford TK, Foote LJ, Makkinje A. The beta4 subunit of the integrin family is displayed on a restricted subset of endothelium in mice. J Cell Sci 1992; 101: 145–150
- Lee EC, Lotz MM, Steele GD, Jr, Mercurio AM. The integrin alpha 6 beta 4 is a laminin receptor. J Cell Biol 1992; 117: 671–678
- Lipscomb EA, Simpson KJ, Lyle SR, Ring JE, Dugan AS, Mercurio AM. The alpha6beta4 integrin maintains the survival of human breast carcinoma cells in vivo. Cancer Res 2005; 65: 10970–10976
- Lu CF, Springer TA. The alpha subunit cytoplasmic domain regulates the assembly and adhesiveness of integrin lymphocyte function-associated antigen-1. J Immunol 1997; 159: 268–278
- Mainiero F, Pepe A, Wary KK, Spinardi L, Mohammadi M, Schlessinger J, Giancotti FG. Signal transduction by the alpha6beta4 integrin: Distinct beta4 subunit sites mediate recruitment of Shc/Grb2 and association with the cytoskeleton of hemidesmosomes. EMBO J 1995; 14: 4470–4481
- Mehta RJ, Diefenbach B, Brown A, Cullen E, Jonczyk A, Gussow D, Luckenbach GA, Goodman SL. Transmembrane-truncated alphavbeta3 integrin retains high affinity for ligand binding: Evidence for an ‘inside-out’ suppressor?. Biochem J 1998; 330: 861–869
- Merdek KD, Yang X, Taglienti CA, Shaw LM, Mercurio AM. Intrinsic signaling functions of the beta4 integrin intracellular domain. J Biol Chem 2007; 282: 30322–30330
- Natali PG, Nicotra MR, Bigotti A, De Martino C. Localization of the alpha6 and beta4 integrin subunits in normal human non-lymphoid tissues. J Cell Sci 1992; 103: 1243–1247
- Niessen CM, Cremona O, Daams H, Ferraresi S, Sonnenberg A, Marchisio PC. Expression of the integrin alpha6beta4 in peripheral nerves: Localization in Schwann and perineural cells and different variants of the beta4 subunit. J Cell Sci 1994; 107: 543–552
- Peterson JA, Visentin GP, Newman PJ, Aster RH. A recombinant soluble form of the integrin alphaIIbbeta3 (GPIIb-IIIa) assumes an active, ligand-binding conformation and is recognized by GPIIb-IIIa-specific monoclonal, allo-, auto-, and drug-dependent platelet antibodies. Blood 1998; 92: 2053–2063
- Rabinovitz I, Toker A, Mercurio AM. Protein kinase C-dependent mobilization of the alpha6beta4 integrin from hemidesmosomes and its association with actin-rich cell protrusions drive the chemotactic migration of carcinoma cells. J Cell Biol 1999; 146: 1147–1160
- Rabinovitz I, Tsomo L, Mercurio AM. Protein kinase C-alpha phosphorylation of specific serines in the connecting segment of the beta 4 integrin regulates the dynamics of type II hemidesmosomes. Mol Cell Biol 2004; 24: 4351–4360
- Russell AJ, Fincher EF, Millman L, Smith R, Vela V, Waterman EA, Dey CN, Guide S, Weaver VM, Marinkovich MP. Alpha6beta4 integrin regulates keratinocyte chemotaxis through differential GTPase activation and antagonism of alpha3beta1 integrin. J Cell Sci 2003; 116: 3543–3556
- Schaapveld RQ, Borradori L, Geerts D, van Leusden MR, Kuikman I, Nievers MG, Niessen CM, Steenbergen RD, Snijders PJ, Sonnenberg A. Hemidesmosome formation is initiated by the beta4 integrin subunit, requires complex formation of beta4 and HD1/plectin, and involves a direct interaction between beta4 and the bullous pemphigoid antigen 180. J Cell Biol 1998; 142: 271–284
- Shaw LM. Identification of insulin receptor substrate 1 (IRS-1) and IRS-2 as signaling intermediates in the alpha6beta4 integrin-dependent activation of phosphoinositide 3-OH kinase and promotion of invasion. Mol Cell Biol 2001; 21: 5082–5093
- Sonnenberg A, Linders CJ, Daams JH, Kennel SJ. The alpha6beta1 (VLA-6) and alpha6beta 4 protein complexes: Tissue distribution and biochemical properties. J Cell Sci 1990; 96: 207–217
- Spinardi L, Ren YL, Sanders R, Giancotti FG. The beta 4 subunit cytoplasmic domain mediates the interaction of alpha6beta4 integrin with the cytoskeleton of hemidesmosomes. Mol Biol Cell 1993; 4: 871–884
- Staatz WD, Peters KJ, Santoro SA. Divalent cation-dependent structure in the platelet membrane glycoprotein Ia-IIa (VLA-2) complex. Biochem Biophys Res Commun 1990; 168: 107–114
- Staatz WD, Rajpara SM, Wayner EA, Carter WG, Santoro SA. The membrane glycoprotein Ia-IIa (VLA-2) complex mediates the Mg+ + -dependent adhesion of platelets to collagen. J Cell Biol 1989; 108: 1917–1924
- Stephens PE, Ortlepp S, Perkins VC, Robinson MK, Kirby H. Expression of a soluble functional form of the integrin alpha4beta1 in mammalian cells. Cell Adhes Commun 2000; 7: 377–390
- Suzuki S, Naitoh Y. Amino acid sequence of a novel integrin beta 4 subunit and primary expression of the mRNA in epithelial cells. EMBO J 1990; 9: 757–763
- Tagliabue E, Ghirelli C, Squicciarini P, Aiello P, Colnaghi MI, Menard S. Prognostic value of alpha6beta4 integrin expression in breast carcinomas is affected by laminin production from tumor cells. Clin Cancer Res 1998; 4: 407–410
- Urlaub G, Chasin LA. Isolation of Chinese hamster cell mutants deficient in dihydrofolate reductase activity. Proc Natl Acad Sci U S A 1980; 77: 4216–4220
- Watt FM. Role of integrins in regulating epidermal adhesion, growth and differentiation. EMBO J 2002; 21: 3919–3926
- Wilhelmsen K, Litjens SH, Sonnenberg A. Multiple functions of the integrin alpha6beta4 in epidermal homeostasis and tumorigenesis. Mol Cell Biol 2006; 26: 2877–2886
- Yoon SO, Shin S, Lipscomb EA. A novel mechanism for integrin-mediated ras activation in breast carcinoma cells: The alpha6beta4 integrin regulates ErbB2 translation and transactivates epidermal growth factor receptor/ErbB2 signaling. Cancer Res 2006; 66: 2732–2739