ABSTRACT
Nucleotide modifications constitute marks in RNA and DNA that contribute to gene regulation, development and other cellular processes. The understanding of their intricate molecular roles has been hampered by the high number of different modifications, the lack of effective methods and tools for their detection and quantification as well as by their complex structure-function relationship. The recent development of RNA and DNA immunoprecipitation followed by high-throughput sequencing (RIP- and DIP-seq) initiated detailed transcriptome- and genome-wide studies. Both techniques depend on highly specific and sensitive antibodies to specifically enrich the targeted modified nucleotides without background or potential biases. Here, we review the challenges and developments when generating and validating antibodies targeting modified nucleotides. We discuss antibody-antigen interactions, different strategies of antigen generation and compare different binder formats suitable for state-of-the-art high resolution mapping and imaging technologies.
Introduction
A wide range of chemically modified nucleic acids is present in DNA and RNA. It is generally accepted that the modification and isomerization of nucleotides serve as a regulatory layer to fine-tune vital cellular processes. More than 150 different modifications have been identified in different RNA families so far, approximately half of them in eukaryotes, but the functions of many of these modifications are still unclear.Citation1-3 In the past, most studies focused on tRNA, rRNA and, to a lower extent, mRNA, mainly because rRNA and tRNA are the most abundant RNA families and also exhibit the largest diversity in modified nucleotides.Citation3 In recent years, modified nucleotides have also been discovered in snRNA and miRNA precursor molecules.Citation4-6 In mRNA, beside various types of N7-methylguanosine (m7G) as 5′-capping nucleotides, only very few types of modified nucleotides have been identified in coding RNA sequences, e.g. 5-methylcytidine (5mC) and its oxidized form 5-hydroxylmethylcytidine (5hmC), N6-methyladenosine (m6A), N1-methyladenosine (m1A), pseudouridine (Ψ) and inosine (I).Citation7-12 A more detailed review on modified RNA nucleotides and their role in gene regulation has been published recently.Citation13
In vertebrate genomes, 5-methyldeoxycytidine (5mdC) was already discovered in 1948.Citation14 5mdC has a relatively high abundance of about 4% in the human genome and is the major heritable modification in DNA.Citation15 Once the 5mdC pattern is established, it has to be maintained in dividing cells to ensure the lineage specific gene expression pattern. Therefore, 5mdC was believed to be a stable modification except during early embryogenesis.Citation16 The fact that the 5mdC mark is actively removed in the paternal genome of the zygote was reported earlier,Citation17,18 but the discovery of TET-proteins in 2009 initiated a wealth of studies that shed light into the dynamic regulatory network which includes several oxidized 5mdC variants.Citation19,20 5mdC clusters in so called CpG islands in transcriptional regulatory regions. The balanced establishment and maintenance of the CpG methylation pattern is vital for development and normal cellular processes. After fusion of sperm and oocyte, an epigenetic reprogramming occurs including a massive reduction of CpG methylation.Citation17,21 The development of the totipotent zygote into pluripotent stem cells and further cell fate decisions correlate with a cell-type specific re-establishment of CpG-methylation patterns (reviewsCitation22,23). CpG methylation is challenged by active and passive processes which can lead to DNA demethylation, e.g., by reduced DNA methyltransferase activity or DNA repair pathways. It is becoming increasingly clear that lifestyle and environmental stress leads to altered methylation patterns, affecting aging and disease development including cancer progression.Citation23-26 5-methylcytidine is also found in tRNA, rRNA, and mRNA, with 5mC stabilizing tRNA, regulating translational fidelity in rRNA, in mRNA it is overrepresented in UTRs and in the neighbuorhood Ago binding sites but the function of 5mC in mRNA is not understood.Citation27-29
With the advent of more sensitive high-throughput profiling techniques, it was demonstrated that adenosine methylation in RNA and DNA provides an additional regulatory layer to many cellular processes including transcription, translation and epigenetic inheritance. For example, early studies proposed the existence of 6-methyldeoxyadenosine (m6dA) in eukaryotes, but direct evidence was only reported recently.Citation30-33 The abundance of m6dA differs in the genomes of various eukaryotic species, but is less frequent than 5mdC.Citation30-33 These studies also revealed that m6dA is functionally involved in transcriptional regulation, albeit using different mechanisms in distinct species. In mammalian mRNA, m6A constitutes 0.1–0.4% of all adenosine nucleotides.Citation34-36 m6A has also been identified in snoRNACitation6 and miRNA.Citation4 In mRNA, m6A is enriched around stop codons and levels increase during development.Citation37,38 Functionally, m6A affects alternative splicing patterns,Citation37 regulates translation by destabilizing mRNACitation38-41 and some steps of the translation process itself.Citation42-44 A related modification, N6–2′O-dimethyladenosine (m6Am), was recently reported as a cap-associated modified nucleotide stabilizing mRNA.Citation6,45 Currently, it is conceivable that some functions that have been originally attributed to m6A are in fact caused by m6Am, as the specificity of the used antibodies was unclear (ibid).
Transcriptome-wide mapping of m1A revealed an enrichment of this modification around the start codon. m1A dynamically acts as a positive regulator of translation upon stress conditions.Citation46,47 Also pseudouridine, a modified nucleobase that has been mainly analyzed in tRNA, is discussed as a modification with a regulatory function in mRNA.Citation48-50
These recent publications show that epitranscriptomics and epigenomics have become two of the most dynamic areas of research in cell biology. RIP- and DIP-seq techniques and also high-resolution imaging approaches to generate landscapes of appearance and dynamics of modified nucleic acids in eukaryotic cells depend on very specific and sensitive antibodies. However, many antibodies are often used without determining potential secondary effects in the experimental setting. In this review, we want to discuss the challenges in generating monoclonal antibodies against modified nucleotides. We will discuss aspects of antibody-antigen interactions, antigen generation, immunization and antibody validation.
Antibody-antigen interactions
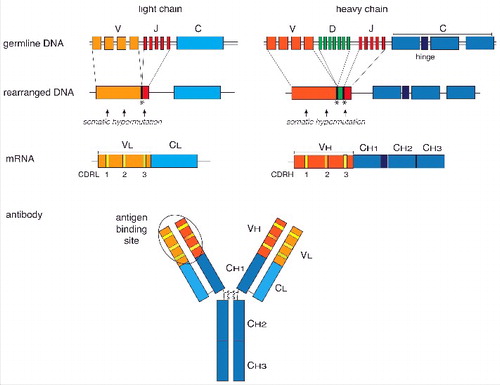
The fundamental properties of specificity and affinity of each antibody molecule are determined by the variable domains of the heavy (VH) and light chains (VL), (). The affinity of the primary B cell repertoire is low and increases if immunoglobulin genes of activated B cells undergo somatic hypermutation upon repeated encounter with antigens.Citation51 These mutations accumulate preferentially in the complementarity-determining regions (CDRs) of both the heavy and the light variable immunoglobulin genes ().Citation52 Each VH and VL domain contains 3 hypervariable CDRs (CDRH1 to 3 and CDRL1 to 3). CDRs are surface-exposed loops that form the antigen-binding site, and hypermutation of the CDRs results in structural changes in the antigen binding sites.Citation53 The CDRH3 loop is highly variable in length, sequence, and structure.Citation54 In humans and mice, the other 5 CDRs are less variable and assume only a limited number of canonical backbone conformations (reviewsCitation55,56). This hypervariability, particularly in CDRH3, determines antigen specificity and affinity.Citation56 In rabbits, however, due to a higher junctional diversity during VJ recombination, the CDRL3 loops are also heterogeneous in sequence and length and further contribute to high-affinity antigen binding.Citation57,58
Figure 1. Schematic overview of the organization and expression of immunoglobulin (Ig) genes. Different germline gene segments coding for the variable Ig heavy and light chains are joined by somatic V(D)J gene rearrangement (upper panels). Addition or removal of nucleotides during recombination at the junctions (symbolised by asterics) and somatic hypermutation (arrows) in the complementary-determining regions (CDR) of the VL and VH genes results in a high diversity of the Ig repertoire. The constant regions of the heavy chain are joined by RNA splicing to the variable regions. The heavy and light chains are covalently linked by disulfide bridges and fold into the typical Y-shaped immunoglobulin molecule. The antigen-binding site is formed by the CDRs of the heavy and light variable chains. A 3D shape of an Ig molecule can be found in the RCSB Protein Data Bank PDB ID: 1IGT (doi: 10.2210/pdb1igt/pdb).
Crystallographic X-ray analyses of antibody-antigen complexes have revealed a closer insight into the residues of CDRs interacting with the antigen.Citation59,60 The number and positions of the residues interacting with the antigen largely depend on the size of the antigen and determine the overall shape of the antigen-binding site.Citation61,62 Comparative studies of more than hundred immune complexes showed that large antigens such as proteins are bound in planar interaction sites, peptides within grooves, whereas small antigens, so-called haptens, are burried in deep cavities (reviewCitation63). Haptens are defined as small molecules of < 1kDa in size.Citation64 Modified nucleotides, which have an average molecular weight of 0.35 kDa, are therefore haptens.
On the molecular level, the interactions between antibody and antigen are non-covalent and reversible. They are based on a combination of hydrogen bonds, hydrophobic interactions, electrostatic and van der Waals forces.Citation65 To the best of our knowledge, no crystal structures of antibody complexes with modified nucleotides have been published yet. So far, analyses of several autoimmune anti-DNA antibody complexes revealed that high-affinity binding to DNA is mainly mediated through electrostatic forces between arginine residues in the CDR3H region and the bases or phosphate groups of the nucleic acid.Citation66 Small changes in the antigen alter the electrostatic and hydrophobic interactions and have a profound effect on the strength of the antibody-antigen interaction. For example, the addition of a methyl group to guanosine completely abolished binding of an anti-ssDNA antibody.Citation67 Similarly, substitution of a single arginine residue in the CDR3H loop by glycine abrogated antibody binding to ssDNA, while introduction of additional arginine residues into CDRH2 improved the binding affinity.Citation68 A study comparing 6 monoclonal antibodies raised against various modified nucleotides (5mC, m7G, Ψ, m1In, m1A, 4AcC) revealed high specificity of each antibody toward the respective antigen and no cross-reactivity against the other nucleotides.Citation69 These data demonstrate that modified positions of a nucleobase are part of the antibody interaction site and any change significantly alters the specificity and affinity of the interaction. Systematic structural analyses of antibodies bound to different modified nucleotides are required to gain insights of how specificity is achieved for nucleic acids that differ only in one position.
Modified nucleosides as antigens for antibody generation
Early attempts to generate antibodies against DNA were largely unsuccessful until it was discovered that carrier molecules improve the immune response (reviewCitation70). It is thought that antibodies against the most common B-DNA-form are generally difficult to generate in experimental settings because responsive B-lymphocytes are eliminated as self from natural repertoires.Citation71,72 Only certain ssDNA and less common DNA forms (e.g., Z-DNA), triplexes or DNA-RNA hybrids are targets of natural antibody responses (summarizedCitation73). Humans and animals therefore exhibit an immunotolerance to B-form DNA and autoantibodies against dsDNA are only found in patients diagnosed with e.g., systemic lupus erythematosus (SLE) or lupus nephritis as a result of progressive hypermutation in the CDR3.Citation74 The first antibodies against unusual and modified nucleotides were generated in the 1970s and early 1980s when it was discovered that tRNA contains several differently modified nucleotidesCitation75,76 and that 5mdC is important in gene regulation and cell development (reviewsCitation77,78).
RNA or DNA nucleotides are too small to elicit an immune response but it is possible to generate antibodies targeting single nucleosides by coupling the latter to an immunogenic carrier protein. Studies in the early 1960s identified BSA, a basic protein with many free –NH2 groups, as an appropriate carrier.Citation70 A stable covalent coupling of ribonucleosides was achieved by oxidation of the ribose ring with sodium periodate followed by a reductive condensation of the resulting aldehyde groups to the NH2-group of the lysine side chain.Citation79 The oxidation step opens the ribose ring between the 2′ and 3′ position and covalently couples the nucleobase via the opened ribose to the carrier protein (). As a consequence, antibodies generated against such antigens cannot discriminate between molecules differing at the ribose moiety, e.g., between RNA and DNA or nucleic acids with modified or non-modified 2′- and 3′-ribose OH-groups.Citation6 All antibodies directed against modified nucleosides generated so far are based on this coupling method. They target single nucleotides in DNA or RNA chains and neighboring nucleotides appear not to influence antibody binding. Consequently, these antibodies allow an unbiased determination of sequences flanking modified nucleotides and their potential consensus motifs. For example, antibodies generated against m6A led to the identification of the DRACH motif flanking m6A.Citation6,37,38,80 Other examples of antibodies currently in the spotlight are those specific for 5mC, m1A and m7G.Citation69,81-83 summarizes available antibodies against modified nucleosides and their applications.
Figure 2. Conjugation of nucleosides to carrier proteins. In a first step the 2′ and 3′ hydroxyl groups of the ribose of the nucleic acid are oxidized with IO4− at pH9–9.5. This allows the coupling to primary amino groups of carrier proteins, e.g., ϵ-NH2-group of lysine residues. The resulting unstable acid is subsequently stabilized by reduction with NaBH4.
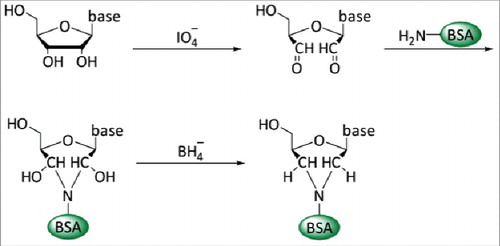
Table 1. Overview of the most commonly studied nucleic acid modifications and their analyses using antibody-based approaches.
Presently, a modified nucleobase-coupling protocol allows a more efficient binding of the hapten and also the coupling of nucleobases sensitive to oxidation or reduction such as m1A (pers. communication R. Hett / G. Meister). The use of cationized BSA, in which the carboxy groups of acidic amino acids are converted to aminoethylamide groups, enhances the resulting T-cell immune response and increases the immunogenicity of poorly immunogenic molecules.Citation84-86 In addition, it increases the number of -NH2 groups available for coupling with the nucleoside. The higher coupling efficiency increases the likelihood that 2 or more modified nucleobases are in close vicinity to each other on the carrier so that antibodies that specifically recognize pairs of adjacent modified nucleobases might be raised. Such antibodies, if undesired, have to be eliminated during the validation process or by affinity purification. Modern Click ChemistryCitation87 provides an alternative strategy to couple DNA or RNA nucleosides to the carrier protein. Here, the 5′OH group of the ribose is activated with azide allowing the copper(I)-catalyzed addition of the alkyne-linker molecule. This coupling method has the advantage that the ribose is kept intact, thus potentially allowing the generation of antibodies that discriminate between DNA and RNA nucleosides or recognize modified ribose OH groups. This is of special importance for the generation of antibodies that allow the discrimination between highly related modified nucleosides, for example nucleosides having the same chemical modification at one position in the nucleobase but differ in an additional modification of the ribose such as a methylated OH-group (e.g., m7G versus m7Gm). We are currently trying to establish monoclonal antibodies using DNA nucleosides coupled by Click chemistry to the carrier protein as antigens (in collaboration with T. Carell). One possible drawback of this method is the generation of antibodies that might bind to the modified nucleoside only in conjunction with the 1,2,3-triazole resulting from the Click reaction. These antibodies need to be excluded during the antibody validation procedure.
Comparison of different antibody formats
The validity of high-throughput approaches in epitranscriptomics and epigenomics directly depends on the specificity and affinity of the used antibody. Currently, polyclonal and monoclonal antibodies are used to target modified nucleic acids (). Both have their advantages and disadvantages. A systematic comparison of monoclonal and polyclonal antibodies targeting modified nucleotides has not been performed so far. However, a detailed comparison of polyclonal and monoclonal antibodies targeting different post-translational histone modifications in ChIP-seq experiments showed that monoclonal antibodies exhibit the same sensitivity as polyclonal antibodies, yet offer higher reproducibility.Citation88 Polyclonal antibodies, which are mainly produced in rabbits, comprise a heterogeneous mixture of antibodies that target different epitopes and bind the antigen with different affinities. They often perform well in multiple applications, as they can bind their targets under conditions of different pH and salt concentrations.Citation89 A major drawback of polyclonal antibodies is batch-to-batch inconsistencies. Affinity column purification, e.g., on modified and unmodified nucleotides is required to reduce carrier-specific and other unspecific antibodies from the immune serum. However, due to their heterogenic nature, validation for cross-reactivity, e.g., against similar nucleotide modifications or binding of 2 adjacent modified nucleotides, is difficult. In general, polyclonal antibodies exhibit a broader but less well characterized interaction pattern, which is the disadvantage of the larger antibody pool. In contrast, monoclonal antibodies, which are secreted from a single B cell clone, recognize a defined epitope with a given affinity and specificity. Their generation is more expensive and time-consuming as compared with polyclonal antibodies.Citation90 However, once a monoclonal antibody is established it can be repeatedly produced in large amounts and with consistent quality between different batches. In addition, cross-reactive or unspecific clones can be eliminated already during primary antibody screening (see next chapter).
The development of a rabbit myeloma cell line allowing the efficient fusion of rabbit B cells from immunised animals was a major improvement on the antibody market.Citation58,91 Because rabbit monoclonal antibodies often display high affinitiesCitation58 they could be a valuable alternative to those from mouse and rat for antibody DIP-and RIPseq studies. At present, we are not aware of rabbit monoclonals generated against modified nucleic acids. Unfortunately, the rabbit myeloma fusion cell line is not freely available for the scientific community. The recombinant cloning of smaller antibody formats, such as Fab or single-chain variable fragments (scFv) from established hybridoma cell linesCitation92,93 or of nanobodies dervied from camelid heavy chain antibodiesCitation94 can be useful especially for high-resolution imaging. Recombinant antibody technologies provide an alternative to the hybridoma technology. Different display technologies are now available to isolate the best binders from either immune or naive antibody libraries (summarizedCitation95). However, as far as we know, these technologies have not been applied for modified nucleotides so far.
Antibody validation
To serve as valuable and reliable research tools, antibodies need to be thoroughly validated regarding their affinity, specificity and reproducibility in the context of their intended use.Citation96 The primary screening process during monoclonal antibody generation already identifies hybridoma candidate clones that produce the strongest and most specific binders. Supernatants from several hundred hybridomas can easily be tested in a high-throughput manner in solid-phase enzyme-linked immunosorbent (ELISA) assays.Citation97 Detection ELISA assays are ideal to identify antibodies that recognize the desired antigen, e.g., by testing the binding of modified nucleobases coupled to a different carrier protein or on small modified oligonucleotides to ensure the identification of hapten-specific antibodies and to exclude carrier-specific ones.Citation69,83,92,98 Capture ELISA identifies antibodies that immunoprecipitate antigen with high affinity, a prerequisite for antibody-based mapping studies of modified nucleic acids. Competitive ELISA can further determine antibody specificity and cross-reactivity. Here, antibodies are pre-incubated with increasing amounts of the target antigen or potentially cross-reactive antigens as competitors before binding to the target antigen is assessed. The combination of these ELISA-based techniques allows identification of antibodies binding the desired antigens with high affinity and to eliminate those showing cross-reactivity.Citation69,83,92,98
Further validation is essential to verify the antibodies' performance in particular applications. Antibodies targeting modified nucleic acids were validated e.g., by DNA or RNA immunoprecipitation,Citation32,46,99 immuno-Northern blottingCitation99 or high-resolution nucleic acid mapping technologies.Citation6,32,37,100 Potential off-target binding and biases can be detected by using methylation-deficient control cells, as recently demonstrated for anti-m6A antibodies in mapping studies with N6-adenine-methylases-negative bacteria or yeast cells.Citation32,100 A study by Linder et al. revealed, that antibodies described as 6mA-specific, which were generated by coupling 6mA to the carrier protein by the reductive coupling method described aboveCitation79 () do not discriminate between m6A and m6Am6. This is not surprising, as the antigen used for immunization does not contain the intact 2′- and 3′-moieties of the ribose. These studies highlight the importance of antibody validation, especially if highly related targets exist in the same molecule, as it is the case in RNA and DNA. Antibodies targeting nucleic acids or modified nucleic acids are generated by coupling single (modified) nucleosides to the BSA carrier protein.Citation79 However, the resulting antibodies are used to detect the respective target in the context of complex structures, e.g., chromatin, dsDNA, protein-bound RNA or structured regions of RNA. These structures may impede antibody-antigen interactions. Most parts of RNA are single-stranded, but in DNA and in structured regions in RNA, nucleic acids pair with each other to make double-strands, and may therefore not be readily accessible for the antibody. For example, we observed that some antibody clones detecting a single modified nucleoside coupled to BSA in ELISA, did not bind to the same modified nucleotide in the context of RNA or DNA (own unpublished). In mammalian cells, the generation of CRISPR/Cas9-targeted methyltransferase knockout cell lines will be instrumental to identify antibodies without or low off-target activity.
Interestingly, apart from specific antigen binding, antibodies can be used to generate signatures on modified RNA after UV-induced crosslinking and mutations introduced during reverse transcription, allowing a precise mapping of modified nucleobases.Citation6 However, although directed against the same modification, some antibodies were shown to induce an inconsistent mutation pattern.Citation6
Summary and perspective
Modifications of nucleic acids are widespread, and the function of many of these modifications is far from understood. Antibodies specific for modified nucleotides are essential tools to address fundamental questions on the molecular regulation of life in this new era of epigenomics and epitranscriptomics. At present, antibodies against a limited number of DNA and RNA modifications are available. The generation of new specific antibodies will be instrumental to decipher the function of those modifications that have been neglected so far. Comparative structural studies of antibody complexes with modified nucleobases are required for an improved understanding of specific target recognition.
Antibodies directed against modified nucleobases are traditionally generated by immunization with nucleosides coupled to a carrier protein using the ribose part as a linker. Modern Click Chemistry now allows coupling by maintaining the sugar intact, thus permitting the generation of antibodies that may, in addition to recognizing specific modified nucleobases, be able to distinguish between different forms of the ribose moiety, such as ribose, deoxyribose, and O-methylated variants. Careful antibody validation is essential to reduce background through off-target activity and also to verify the applicability in a particular technique. Whenever possible, cellular knockout controls should be part of the validation process. Polyclonal antibodies are often not sufficiently characterized, and their functionality may vary from batch to batch, which may hamper reproducibility and comparability of results. Monoclonal antibodies from stable hybridoma cell lines have unlimited availability, allow for precise definition of specificity and affinity, and therefore more consistent experimentation. Several monoclonal antibody clones should be used to validate nucleotide mapping in RIP- and DIP-based experiments. The generation of high-affinity nucleotide-specific monoclonal antibodies in rabbits might further advance the epitranscriptomics field, especially for very rare modifications or in single-cell settings. Smaller binder formats, e.g., scFv that can be obtained by recombinant cloning of nucleoside-specific monoclonal antibodies or by recombinant display technologies, will be useful tools for high-resolution imaging.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed. Some antibodies described in this review are from commercial sources. We are not recommending a particular antibody for a specific experiment but are only citing publications where they have been used. We have no competing financial interest.
Acknowledgments
We thank Andreas Moosmann and Gunter Meister for critical reading of the manuscript, Gunter Meister, Robert Hett and Thomas Carell for personal communications and sharing unpublished, and Matthias Q. Kurz for the chemical drawings in and .
Funding
This work was supported by DFG grants SPP1784 and SFB1064 and by Institutional grants.
References
- Machnicka MA, Milanowska K, Osman Oglou O, Purta E, Kurkowska M, Olchowik A, Januszewski W, Kalinowski S, Dunin-Horkawicz S, Rother KM, et al. MODOMICS: a database of RNA modification pathways–2013 update. Nucleic Acids Res 2013; 41:D262-7; PMID: 23118484; http://dx.doi.org/10.1093/nar/gks1007
- Frye M, Jaffrey SR, Pan T, Rechavi G, Suzuki T. RNA modifications: what have we learned and where are we headed? Nat Rev Genet 2016; 17:365-72; PMID: 27140282; http://dx.doi.org/10.1038/nrg.2016.47
- Cantara WA, Crain PF, Rozenski J, McCloskey JA, Harris KA, Zhang X, Vendeix FA, Fabris D, Agris PF. The RNA Modification Database, RNAMDB: 2011 update. Nucleic Acids Res 2011; 39:D195-201; PMID: 21071406; http://dx.doi.org/10.1093/nar/gkq1028
- Alarcon CR, Lee H, Goodarzi H, Halberg N, Tavazoie SF. N6-methyladenosine marks primary microRNAs for processing. Nature 2015; 519:482-5; PMID: 25799998; http://dx.doi.org/10.1038/nature14281
- Gu J, Patton JR, Shimba S, Reddy R. Localization of modified nucleotides in Schizosaccharomyces pombe spliceosomal small nuclear RNAs: modified nucleotides are clustered in functionally important regions. RNA 1996; 2:909-18; PMID:8809017; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1369425/
- Linder B, Grozhik AV, Olarerin-George AO, Meydan C, Mason CE, Jaffrey SR. Single-nucleotide-resolution mapping of m6A and m6Am throughout the transcriptome. Nat Methods 2015; 12:767-72; PMID: 26121403; http://dx.doi.org/10.1038/nmeth.3453
- Delatte B, Wang F, Ngoc LV, Collignon E, Bonvin E, Deplus R, Calonne E, Hassabi B, Putmans P, Awe S, et al. RNA biochemistry. Transcriptome-wide distribution and function of RNA hydroxymethylcytosine. Science 2016; 351:282-5; PMID: 26816380; http://dx.doi.org/10.1126/science.aac5253
- Ge J, Yu YT. RNA pseudouridylation: new insights into an old modification. Trends Biochem Sci 2013; 38:210-8; PMID: 23391857; http://dx.doi.org/10.1016/j.tibs.2013.01.002
- Gilbert WV, Bell TA, Schaening C. Messenger RNA modifications: Form, distribution, and function. Science 2016; 352:1408-12; PMID: 27313037; http://dx.doi.org/10.1126/science.aad8711
- Pan T. N6-methyl-adenosine modification in messenger and long non-coding RNA. Trends Biochem Sci 2013; 38:204-9; PMID: 23337769; http://dx.doi.org/10.1016/j.tibs.2012.12.006
- Qiu S, Li W, Xiong H, Liu D, Bai Y, Wu K, Zhang X, Yang H, Ma K, Hou Y, et al. Single-cell RNA sequencing reveals dynamic changes in A-to-I RNA editome during early human embryogenesis. BMC Genomics 2016; 17:766; PMID: 27687780; http://dx.doi.org/10.1186/s12864-016-3115-2
- Zhao BS, Roundtree IA, He C. Post-transcriptional gene regulation by mRNA modifications. Nat Rev Mol Cell Biol 2017; 18:31-42; PMID: 27808276; http://dx.doi.org/10.1038/nrm.2016.132
- Chen K, Zhao BS, He C. Nucleic Acid Modifications in Regulation of Gene Expression. Cell Chem Biol 2016; 23:74-85; PMID: 26933737; http://dx.doi.org/10.1016/j.chembiol.2015.11.007
- Hotchkiss RD. The quantitative separation of purines, pyrimidines, and nucleosides by paper chromatography. J Biol Chem 1948; 175:315-32; PMID:18873306
- Breiling A, Lyko F. Epigenetic regulatory functions of DNA modifications: 5-methylcytosine and beyond. Epigenetics Chromatin 2015; 8:24; PMID: 26195987; http://dx.doi.org/10.1186/s13072-015-0016-6
- Monk M, Boubelik M, Lehnert S. Temporal and regional changes in DNA methylation in the embryonic, extraembryonic and germ cell lineages during mouse embryo development. Development 1987; 99:371-82; PMID:3653008; http://dev.biologists.org/content/99/3/371.long
- Oswald J, Engemann S, Lane N, Mayer W, Olek A, Fundele R, Dean W, Reik W, Walter J. Active demethylation of the paternal genome in the mouse zygote. Curr Biol 2000; 10:475-8; PMID: 10801417; http://dx.doi.org/10.1016/S0960-9822(00)00448-6
- Mayer W, Niveleau A, Walter J, Fundele R, Haaf T. Demethylation of the zygotic paternal genome. Nature 2000; 403:501-2; PMID: 10676950; http://dx.doi.org/10.1038/35000656
- Kriaucionis S, Heintz N. The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science 2009; 324:929-30; PMID: 19372393; http://dx.doi.org/10.1126/science.1169786
- Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, Agarwal S, Iyer LM, Liu DR, Aravind L, et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 2009; 324:930-5; PMID: 19372391; http://dx.doi.org/10.1126/science.1170116
- Santos F, Hendrich B, Reik W, Dean W. Dynamic reprogramming of DNA methylation in the early mouse embryo. Dev Biol 2002; 241:172-82; PMID: 11784103; http://dx.doi.org/10.1006/dbio.2001.0501
- Hemberger M, Dean W, Reik W. Epigenetic dynamics of stem cells and cell lineage commitment: digging Waddington's canal. Nat Rev Mol Cell Biol 2009; 10:526-37; PMID: 19603040; http://dx.doi.org/10.1038/nrm2727
- Tan L, Shi YG. Tet family proteins and 5-hydroxymethylcytosine in development and disease. Development 2012; 139:1895-902; PMID: 22569552; http://dx.doi.org/10.1242/dev.070771
- Jung M, Pfeifer GP. Aging and DNA methylation. BMC Biol 2015; 13:7; PMID: 25637097; http://dx.doi.org/10.1186/s12915-015-0118-4
- Zampieri M, Ciccarone F, Calabrese R, Franceschi C, Burkle A, Caiafa P. Reconfiguration of DNA methylation in aging. Mech Ageing Dev 2015; 151:60-70; PMID: 25708826; http://dx.doi.org/10.1016/j.mad.2015.02.002
- Ko M, An J, Rao A. DNA methylation and hydroxymethylation in hematologic differentiation and transformation. Curr Opin Cell Biol 2015; 37:91-101; PMID: 26595486; http://dx.doi.org/10.1016/j.ceb.2015.10.009
- Hussain S, Aleksic J, Blanco S, Dietmann S, Frye M. Characterizing 5-methylcytosine in the mammalian epitranscriptome. Genome Biol 2013; 14:215; PMID: 24286375; http://dx.doi.org/10.1186/gb4143
- Liu J, Jia G. Methylation modifications in eukaryotic messenger RNA. J Genet Genomics 2014; 41:21-33; PMID: 24480744; http://dx.doi.org/10.1016/j.jgg.2013.10.002
- Squires JE, Patel HR, Nousch M, Sibbritt T, Humphreys DT, Parker BJ, Suter CM, Preiss T. Widespread occurrence of 5-methylcytosine in human coding and non-coding RNA. Nucleic Acids Res 2012; 40:5023-33; PMID: 22344696; http://dx.doi.org/10.1093/nar/gks144
- Fu Y, Luo GZ, Chen K, Deng X, Yu M, Han D, Hao Z, Liu J, Lu X, Doré LC, et al. N6-methyldeoxyadenosine marks active transcription start sites in Chlamydomonas. Cell 2015; 161:879-92; PMID: 25936837; http://dx.doi.org/10.1016/j.cell.2015.04.010
- Greer EL, Blanco MA, Gu L, Sendinc E, Liu J, Aristizabal-Corrales D, Hsu CH, Aravind L, He C, Shi Y. DNA Methylation on N6-Adenine in C. elegans. Cell 2015; 161:868-78; ; PMID:25936839; http://dx.doi.org/10.1016/j.cell.2015.04.005
- Koziol MJ, Bradshaw CR, Allen GE, Costa AS, Frezza C, Gurdon JB. Identification of methylated deoxyadenosines in vertebrates reveals diversity in DNA modifications. Nat Struct Mol Biol 2016; 23:24-30; PMID: 26689968; http://dx.doi.org/10.1038/nsmb.3145
- Zhang G, Huang H, Liu D, Cheng Y, Liu X, Zhang W, Yin R, Zhang D, Zhang P, Liu J, et al. N6-methyladenine DNA modification in Drosophila. Cell 2015; 161:893-906; PMID: 25936838; http://dx.doi.org/10.1016/j.cell.2015.04.018
- Desrosiers R, Friderici K, Rottman F. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc Natl Acad Sci U S A 1974; 71:3971-5; PMID: 4372599; http://dx.doi.org/10.1073/pnas.71.10.3971
- Rottman F, Shatkin AJ, Perry RP. Sequences containing methylated nucleotides at the 5′ termini of messenger RNAs: possible implications for processing. Cell 1974; 3:197-9; PMID: 4373171; http://dx.doi.org/10.1016/0092-8674(74)90131-7
- Wei CM, Gershowitz A, Moss B. Methylated nucleotides block 5′ terminus of HeLa cell messenger RNA. Cell 1975; 4:379-86; PMID: 164293; http://dx.doi.org/10.1016/0092-8674(75)90158-0
- Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, Cesarkas K, Jacob-Hirsch J, Amariglio N, Kupiec M, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 2012; 485:201-6; PMID: 22575960; http://dx.doi.org/10.1038/nature11112
- Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell 2012; 149:1635-46; PMID: 22608085; http://dx.doi.org/10.1016/j.cell.2012.05.003
- Wang X, Lu Z, Gomez A, Hon GC, Yue Y, Han D, Fu Y, Parisien M, Dai Q, Jia G, et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature 2014; 505:117-20; PMID: 24284625; http://dx.doi.org/10.1038/nature12730
- Liu N, Dai Q, Zheng G, He C, Parisien M, Pan T. N(6)-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature 2015; 518:560-4; PMID: 25719671; http://dx.doi.org/10.1038/nature14234
- Yue Y, Liu J, He C. RNA N6-methyladenosine methylation in post-transcriptional gene expression regulation. Genes Dev 2015; 29:1343-55; PMID: 26159994; http://dx.doi.org/10.1101/gad.262766.115
- Choi J, Ieong KW, Demirci H, Chen J, Petrov A, Prabhakar A, O'Leary SE, Dominissini D, Rechavi G, Soltis SM, et al. N(6)-methyladenosine in mRNA disrupts tRNA selection and translation-elongation dynamics. Nat Struct Mol Biol 2016; 23:110-5; PMID: 26751643; http://dx.doi.org/10.1038/nsmb.3148
- Meyer KD, Patil DP, Zhou J, Zinoviev A, Skabkin MA, Elemento O, Pestova TV, Qian SB, Jaffrey SR. 5′ UTR m(6)A Promotes Cap-Independent Translation. Cell 2015; 163:999-1010; PMID: 26593424; http://dx.doi.org/10.1016/j.cell.2015.10.012
- Wang X, Zhao BS, Roundtree IA, Lu Z, Han D, Ma H, Weng X, Chen K, Shi H, He C. N(6)-methyladenosine Modulates Messenger RNA Translation Efficiency. Cell 2015; 161:1388-99; PMID: 26046440; http://dx.doi.org/10.1016/j.cell.2015.05.014
- Mauer J, Luo X, Blanjoie A, Jiao X, Grozhik AV, Patil DP, Linder B, Pickering BF, Vasseur JJ, Chen Q. Reversible methylation of m6Am in the 5′ cap controls mRNA stability. Nature 2017; 541:371-5; PMID: 28002401; http://dx.doi.org/10.1038/nature21022
- Dominissini D, Nachtergaele S, Moshitch-Moshkovitz S, Peer E, Kol N, Ben-Haim MS, Dai Q, Di Segni A, Salmon-Divon M, Clark WC, et al. The dynamic N(1)-methyladenosine methylome in eukaryotic messenger RNA. Nature 2016; 530:441-6; PMID: 26863196; http://dx.doi.org/10.1038/nature16998
- Li X, Xiong X, Wang K, Wang L, Shu X, Ma S, Yi C. Transcriptome-wide mapping reveals reversible and dynamic N(1)-methyladenosine methylome. Nat Chem Biol 2016; 12:311-6; PMID: 26863410; http://dx.doi.org/10.1038/nchembio.2040
- Carlile TM, Rojas-Duran MF, Zinshteyn B, Shin H, Bartoli KM, Gilbert WV. Pseudouridine profiling reveals regulated mRNA pseudouridylation in yeast and human cells. Nature 2014; 515:143-6; PMID: 25192136; http://dx.doi.org/10.1038/nature13802
- Li X, Zhu P, Ma S, Song J, Bai J, Sun F, Yi C. Chemical pulldown reveals dynamic pseudouridylation of the mammalian transcriptome. Nat Chem Biol 2015; 11:592-7; PMID: 26075521; http://dx.doi.org/10.1038/nchembio.1836
- Schwartz S, Bernstein DA, Mumbach MR, Jovanovic M, Herbst RH, Leon-Ricardo BX, Engreitz JM, Guttman M, Satija R, Lander ES, et al. Transcriptome-wide mapping reveals widespread dynamic-regulated pseudouridylation of ncRNA and mRNA. Cell 2014; 159:148-62; PMID: 25219674; http://dx.doi.org/10.1016/j.cell.2014.08.028
- Li Z, Woo CJ, Iglesias-Ussel MD, Ronai D, Scharff MD. The generation of antibody diversity through somatic hypermutation and class switch recombination. Genes Dev 2004; 18:1-11; PMID: 14724175; http://dx.doi.org/10.1101/gad.1161904
- Murphy K, Weaver C. Janeway's Immunobiology. New York: Garland Science, 2016:173-213.
- Manivel V, Sahoo NC, Salunke DM, Rao KV. Maturation of an antibody response is governed by modulations in flexibility of the antigen-combining site. Immunity 2000; 13:611-20; PMID: 11114374; http://dx.doi.org/10.1016/S1074-7613(00)00061-3
- Rock EP, Sibbald PR, Davis MM, Chien YH. CDR3 length in antigen-specific immune receptors. J Exp Med 1994; 179:323-8; PMID: 8270877; http://dx.doi.org/10.1084/jem.179.1.323
- Chothia C, Lesk AM, Tramontano A, Levitt M, Smith-Gill SJ, Air G, Sheriff S, Padlan EA, Davies D, Tulip WR, et al. Conformations of immunoglobulin hypervariable regions. Nature 1989; 342:877-83; PMID: 2687698; http://dx.doi.org/10.1038/342877a0
- Xu JL, Davis MM. Diversity in the CDR3 region of V(H) is sufficient for most antibody specificities. Immunity 2000; 13:37-45; PMID: 10933393; http://dx.doi.org/10.1016/S1074-7613(00)00006-6
- Kodangattil S, Huard C, Ross C, Li J, Gao H, Mascioni A, Hodawadekar S, Naik S, Min-debartolo J, Visintin A, et al. The functional repertoire of rabbit antibodies and antibody discovery via next-generation sequencing. MAbs 2014; 6:628-36; PMID: 24481222; http://dx.doi.org/10.4161/mabs.28059
- Zhu W, Yu GL. Rabbit Hybridoma. In: An Z, ed. Therapeutic Monoclonal Antibodies: From Bench to Clinic John Wiley and Sons, 2009:151-68; http://dx.doi.org/10.1002/9780470485408
- Davies DR, Padlan EA, Sheriff S. Antibody-antigen complexes. Annu Rev Biochem 1990; 59:439-73; PMID: 2197980; http://dx.doi.org/10.1146/annurev.bi.59.070190.002255
- Dunbar J, Krawczyk K, Leem J, Baker T, Fuchs A, Georges G, Shi J, Deane CM. SAbDab: the structural antibody database. Nucleic Acids Res 2014; 42:D1140-6; PMID: 24214988; http://dx.doi.org/10.1093/nar/gkt1043
- Almagro JC. Identification of differences in the specificity-determining residues of antibodies that recognize antigens of different size: implications for the rational design of antibody repertoires. J Mol Recognit 2004; 17:132-43; PMID: 15027033; http://dx.doi.org/10.1002/jmr.659
- Raghunathan G, Smart J, Williams J, Almagro JC. Antigen-binding site anatomy and somatic mutations in antibodies that recognize different types of antigens. J Mol Recognit 2012; 25:103-13; PMID: 22407974; http://dx.doi.org/10.1002/jmr.2158
- Finlay WJ, Almagro JC. Natural and man-made V-gene repertoires for antibody discovery. Front Immunol 2012; 3:342. PMID: 23162556; http://dx.doi.org/10.3389/fimmu.2012.00342
- Erkes DA, Selvan SR. Hapten-induced contact hypersensitivity, autoimmune reactions, and tumor regression: plausibility of mediating antitumor immunity. J Immunol Res 2014; 2014:175265; PMID: 24949488; http://dx.doi.org/10.1155/2014/175265
- Garcia C, Reinherz E, R. S, Wilson I. Antigen Recognition by B-cell and T-cell receptors. In: Murphy K, Weaver C, eds. Janeway's Immunobiology. New York: Garland Sciences, 2016:139-73.
- Krishnan MR, Jou NT, Marion TN. Correlation between the amino acid position of arginine in VH-CDR3 and specificity for native DNA among autoimmune antibodies. J Immunol 1996; 157:2430-9; PMID:8805642; http://www.jimmunol.org/content/157/6/2430.long
- Munns TW, Liszewski MK, Tellam JT, Ebling FM, Hahn BH. Antibody-nucleic acid complexes. Identification of antigenic determinant of a murine monoclonal antibody specific for single-stranded nucleic acids. Biochemistry 1982; 21:2929-36; PMID: 6179538; http://dx.doi.org/10.1021/bi00541a019
- Radic MZ, Mackle J, Erikson J, Mol C, Anderson WF, Weigert M. Residues that mediate DNA binding of autoimmune antibodies. J Immunol 1993; 150:4966-77. PMID: 8496598.
- Reynaud C, Bruno C, Boullanger P, Grange J, Barbesti S, Niveleau A. Monitoring of urinary excretion of modified nucleosides in cancer patients using a set of six monoclonal antibodies. Cancer Lett 1992; 61:255-62; PMID: 1739950; http://dx.doi.org/10.1016/0304-3835(92)90296-8
- Plescia OJ, Braun W. Nucleic acids as antigens. Adv Immunol 1967; 6:231-52. PMID: 4860247.
- Di Pietro SM, Centeno JM, Cerutti ML, Lodeiro MF, Ferreiro DU, Alonso LG, Schwarz FP, Goldbaum FA, de Prat-Gay G. Specific antibody - DNA interaction: A novel strategy for fight dna recognition. Biochemistry 2003; 42:6218-27; PMID: 12755625; http://dx.doi.org/10.1021/bi026866u
- Stollar BD. Antibodies to DNA. CRC Crit Rev Biochem 1986; 20:1-36; PMID: 3514122; http://dx.doi.org/10.3109/10409238609115899
- Anderson WF, Cygler M, Braun RP, Lee JS. Antibodies to DNA. Bioessays 1988; 8:69-74; PMID: 3282507; http://dx.doi.org/10.1002/bies.950080206
- Rekvig OP, Andreassen K, Moens U. Antibodies to DNA–towards an understanding of their origin and pathophysiological impact in systemic lupus erythematosus. Scand J Rheumatol 1998; 27:1-6. PMID: 9506871.
- Munns TW, Liszewski MK, Sims HF. Characterization of antibodies specific for N6-methyladenosine and for 7-methylguanosine. Biochemistry 1977; 16:2163-8; PMID: 861202; http://dx.doi.org/10.1021/bi00629a019
- Munns TW, Liszewski MK, Oberst RJ, Sims HF. Antibody nucleic acid complexes. Immunospecific retention of N6-methyladenosine-containing transfer ribonucleic acid. Biochemistry 1978; 17:2573-8; PMID: 354691; http://dx.doi.org/10.1021/bi00606a018
- Chandler LA, Jones PA. Hypomethylation of DNA in the regulation of gene expression. Dev Biol (N Y 1985) 1988; 5:335-49. PMID: 2481475.
- Razin A, Riggs AD. DNA methylation and gene function. Science 1980; 210:604-10; PMID: 6254144; http://dx.doi.org/10.1126/science.6254144
- Erlanger BF, Beiser SM. Antibodies Specific for Ribonucleosides and Ribonucleotides and Their Reaction with DNA. Proc Natl Acad Sci U S A 1964; 52:68-74; PMID: 14192660; http://dx.doi.org/10.1073/pnas.52.1.68
- Chen K, Lu Z, Wang X, Fu Y, Luo GZ, Liu N, Han D, Dominissini D, Dai Q, Pan T, et al. High-resolution N(6) -methyladenosine (m(6) A) map using photo-crosslinking-assisted m(6) A sequencing. Angew Chem Int Ed Engl 2015; 54:1587-90; PMID: 25491922; http://dx.doi.org/10.1002/anie.201410647
- Achwal CW, Iyer CA, Chandra HS. Immunochemical evidence for the presence of 5mC, 6mA and 7mG in human, Drosophila and mealybug DNA. FEBS Lett 1983; 158:353-8; PMID: 6409666; http://dx.doi.org/10.1016/0014-5793(83)80612-7
- Sano H, Royer HD, Sager R. Identification of 5-methylcytosine in DNA fragments immobilized on nitrocellulose paper. Proc Natl Acad Sci U S A 1980; 77:3581-5; PMID: 6251470; http://dx.doi.org/10.1073/pnas.77.6.3581
- Itoh K, Mizugaki M, Ishida N. Preparation of a monoclonal antibody specific for 1-methyladenosine and its application for the detection of elevated levels of 1-methyladenosine in urines from cancer patients. Jpn J Cancer Res 1988; 79:1130-8; PMID: 3143701; http://dx.doi.org/10.1111/j.1349-7006.1988.tb01536.x
- Apple RJ, Domen PL, Muckerheide A, Michael JG. Cationization of protein antigens. IV. Increased antigen uptake by antigen-presenting cells. J Immunol 1988; 140:3290-5. PMID: 3258879.
- Muckerheide A, Domen PL, Michael JG. Cationization of protein antigens. II. Alteration of regulatory properties. J Immunol 1987; 138:2800-4. PMID: 3494770.
- Muckerheide A, Apple RJ, Pesce AJ, Michael JG. Cationization of protein antigens. I. Alteration of immunogenic properties. J Immunol 1987; 138:833-7. PMID: 2433331.
- Himo F, Lovell T, Hilgraf R, Rostovtsev VV, Noodleman L, Sharpless KB, Fokin VV. Copper(I)-catalyzed synthesis of azoles. DFT study predicts unprecedented reactivity and intermediates. J Am Chem Soc 2005; 127:210-6; PMID: 15631470; http://dx.doi.org/10.1021/ja0471525
- Busby M, Xue C, Li C, Farjoun Y, Gienger E, Yofe I, Gladden A, Epstein CB, Cornett EM, Rothbart SB, et al. Systematic comparison of monoclonal versus polyclonal antibodies for mapping histone modifications by ChIP-seq. Epigenetics Chromatin 2016; 9:49; PMID: 27826357; http://dx.doi.org/10.1186/s13072-016-0100-6
- Lipman NS, Jackson LR, Trudel LJ, Weis-Garcia F. Monoclonal versus polyclonal antibodies: distinguishing characteristics, applications, and information resources. ILAR J 2005; 46:258-68; PMID: 15953833; http://dx.doi.org/10.1093/ilar.46.3.258
- Greenfield EA. Generating Monoclonal Antibodies. In: Greenfield EA, ed. Antibodies - A Laboratory Manual. New York: Cold Spring Harbor Laboratory Press, 2014:201-37.
- Spieker-Polet H, Sethupathi P, Yam PC, Knight KL. Rabbit monoclonal antibodies: generating a fusion partner to produce rabbit-rabbit hybridomas. Proc Natl Acad Sci U S A 1995; 92:9348-52; PMID: 7568130; http://dx.doi.org/10.1073/pnas.92.20.9348
- Mizugaki M, Itoh K, Yamaguchi T, Ishiwata S, Hishinuma T, Nozaki S, Ishida N. Preparation of a monoclonal antibody specific for 5-methyl-2′-deoxycytidine and its application for the detection of DNA methylation levels in human peripheral blood cells. Biol Pharm Bull 1996; 19:1537-40; PMID: 8996634; http://dx.doi.org/10.1248/bpb.19.1537
- Ohshima M, Tadakuma T, Hayashi H, Inoue K, Itoh K. Generation of a recombinant single-chain variable fragment (scFv) targeting 5-methyl-2′-deoxycytidine. J Biochem 2010; 147:135-41; PMID: 19815683; http://dx.doi.org/10.1093/jb/mvp151
- Pleiner T, Bates M, Trakhanov S, Lee CT, Schliep JE, Chug H, Böhning M, Stark H, Urlaub H, Görlich D. Nanobodies: site-specific labeling for super-resolution imaging, rapid epitope-mapping and native protein complex isolation. Elife 2015; 4:e11349; PMID: 26633879; http://dx.doi.org/10.7554/eLife.11349
- Ponsel D, Neugebauer J, Ladetzki-Baehs K, Tissot K. High affinity, developability and functional size: the holy grail of combinatorial antibody library generation. Molecules 2011; 16:3675-700; PMID: 21540796; http://dx.doi.org/10.3390/molecules16053675
- Bordeaux J, Welsh A, Agarwal S, Killiam E, Baquero M, Hanna J, Anagnostou V, Rimm D.. Antibody validation. Biotechniques 2010; 48:197-209; PMID: 20359301; http://dx.doi.org/10.2144/000113382
- O'Kennedy RJ, Reading CL. Rapid simple cell suspension enzyme-linked immunosorbent assay to demonstrate and measure antibody binding. Analyst 1990; 115:1145-6; PMID: 2256557; http://dx.doi.org/10.1039/an9901501145
- Itoh K, Mizugaki M, Ishida N. Detection of elevated amounts of urinary pseudouridine in cancer patients by use of a monoclonal antibody. Clin Chim Acta 1989; 181:305-15; PMID: 2758683; http://dx.doi.org/10.1016/0009-8981(89)90236-2
- Mishima E, Jinno D, Akiyama Y, Itoh K, Nankumo S, Shima H, Kikuchi K, Takeuchi Y, Elkordy A, Suzuki T, et al. Immuno-Northern Blotting: Detection of RNA Modifications by Using Antibodies against Modified Nucleosides. PLoS One 2015; 10:e0143756; PMID: 26606401; http://dx.doi.org/10.1371/journal.pone.0143756
- Schwartz S, Agarwala SD, Mumbach MR, Jovanovic M, Mertins P, Shishkin A, Tabach Y, Mikkelsen TS, Satija R, Ruvkun G, et al. High-resolution mapping reveals a conserved, widespread, dynamic mRNA methylation program in yeast meiosis. Cell 2013; 155:1409-21; PMID: 24269006; http://dx.doi.org/10.1016/j.cell.2013.10.047
- Amouroux R, Nashun B, Shirane K, Nakagawa S, Hill PW, D'Souza Z, Nakayama M, Matsuda M, Turp A, Ndjetehe E, et al. De novo DNA methylation drives 5hmC accumulation in mouse zygotes. Nat Cell Biol 2016; 18:225-33; PMID: 26751286; http://dx.doi.org/10.1038/ncb3296
- Gerlitz G, Bustin M. Efficient cell migration requires global chromatin condensation. J Cell Sci 2010; 123:2207-17; PMID: 20530575; http://dx.doi.org/10.1242/jcs.058271
- Sakai Y, Suetake I, Itoh K, Mizugaki M, Tajima S, Yamashina S. Expression of DNA methyltransferase (Dnmt1) in testicular germ cells during development of mouse embryo. Cell Struct Funct 2001; 26:685-91; PMID: 11942627; http://dx.doi.org/10.1247/csf.26.685
- Ficz G, Branco MR, Seisenberger S, Santos F, Krueger F, Hore TA, Marques CJ, Andrews S, Reik W. Dynamic regulation of 5-hydroxymethylcytosine in mouse ES cells and during differentiation. Nature 2011; 473:398-402; PMID: 21460836; http://dx.doi.org/10.1038/nature10008
- Jin SG, Kadam S, Pfeifer GP. Examination of the specificity of DNA methylation profiling techniques towards 5-methylcytosine and 5-hydroxymethylcytosine. Nucleic Acids Res 2010; 38:e125; PMID: 20371518; http://dx.doi.org/10.1093/nar/gkq223
- Wossidlo M, Nakamura T, Lepikhov K, Marques CJ, Zakhartchenko V, Boiani M, Arand J, Nakano T, Reik W, Walter J. 5-Hydroxymethylcytosine in the mammalian zygote is linked with epigenetic reprogramming. Nat Commun 2011; 2:241; PMID: 21407207; http://dx.doi.org/10.1038/ncomms1240
- Pastor WA, Pape UJ, Huang Y, Henderson HR, Lister R, Ko M, McLoughlin EM, Brudno Y, Mahapatra S, Kapranov P, et al. Genome-wide mapping of 5-hydroxymethylcytosine in embryonic stem cells. Nature 2011; 473:394-7; PMID: 21552279; http://dx.doi.org/10.1038/nature10102
- Wu H, D'Alessio AC, Ito S, Wang Z, Cui K, Zhao K, Sun YE, Zhang Y. Genome-wide analysis of 5-hydroxymethylcytosine distribution reveals its dual function in transcriptional regulation in mouse embryonic stem cells. Genes Dev 2011; 25:679-84; PMID: 21460036; http://dx.doi.org/10.1101/gad.2036011
- Masuda M, Nishihira T, Itoh K, Mizugaki M, Ishida N, Mori S. An immunohistochemical analysis for cancer of the esophagus using monoclonal antibodies specific for modified nucleosides. Cancer 1993; 72:3571-8; PMID: 8252470; http://dx.doi.org/10.1002/1097-0142(19931215)72:12%3c3571::AID-CNCR2820721205%3e3.0.CO;2-9
- Mishima E, Inoue C, Saigusa D, Inoue R, Ito K, Suzuki Y, Jinno D, Tsukui Y, Akamatsu Y, Araki M, et al. Conformational change in transfer RNA is an early indicator of acute cellular damage. J Am Soc Nephrol 2014; 25:2316-26; PMID: 24833129; http://dx.doi.org/10.1681/ASN.2013091001
- Bodi Z, Button JD, Grierson D, Fray RG. Yeast targets for mRNA methylation. Nucleic Acids Res 2010; 38:5327-35; PMID: 20421205; http://dx.doi.org/10.1093/nar/gkq266
- Bochnig P, Reuter R, Bringmann P, Luhrmann R. A monoclonal antibody against 2,2,7-trimethylguanosine that reacts with intact, class U, small nuclear ribonucleoproteins as well as with 7-methylguanosine-capped RNAs. Eur J Biochem 1987; 168:461-7; PMID: 2959477; http://dx.doi.org/10.1111/j.1432-1033.1987.tb13439.x
