?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
A study of biochar/polypropylene composites has been conducted to describe the influence of biochars on the supermolecular structure of PP. The first stage of the work described the influence of carbonization on the properties of carbon fillers (tomato and miscanthus). The biochar was added to the polymer matrix, and the composite samples were manufactured by extrusion and injection molding. The influence of the fillers was investigated using WAXS, hot-stage optical microscopy, PLM microscopy, FTIR and ASTM designation. There were showed the strong influence of the biochar fillers on the properties of polypropylene matrix in composite materials. Obtained composites were characterized by the satisfying properties.
摘要
对生物炭/聚丙烯复合材料进行了研究,以描述生物炭对聚丙烯超分子结构的影响. 工作的第一阶段描述了碳化对碳填料(番茄和芒属植物)性能的影响. 将生物炭添加到聚合物基体中,并通过挤出和注射成型制备复合材料样品. 使用WAXS、热台光学显微镜、PLM显微镜、FTIR和ASTM名称研究了填料的影响. 在复合材料中,生物炭填料对聚丙烯基体的性能有很大的影响. 所获得的复合材料具有令人满意的性能.
Introduction
Composites of thermoplastic polymers filled with various plant materials – wood polymer composites (WPC) are widely used due to their processing and application properties. Such composite systems are also an interesting issue from the point of view of modern material science. It is related to the influence of plant additives on the properties, structure, and processing of polymer matrices. Well-known and analyzed fillers include materials such as wood, flax, hemp, jute, or rapeseed straw (Bledzki and Faruk Citation2006; Borysiak Citation2012; Le Digabel et al. Citation2004; Pracella et al. Citation2006; Kandola, Pornwannachai, and Ebdon Citation2020). Currently, many scientists are researching the influence of the use and crumbling of these fillers on the composite material. Also, another group of researchers is working to find and describe the effects of innovative plant-based lignocellulosic materials and use them as fillers for composites. Theoretically, all plant parts can be applied as fillers in WPC materials: leaves, stems, roots, and seeds. The common element for all these materials from various plant species is the content of cellulose, hemicellulose, and lignin as basic building materials. An important feature for all of these fibers is the chain structure and similar elemental composition. Cellulose fibers are a complex mixture of a homogeneous polymer with the elemental formula (C6H10O5)n.
Cellulose is often subjected to a variety of physicochemical modifications before being used as a filler in polymer composites. There are many methods to modify and functionalizing cellulose and nanocellulose. The vast majority of these methods focus on improving the dispersibility of cellulose in various solvents and the compatibility with the polymer matrix. The most commonly used methods include oxidation with, for example, acetic acid anhydride, polymer grafting, as well as the addition of functional groups to the surface, acetyl, ester, carboxyl, or siloxane groups (Schilling et al. Citation2012).
Biomass-based pyrolyzates and carbonizates (Das, Sarmah, and Bhattacharyya Citation2015), called simultaneously biocarbon or biochar, consist of a relatively new kind of fillers used in composite manufacturing. The pyrolysis, carbonization, and gasification processes allow materials to be completely different from those of biomass. In the literature, many types of carbonizates obtained from, e.g., wheat straw, wood shaving, pine cones, and many more (Cha et al. Citation2016; Doczekalska et al. Citation2020; Weber and Quicker Citation2018). The structure of biochar is strongly dependent on the physicochemical structure of the source material. The conversion of biomass into biochar eliminates the water consistence, decreases the ash content, improves the thermal stability, increases the elemental carbon content in the materials, and modifies the hydrophobic properties of biomaterials as well as their surface and porous properties, removing organic pollutants. The biochars may be used successfully during filling the composites based on: thermoplastics, rubbers, and thermosets and chemosets (Doczekalska et al. Citation2020).
Andrzejewski et al. (Citation2022) presented a complex study of polyoxymethylene (POM) and polyamide (PA) compounds filled with 10–30 wt% of biocarbon, obtained from pyrolyzed wood chips. The authors obtained materials with more than satisfactory properties. In addition, planned research includes the purchase of POM and PA composites with biochar content of more than 50% by weight. Andrzejewski and coworkers (Citation2022) investigated the following: polycarbonate (PC), acrylonitrile butadiene styrene (ABS), and polycarbonate/acrylonitrile-butadiene-styrene (PC/ABS) hybrid composites with biocarbon and carbon fiber. The results confirmed the beneficial effect of using a hybrid filler system on the properties of PC/ABS blends. For example, the results of the HDT tests were closely dependent on the presence or absence of carbon fibers in the PC/ABS/biocarbon composition. Moreover, the results clearly indicated a significant improvement in the uniformity of the structure for hybrid composites. Watt et al. (Citation2020) have also analyzed hybrid composites with biochar, but with the isotactic polypropylene (iPP) matrix and graphene nanoplatelets. The authors obtained composites with the potential to benefit the automotive field while simultaneously reducing the carbon footprint. The rigid polyurethane (PU) foam composites with biochar obtained by Uram et al. (Citation2021) were characterized with satisfactory properties. The thermal conductivity of PU composites with 20% weight of biocarbon did not worsen. In addition, the addition of biochar improved the dimensional and thermal stability. Biodegradable composites with biochar have been described in the literature (Musioł et al. Citation2022). The incorporation of biochar into the poly(butylene-terephthalate-adipate)/polylactic acid (PBAT/PLA) blend reduced the resistivity of the materials. In addition, the presence of biochar did not affect the thermal stability without influencing the biodegradation of the material. In the paper presented by Pudełko et al. (Citation2021) were described the polylactic acid (PLA) composites with up to 20% by weight biochar from sewage sludge or wood residues were described. The biochars decreased the impact strength of the materials compared to those of the materials made from native polymers. They have paid attention to the tendency to agglomeration of biochar’s particles in composite materials. That kind of composites can be successfully used as biodegradable material with low strength requirements.
Biocarbon can not only be waste-derived and may be obtained from renewable biomass, such as, eg, Giant miscanthus grass’ carbonizates (Anstey et al. Citation2016). The obtained material may be successfully modified and functionalized. The addition of biochar obtained from miscanthus to iPP improves the impact strength and material stiffness (Behazin, Misra, and Mohanty Citation2017). Wang et al. (Citation2018) reported the improvement of properties of composites with ball milled biochar from miscanthus comparing with material including nontreated biocarbon. Furthermore, increasing the milling time influences beneficially on composites’ impact strength of composites positively. The milling process also changes the porous structure of biochar.
So far, the effect on the properties of thermoplastic composites of the addition of carbonizates of crops characterized by a rapid growth of biomass has not been obtained or studied. In this work, the influence of the addition of waste-derived biochar from the stalks and leaves of tomato (Solanum lycopersicum L.) and miscanthus (Miscanthus giganteus) during the carbonization process. Therefore, the aim of this work is to examine the influence of carbonized parts of rapid growth biomass on the crystallization process of polypropylene, as well as the thermochemical properties. The influence of structure on the mechanical properties of biochar/iPP composites will be discussed in the second part of the paper.
Materials
The matrix of the composites is a TATREN HT 3 06. PP are produced by the Slovak company Slovnaft that are characterized by good processing stability. The basic processing parameters for this material are: melting temperature 165°C and Melt Flow Rate MFR 3 g/10 min (230°C/2.16 kg).
The fillers used in the production of composites were Giant miscanthus (Miscanthus giganteus) and tomato stems (Solanum lycopersicum L.). In the first stage, the plant material was subjected to a carbonization process (Das et al. Citation2018). The carbonization process was carried out in a chamber reactor in an oxygen-free atmosphere by heating to 600°C at a rate of 3°C/min and then maintained under stable conditions for 1 h. The carbonizates obtained () were ground mechanically to particles of size 0.5 to 1 mm ().
Obtaining of composite materials
In the first step, the filler was dried at 120°C for 8 h in a convectional laboratory dryer. Then, a mixture of fillers and isotactic iPP was prepared. Based on the preliminary analyses performed and the available literature data, it was decided to add a filler to the composite at a level of 25% by weight. Three types of samples were made:
PP_t − 25% carbonized tomato stalks + 75% PP;
PP_m − 25% carbonized giant miscanthus stalks + 75% PP;
PP – reference samples of isotactic PP.
The polymer with filler was processed in the Fairex single-screw extruder (McNeil Akron Repiquet L/D = 24, D = 25 mm). The particular temperature in the extruder zones is between 185°C and 205°C depending on the extruder zone.
The extruded composite material was cut with a knife granulator. The injection molding was performed in an ENGELES 80/20 HLS machine. Then, it was subjected to the injection process with operating parameters: injection speed 30 mm/s, nozzle temperature 220°C, zone 1 temperature 210°C, zone 2 temperature 200°C, zone 3 temperature 190°C and mold temperature 35°C. The samples were made in accordance with the PN-EN ISO 527–4:2000.
Elemental analysis
The content of carbon, hydrogen and nitrogen in the samples was determined using the Flash 2000 elemental analyzer (Thermo Fisher Scientific, USA). The calibration of the instrument was performed with the standards BBOT (2,5-bis-(tert-butyl-benzoxazol-2-yl)thiophene) and benzoic acid (Thermo Fisher Scientific, USA) and the certified reference material Alfalfa (Elemental Microanalysis Ltd., UK). For each element (C, H, N), the 6-point calibration curves were plotted using the K factor as the calibration method. The correctness of the method was verified by analysis of the C, H, and N content in the certified reference material Birch leaf (Elemental Microanalysis Ltd., UK).
Iodine numbers
Iodine numbers (IN) of biochars were determined on the basis of the standard test method ASTM Designation: ASTM-D4607-94. Therefore, the iodine number (mg I2/g carbon) was measured by titration at 30°C. From each biochar, three dried samples (0.1 g) were placed in separated flasks and fully wetted with 10 ml of 5% HCl. Then, 100 ml of 0.025 M standard iodine solution was poured into the flask, and the content was vigorously shaken for 30 s. After quick filtration, 50 ml of the solution was titrated using 0.1 M sodium thiosulfate with starch as an indicator. The concentration of iodine in the solution was calculated according to the formula of the total volume of sodium thiosulfate used:
V0, Vx – volumes of sodium thiosulfate solution used for the titration of the tested (Vx) and blank samples (V0) (mL);
cthio – concentration of sodium thiosulfate solution (M);
m – biochar sample (g);
126.92 – mass of 1 mole of iodine (g).
Fourier transform infrared spectroscopy (FTIR)
Infrared spectra were collected using a Vertex 70 spectrophotometer with Fourier transformation (Bruker, Germany). The ATR technique was used. The stem samples of tomato and miscanthus and biochar obtained from them were tested as fibers received without any preparation for measurement. This enables to study the microsurface of all samples as received. The spectra were collected at an air atmosphere. The resolution was 4 cm−1.
Structural investigations
In order to perform a more accurate structural analysis of the composite materials, Wide Angle X-Ray Scattering (WAXS) tests were performed. WAXS studies were performed using a TUR-M62 (Germany) wide-angle X-ray diffractometer with a HZG-3 goniometer (CuKα radiation, 30 kV and 20 mA anode excitation). The measured was investigated in a range of 2θ: 10–30° with step of 0.04°. The diffraction curve analysis was determined by usage of Hindeleh and Johnson model (modified by Rabiej) (Rabiej Citation2014).
The crystallinity degree Xc has been calculated based on the dependence: Xc = ∑Pc ∙ (∑Pc + ∑Pa)−1 where: ∑Pc is the sum of areas* of crystalline peaks and Pa is the area of the amorphous peak. The areas were determined using a graphical method, where the peaks are separated, deconvolution is applied, and the functions are integrated. There have been taken seven diffraction maxima to calculate Xc. Seven diffraction maxima have been taken into account for the Xc calculation, appropriate:
2θ = 14°, α plane (110)
2θ = 16°, β plane (300)
2θ = 17°, α plane (040)
2θ = 18°, α plane (130)
2θ = 21°, α plane (111)
2θ = 22°, α plane (131 + 041)
2θ = 25°, α plane (060)
Turner-Jones equation was used to calculate the amount of triclinic β phase in iPP and composites: k = Pβ ∙ (Pβ + Pα1 + Pα2 + Pα3)−1 where: k is the quantity of the β-form in the examined material, Pβ is the surface area of the diffractive maximum that derives from a diffraction on a surface (300) of the β-form, Pα1 is the surface area of the diffractive maximum deriving from a diffraction on a surface (110) of the α-form, Pα2 is the surface area of the diffractive maximum deriving from a diffraction on a surface (040) of the α-form, and Pα3 is the surface area of the diffractive maximum deriving from a diffraction on a surface (130) of the α-form – Pβ1, Pα1, Pα2, Pα3 (Rabiej, n.d.).
Thermal characterization
The materials were thermally characterized using the Differential Scanning Calorimetry (DSC) technique. The Netzsch DSC 200 (Germany) equipment has been used. The 8 mg of each sample were placed in the measuring can and heated to 200°C at a rate of 10°C/min under a nitrogen atmosphere and kept at this temperature for 5 min to erase their thermal history. The samples were cooled from 200°C to 40°C at a cooling rate of 5°C/min. This procedure was repeated twice, and the measurements of the second cycle were supplied to the further analysis. The crystallization temperatures (Tc) of samples were obtained from the maximum of the exothermic peaks, as well as the melting temperatures (Tm) from endothermic peaks’ maxima. The half-time of the crystallization and the crystal conversion course were also determined.
Thermogravimetric analysis
Thermogravimetric analysis (TG) was carried out on STA 449 F5 Jupiter-QMS of the NETZSCH in the following conditions: final temperature 600°C, rate of temperature increase − 10°C/min, atmosphere – helium flowing at the rate of about 20 ml/min. Weight of the sample was 20 mg ±1 mg.
Polarized light microscopy (PLM)
The PLM investigations were performed using a Polarized Light Microscope (PLM) – Labophot-2 (Nikon, Japan), equipped with TP93 (Linkam Scientific, UK) hot stage. The device was connected to Panasonic CCD camera (KR222, Japan). All of the composites were first heated to 210°C (rate of 40°C/min) and kept at this temperature for 5 min in order to erase a thermal history, especially for destroy the remaining nuclei. Then, the samples were cooled down to 145°C (rate of 40°C/min) at which the crystallization process was observed during 20 min.
Results and discussion – characterization of biochars
The results of the elemental analysis are summarized in . Differences in carbon content were found. The analysis showed that Miscanthus giganteus contained 15% more carbon than the tomato stalks. Miscanthus biochar also contains about 26% more carbon than biochar from tomato stalks. Raw materials have a strong influence on the chemical composition of biochar obtained from them.
Table 1. The results of elemental analysis of biomass and biochar.
Iodine number
In this study, the iodine number of biochar was determined. The iodine adsorption number or iodine number is one of the most important parameters to characterize carbon performance. The iodine number is expressed in mg/g. It is also the measurement of the micropore (0–20 Å) content of carbon samples by adsorption of iodine molecules from a solution. The iodine number is also depicted as an indication of the total surface area. The typical range for iodine number required for waste water application is 500–1200 mg/g, which is equivalent to surface area of carbon between 900 and 1100 m2/g (Mopoung et al. Citation2015; Saka Citation2012). Such IN values are characterized by activated carbons. In contrast, biochar does not have such a strongly developed specific surface area, resulting in relatively poor adsorption properties. The IN for Miscanthus giganteus biochar was found to be 170 mg/g, and for tomato stem biochar it was 210 mg/g. The values of the iodine number of obtained biochars indicate a heterogeneous structure and a less well-developed surface area. It should be emphasized that morphological and chemical structure of lignocellulose materials (precursors) strongly influence on physical and chemical properties of biochar. Literature shows that each natural material requires a specific attitude because of the variety of lignocellulose material composition, i.e. different chemical composition and anatomic structure. The elemental composition consists of more than 40% elemental carbon (calculated per dry mass), about 50% oxygen and up to 8% hydrogen. From among other elements, only nitrogen content exceeds 1%. The content of the major components like cellulose, hemicellulose and lignin varies in a wide range depending on the type of material and its origin. Cellulose content usually exceeds 40% in wood, 50% in straw and 60% in jute and hemp. Lignin content is 20–30%, about 19% and 14–20%, accordingly. Lignin content in the shells of fruit stones (plum and cherry) is much above 50%, while cellulose, only below 40%. Another major component of the lignocellulose materials is hemicelluloses − 15–35% (Antal Citation1983). High lignin content in the lignocellulose materials increases the efficiency of carbon residue since it is considered as one of the most thermally stable components of biomass. Hemicelluloses decompose at 180–350°C, cellulose at 275–350°C, while lignin at 250–500°C (Antal Citation1983). Lignin is a very important component which affects the efficiency of the carbonization process. Lower stability and high mass loss of polysaccharides could ascribed to high oxygen content in the molecules and structural factors. It should be emphasized that the mechanism of pyrolysis of the plant material is specific to each material. It is believed that in the early stage of carbonization (300–350°C), less stable bonds of the polymeric structure are decomposed and free macromolecules are formed. It is accompanied by the loss of volatile substances in form of water, carbon dioxide, methanol and methane and regrouping of carbon atoms into more stable form of a six-membered ring. Also, crosswise bonds between macromolecules are formed and due to a rigid, cross-linked carbon structure, microporosity of the material is observed. When the carbonization temperature exceeds 500°C, the increase of carbonization and aromatization of the structure due to the elimination of hydrogen and oxygen atoms is observed. Above 700°C, small clusters of layers of polycondensed six-membered carbon rings. However, strong crosswise bonds preclude their arrangement and graphitization process (Byrne and Marsh Citation1995).
Fourier-Transform Infrared Spectroscopy (FTIR)
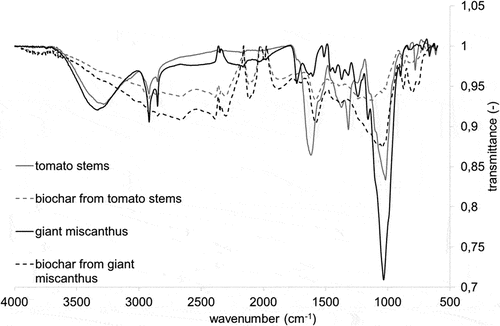
The FTIR spectra are presented in .
The tomato and miscanthus stems possess in their structure OH groups (bands at 3600–3000 cm−1). No aromatic CH bonds are seen in the spectra. The bands of CH bonds are present at ~2950–2840 cm−1 and indicate the presence of alkanes CH bonds (C sp3). They might also be characteristic for C-H of aldehydes. Bands for C=O bonds are also present; however, for miscanthus the band is at 1700 cm−1 whereas for tomato it is at 1615 cm−1 and it is larger and broader. This suggests that carbonyl from ketone is present in the structure of miscanthus stems, as regards tomato stems it might be amide bonds. The differences in the chemical structure of the tomato and miscanthus stems do not significantly reflect in the differences in the structure of biochar obtained from these two materials. The spectra in the range about ~3500–2500 cm−1 are characteristic of active carbon. The presence of different CO groups in two biochars can be seen in the range ~2200–2100 cm−1, 2000–1600 cm−1, 1600–1500 cm−1. The band at 1600–1500 cm−1 can suggest the presence of amide, amine, or nitro groups. More sophisticated techniques should be used for more in-depth analysis of the chemical structure of tested stems and biochar obtained from them. The most important conclusions from FTIR analysis are as follows:
stems from tomatoes and miscanthus differ significantly in the chemical structure,
biochar from both stems is chemically similar as regard microsurface structure and is mainly from carbon.
Thermal and structural properties of composites
The differences in the crystalline structure of PP in the materials examined can be observed in the . Three very important conclusions should be underlined during the interpretation of WAXS results.
Figure 3. XRD patterns of the examined samples, obtained for the skin and core layers a) PP skin, b) PP core, c) PP_m skin, d) PP_m core, e) PP_t skin, PP_t core.
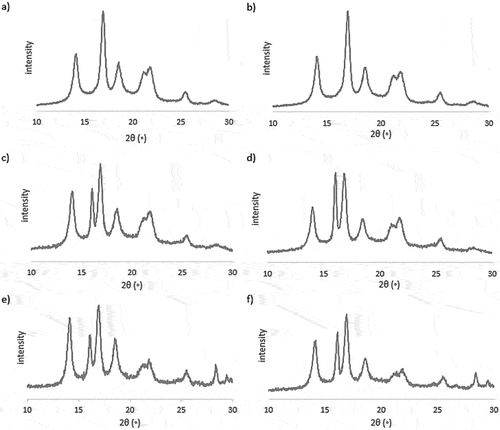
Firstly, the various values of intensities of appropriate maxima are closely connected with the formation of ordered structures. The increase in intensity will be dependent on the number of crystal planes oriented in the appropriate direction.
The intensity values are the highest for native PP, in the skin layer. It proves the high orientation of chains and their aggregates in the Y-axis of the sample that suits the flow direction during injection molding (Makhlouf et al. Citation2016). However, in the skin layer, the presence of miscanthus carbonizate caused the slight decrease in intensities. The flow of filler particles in the border of the skin and the intermediate layer blocks the arrangement of the linear chains. In the core layer of the sample, the effect is opposite – the intensity of maxima increases there with the addition of biochars. In that region, the orientation is forced by the oriented flow of miscanthus biochar particles. In addition, the intense shear between the filler and the PP matrix promotes the alignment. The strong decrease of intensities observed in the case of the PP_t material is unusual. The addition of 25% by weight of tomato biochar has obstacle drastically the orientation and stable alignment, in both of layers, skin and core.
The second important observation is connected with studying the values of degree of crystallinity of the Xc parameter. In the examined samples, the Xc is always higher in the core layer. The highest percentage of crystalline phase was noticed for the PP_m sample in both layers. Especially, in the core layer, the Xc has increased significantly – from 57% in unfilled iPP to 72% in PP_m. The addition of biochar from tomato steams does not practically change the Xc in skin and core layers, comparing with the unfilled polymer. It is surprising if one takes into account the interpretation of FTIR spectra (see 2.2 paragraph), confirming the similar chemical structure of biochars. However, the surface properties are different, which has resulted in a various nucleating ability in the composites.
The amount of β-iPP crystals in the crystalline phase of the polymer () is closely dependent on the applied biochar. In unfilled PP, the unfilled PP the β-crystals are spontaneously formed and occurs in the core region, which may be connected with the intense shear flow in the matrix (Bednarek et al. Citation2021; Garbarczyk and Borysiak Citation2004). Importantly, the incorporation of biochar from miscanthus has resulted in the formation of a high amount of beta phase in the skin layer in the composite. Perhaps, the flow of biochar particles in the interlayer promotes the formation of metastable crystallites in the skin layer through intense shear stress, except that the expansion of chains’ orientation (Zhao et al. Citation2018, Citation2019) (comp. and its interpretation).
Table 2. The degree of crystallinity (Xc) measured for samples and the amount of β-iPP phase in the overall crystalline volume in samples.
Analyzing the DSC results ( and ) it is possible to see the influence of biochars on the phase transitions in the crystallization and melting PP. There is not any difference in the values of the Tm of PP, PP_m, and PP_t samples. However, the crystallization temperature Tc increases with the incorporation of lignocellulosic fibers in the form of biochar from miscanthus and tomato steams (from 120°C for native iPP to 128–129°C for composites). It is a typical effect of the incorporation of lignocellulosic filler into PP matrix (Borysiak et al. Citation2016).
Figure 4. DSC thermograms for the examined samples obtained during: a) heating, b) cooling (the results were obtained during the second heating-cooling cycles).
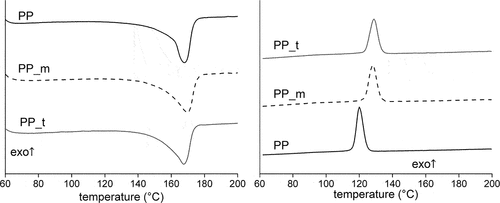
Figure 5. The crystalline conversion in samples during the nonisothermal crystallization process as a function of the crystallization time. Data were obtained using DSC method.
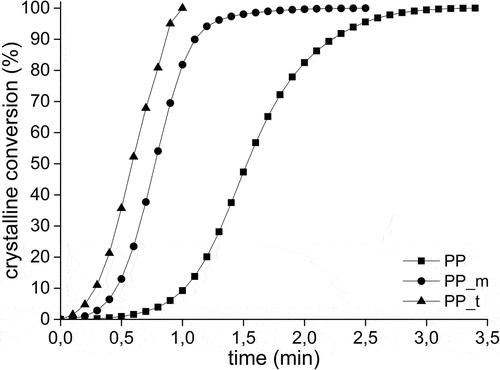
The course of the crystallization in the composites with both of used biochars is very similar (); however, the change of t0.5 value () is significant, from 93 s for unfilled PP, to 33 s for PP_m to 4 s for PP_t. The crystallization of the iPP matrix takes place in the fastest way, which theoretically is the most beneficial from the viewpoint of plastics processing. However, the reduction in the time available for ordering of macromolecules has resulted in the “quality” or “perfection” of the crystalline phase in the material and in the decrease of degree of the crystallinity (comp. ). Changes in t0.5 suggests the occurrence of differences in the nucleation and crystallization processes in the materials (Ichazo Citation2001).
Table 3. Selected parameters obtained during DSC analysis: Tc – crystallization temperature (°C); Tm – melting temperature (°C), t0.5 – half crystallization time (seconds).
Thermogravimetric analysis
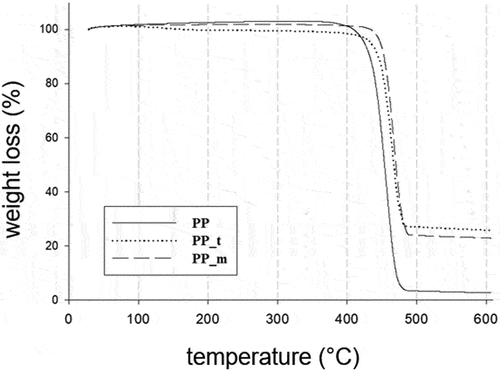
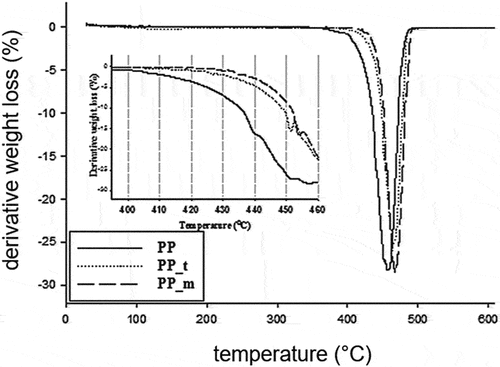
TGA and DTG curves of composites and PP samples are shown in (). Pure PP shows a single degradation step, while the composites show two degradation steps ().
Table 4. Results of thermogravimetric analysis of composites and PP.
The main stage of thermal decomposition of PP takes place in the temperature range of 350–500°C. Almost all PP decomposes in this area. Similar behavior of PP was described in their work by Mofokeng et al. (Citation2011).
In contrast, the decomposition of composites begins at higher temperatures at about 400°C and proceeds in two stages. The first small peak is observed for composites in the temperature range between about 405°C and 455°C. Weight losses in this area are 14% to 16%. Most probably, this stage is related to the thermal decomposition of biochar. The second stage of decomposition of the composites takes place in the temperature range at about 450°C to 490°C. Mass losses in this area are much higher, amounting for the PP_m composite to about 62% and for the PP_t composite to about 54%. The second stage of decomposition of the composites partially overlaps with that of PP and can be caused by thermolysis of the polymer matrix.
Crystallization of PP in the presence of biochars – PLM investigations
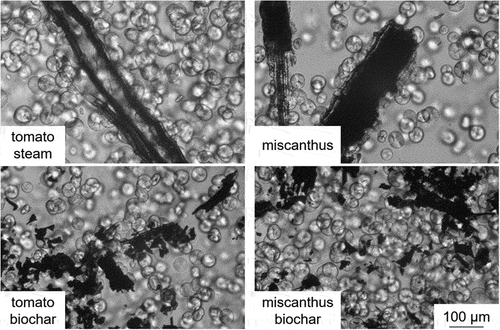
Microphotographs taken during PLM observations of crystallizing samples are presented in . In case of native additives, such as tomato steams and miscanthus, the intensified growth of spherulites on the border between the additive and the polymer matrix can be observed. The occurrence of a transcrystalline layer (TCL), which is characteristic of the native filler, where the cellulose I form is present (Odalanowska and Borysiak Citation2018, Citation2013). In the samples with biochars, the TCL does not occur and the spherulites grow spontaneously in all of the observed volume.
Conclusions
The presented research was focused on the issue of obtaining biochars from two various biomaterials: tomato stems and giant miscanthus. It is worth noting that in case of tomato steams, we are dealing with waste material, which may be troublesome in the cultivation of tomatoes. The leafs and steams should be utilized annually due to the risk of accumulation of the spores of Phytophthora infestans fungus. Furthermore, in case of giant miscanthus, the growth of biomass is very fast, and it is a plant useful during soil reclamation.
Analyzing the results: FTIR, iodine number determination and elemental analysis, it can be concluded that the plant materials used in the carbonization process, differed in chemical composition, which affected the structure of the obtained biochars. This also affected the structure and properties of polypropylene composites. Nevertheless, the supermolecular structure of PP in the composites is various in the case of both composites. The analyzed materials are active nucleating agents for iPP. However, the mechanism of formation of the β-iPP is different. The analyzed materials are not active nucleating agents for β crystallites of iPP. The formation of a polymorphic β form was observed, which is related to the occurrence of shear forces in the presence of biochars during processing. The addition of both fillers also affects the crystallization temperature and time. This observation will have direct application in the selection of industrial processing parameters.
Highlights
Carbonization of plant material (tomato and miscanthus stalks).
Examination of native filler properties and FTIR technique after the carbonization process, elemental analysis and iodine number.
Fabrication of composites by extrusion and injection of isotactic polypropylene and carbonized plant fillers.
Structural analysis of composites: DSC (Differential Scanning Calorimetry), WAXS (Wide Angle X-Ray Scattering), and
Microscopic observations of the effect of the addition of native and carbonized fibers on the crystallization of the matrix – Polarized Light Microscopy (PLM).
Summary and analysis of the results obtained.
Description of the conducted research and comparison of the results with data from the available literature.
Ethical approval
We confirm that all the research meets ethical guidelines and adheres to the legal requirements of the study country. The research does not involve any human or animal welfare related issues.
Acknowledgments
The authors would like to express their gratitude to Mrs. Klaudia Owczarzak.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Andrzejewski, J., J. Aniśko, and J. Szulc. 2022. “A Comparative Study of Biocarbon Reinforced Polyoxymethylene and Polyamide: Materials Performance and Durability.” Composites Part A: Applied Science and Manufacturing 152 (January): 106715. https://doi.org/10.1016/j.compositesa.2021.106715.
- Anstey, A., S. Vivekanandhan, A. Rodriguez-Uribe, M. Misra, and A. K. Mohanty. 2016. “Oxidative Acid Treatment and Characterization of New Biocarbon from Sustainable Miscanthus Biomass.” Science of the Total Environment 550 (April): 241–15. https://doi.org/10.1016/j.scitotenv.2016.01.015.
- Antal, M. J. 1983. “Biomass Pyrolysis: A Review of the Literature Part 1—Carbohydrate Pyrolysis.” In Advances in Solar Energy, edited by K. W. Böer and J. A. Duffie. Boston, MA: Springer. https://doi.org/10.1007/978-1-4684-8992-7_3.
- Bednarek, W. H., D. Paukszta, M. Szostak, and J. Szymańska. 2021. “Fundamental Studies on Shear-Induced Nucleation and Beta-Phase Formation in the Isotactic Polypropylene—Effect of the Temperature.” Journal of Polymer Research 28 (11): 439. https://doi.org/10.1007/s10965-021-02652-5.
- Behazin, E., M. Misra, and A. K. Mohanty. 2017. “Compatibilization of Toughened Polypropylene/Biocarbon Biocomposites: A Full Factorial Design Optimization of Mechanical Properties.” Polymer Testing 61 (August): 364–372. https://doi.org/10.1016/j.polymertesting.2017.05.031.
- Bledzki, A. K., and O. Faruk. 2006. “Injection Moulded Microcellular Wood Fibre–Polypropylene Composites.” Composites Part A: Applied Science and Manufacturing 37 (9): 1358–1367. https://doi.org/10.1016/j.compositesa.2005.08.010.
- Borysiak, S. 2012. “Influence of Wood Mercerization on the Crystallization of Polypropylene in Wood/PP Composites.” Journal of Thermal Analysis and Calorimetry 109 (2): 595–603. https://doi.org/10.1007/s10973-012-2221-x.
- Borysiak, S., Ł. Klapiszewski, K. Bula, and T. Jesionowski. 2016. “Nucleation Ability of Advanced Functional Silica/Lignin Hybrid Fillers in Polypropylene Composites.” Journal of Thermal Analysis and Calorimetry 126 (1): 251–262. https://doi.org/10.1007/s10973-016-5390-1.
- Byrne, J. F., and H. Marsh. 1995. Introductory Overview. Porosity in Carbons: Characterization and Applications, 1–48. London: Edward Arnold.
- Cha, J. S., S. H. Park, S. Jung, C. Ryu, J.-K. Jeon, S. Min-Chul, and Y. Park. 2016. “Production and Utilization of Biochar: A Review.” Journal of Industrial & Engineering Chemistry 40 (August): 1–15. https://doi.org/10.1016/j.jiec.2016.06.002.
- Das, O., N. K. Kim, M. S. Hedenqvist, R. J. T. Lin, A. K. Sarmah, and D. Bhattacharyya. 2018. “An Attempt to Find a Suitable Biomass for Biochar-Based Polypropylene Biocomposites.” Environmental Management 62 (2): 403–413. https://doi.org/10.1007/s00267-018-1033-6.
- Das, O., A. K. Sarmah, and D. Bhattacharyya. 2015. “A Sustainable and Resilient Approach Through Biochar Addition in Wood Polymer Composites.” Science of the Total Environment 512–513:326–336. https://doi.org/10.1016/j.scitotenv.2015.01.063.
- Doczekalska, B., M. Bartkowiak, B. Waliszewska, G. Orszulak, J. Cerazy-Waliszewska, and T. Pniewski. 2020. “Characterization of Chemically Activated Carbons Prepared from Miscanthus and Switchgrass Biomass.” Materials 13 (7): 1654. https://doi.org/10.3390/ma13071654.
- Garbarczyk, J., and S. Borysiak. 2004. “Composites of Polypropylene with Cellulose Fibres. 1. Influence of Conditions of Extrusion and Injection Processes on the Structure of the Polypropylene Matrix.” International Polymer Science and Technology 31 (11): 60–65. https://doi.org/10.1177/0307174X0403101114.
- Ichazo, M. N., C. Albano, J. Gonz, R. Perera, and M. V. Candal. 2001. “Polypropylene/Wood ¯our Composites: Treatments and Properties.” Composite Structures 8 (2–3): 207–214. https://doi.org/10.1016/S0263-8223(01)00089-7.
- Le Digabel, F., N. Boquillon, P. Dole, B. Monties, and L. Averous. 2004. “Properties of Thermoplastic Composites Based on Wheat-Straw Lignocellulosic Fillers.” Journal of Applied Polymer Science 93 (1): 428–436. https://doi.org/10.1002/app.20426.
- Makhlouf, A., H. Satha, D. Frihi, S. Gherib, and R. Seguela. 2016. “Optimization of the Crystallinity of Polypropylene/submicronic-Talc Composites: The Role of Filler Ratio and Cooling Rate.” Express Polymer Letters 10 (3): 237–247. https://doi.org/10.3144/expresspolymlett.2016.22.
- Mofokeng, J. P., A. S. Luyt, T. Tabi, and J. Kovacs. 2011. “Comparison of Injection Moulded, Natural Fibre-Reinforced Composites with PP and PLA as Matrices.” Journal of Thermoplastic Composite Materials 25 (8): 927–948. https://doi.org/10.1177/0892705711423291.
- Mopoung, S., P. Moonsri, W. Palas, and S. Khumpai. 2015. “Characterization and Properties of Activated Carbon Prepared from Tamarind Seeds by KOH Activation for Fe(iii) Adsorption from Aqueous Solution.” Scientific World Journal 1–9. https://doi.org/10.1155/2015/415961.
- Musioł, M., J. Rydz, H. Janeczek, A. Kordyka, J. Andrzejewski, T. Sterzyński, S. Jurczyk, et al. 2022. “(Bio)degradable Biochar Composites – Studies on Degradation and Electrostatic Properties.” Materials Science and Engineering: B 275 (January): 115515. https://doi.org/10.1016/j.mseb.2021.115515.
- Odalanowska, M., and S. Borysiak. 2018. “Analysis of the Nucleation Activity of Wood Fillers for Green Polymer Composites.” Fibres & Textiles in Eastern Europe 26 (2): 66–72. https://doi.org/10.5604/01.3001.0011.5741.
- Odolanowska, M., and S. Borysiak. 2013. “Fundamental Studies on Lignocellulose/Polypropylene Composites: Effects of Wood Treatment on the Transcrystalline Morphology and Mechanical Properties.” Journal of Applied Polymer Science 127 (2): 1309–1322. https://doi.org/10.1002/app.37651.
- Pornwannachai, W., J. R. Ebdon, and B. K. Kandola. 2020. “Fire-Resistant Flax-Reinforced Polypropylene/Polylactic Acid Composites with Optimized Fire and Mechanical Performances.” Journal of Thermoplastic Composite Materials 33 (7): 898–914. https://doi.org/10.1177/0892705718815538.
- Pracella, M., D. Chionna, I. Anguillesi, Z. Kulinski, and E. Piorkowska. 2006. “Functionalization, Compatibilization and Properties of Polypropylene Composites with Hemp Fibres.” Composites Science and Technology 66 (13): 2218–2230. https://doi.org/10.1016/j.compscitech.2005.12.006.
- Pudełko, A., P. Postawa, T. Stachowiak, K. Malińska, and D. Dróżdż. 2021. “Waste Derived Biochar as an Alternative Filler in Biocomposites - Mechanical, Thermal and Morphological Properties of Biochar Added Biocomposites.” Journal of Cleaner Production 278 (January): 123850. https://doi.org/10.1016/j.jclepro.2020.123850.
- Rabiej, M. 2014. “A Hybrid Immune–Evolutionary Strategy Algorithm for the Analysis of the Wide-Angle X-Ray Diffraction Curves of Semicrystalline Polymers.” Journal of Applied Crystallography 47 (5): 1502–1511. https://doi.org/10.1107/S1600576714014782.
- Saka, C. 2012. “BET, TG-DTG, FT-IR, SEM, Iodine Number Analysis and Preparation of Activated Carbon from Acorn Shell by Chemical Activation with ZnCl2.” Journal of Analytical and Applied Pyrolysis 95:21–24. https://doi.org/10.1016/j.jaap.2011.12.020.
- Schilling, J. S., J. Ai, R. A. Blanchette, S. M. Duncan, T. R. Filley, and U. W. Tschirner. 2012. “Lignocellulose Modifications by Brown Rot Fungi and Their Effects, as Pretreatments, on Cellulolysis.” Bioresource Technology 116 (July): 147–154. https://doi.org/10.1016/j.biortech.2012.04.018.
- Uram, K., M. Kurańska, J. Andrzejewski, and A. Prociak. 2021. “Rigid Polyurethane Foams Modified with Biochar.” Materials 14 (19): 5616. https://doi.org/10.3390/ma14195616.
- Wang, T., A. Rodriguez-Uribe, M. Misra, and A. K. Mohanty. 2018. “Sustainable Carbonaceous Biofiller from Miscanthus: Size Reduction, Characterization, and Potential Bio-Composites Applications.” BioResources 13 (2): 3720–3739. https://doi.org/10.15376/biores.13.2.3720-3739.
- Watt, E., M. A. Abdelwahab, M. R. Snowdon, A. K. Mohanty, H. Khalil, and M. Misra. 2020. “Hybrid Biocomposites from Polypropylene, Sustainable Biocarbon and Graphene Nanoplatelets.” Scientific Reports 10 (1): 10714. https://doi.org/10.1038/s41598-020-66855-4.
- Weber, K., and P. Quicker. 2018. “Properties of Biochar.” Fuel 217 (April): 240–261. https://doi.org/10.1016/j.fuel.2017.12.054.
- Zhao, J., C. Lu, S. Guo, K. Wang, and Q. Fu. 2018. “Polymorphic Structures Phase Diagram of Shear-Induced Isotactic Polypropylene/Carbon Fiber Cylindrites.” Materials & Design 150 (July): 40–48. https://doi.org/10.1016/j.matdes.2018.04.030.
- Zhao, J., J. Zhang, M. Jing, G. Sui, D. Liu, K. Wang, and Q. Fu. 2019. “Correlations Between Microstructure of α-Row Nuclei and Polymorphism of Shear-Induced IPP/Carbon Fiber Cylindrite.” Journal of Polymer Science Part B: Polymer Physics 57 (7): 368–377. https://doi.org/10.1002/polb.24790.