ABSTRACT
Previously believed to be mere random degradation products, tRNA-derived small RNAs have been lately connected to a series of functions that include, surprisingly, genome protection against retrotransposons. tRNAs have been known for a long time to be involved in the replication cycle of retroviruses, pararetroviruses and retrotransposons as primers of their reverse transcription. tRNA-derived small RNAs, as functional small RNAs or as mere tRNA degradation products, have emerged as important players in the regulation of genic transcription. Nevertheless, the involvement of functional sRNAs derived from tRNA transcripts in transposon posttranscriptional control is a regulatory layer that remained elusive until now. Here I review the recent discoveries in the field that connect tRNA-derived small RNAs and retrotransposon control.
Genome structure and its dynamics are strongly influenced by the presence and activity of transposable elements (TEs). Genomes have allowed the extensive presence of TEs and, in exchange, TEs are used by genomes as structural parts that help them determine heterochromatic regions (like the centromere or the telomeres) and regulate gene expression [Citation1,2]. Counterintuitively, the known examples of TE-regulated genes are rare successful examples of ancient transposition events and, most of the times, the uncontrolled activity of TEs causes genome instability [Citation1]. Containing such potentially dangerous fragments within the genome requires mechanisms that help to avoid their potential transposition. These mechanisms are very efficient since most TEs are silent during most developmental stages and, moreover, their silenced state is efficiently inherited to the next generation [Citation2]. Despite that tight control, TE transcription is sporadically reactivated at specific times during development concurrent with the resetting of epigenetic marks in a process termed developmental relaxation of transposable element silencing (DRTS)[Citation3]. DRTS has been hypothesized to serve as a way of revealing TE transcriptional status or presence in the forms of messenger RNA (mRNA) or small RNAs (sRNAs) [Citation4]. Alternatively, DRTS can take place during very specific developmental processes due to the ability of TEs to use developmental signals, like transcription factors, to promote their expression [Citation5]. In order to protect genome stability against stress-induced or naturally reactivated TEs most eukaryotic organisms have evolved TE-controlling mechanisms based on RNAi [Citation2]. All these protection pathways involve the production of TE-derived sRNAs (piRNAs in animals or epigenetically activated small interfering RNAs [easiRNAs] in plants) that can degrade TE transcripts or induce DNA methylation at their genomic loci in a sequence specific manner [Citation6-10]. Although these mechanisms are relatively well known, the initial trigger of RNAi against TEs has been an enigma thus far. Recent work revealed that the initiation of TE silencing involves sRNAs derived from transfer RNA (tRNAs), as will be outlined in detail below.
Overview of tRNA-derived small RNAs: tRNA halves and tRNA-derived fragments
tRNAs are one of the best characterized RNA molecules. Extensively studied for more than 50 years [Citation11], they have a well defined role as mediators of messenger RNA (mRNA) translation into proteins. Nevertheless, new regulatory mechanisms of their transcription and stability are still being discovered [Citation12]. For example, in the last years the interaction between RNAi and tRNAs have been explored due to the increase in depth of high-throughput sRNA sequencing. Multiple studies in different species have defined two different classes of tRNA-derived sRNAs: tRNA-derived fragments (termed tRFs) which are between 18–28 nt in length, and tRNA-halves which are 30–35 nt fragments derived from cleavage within the anticodon loop [Citation13,Citation14]. Both classes of sRNAs accumulate during stress in several species [Citation13-16], tRFs and tRNA halves have very different biogenesis pathways and modes of action that, although yet unknown, are slowly being uncovered (summarized in ).
Figure 1. Distinct biogenesis pathways mediate biogenesis of tRFs and tRNA halves. Active tRNA genes transcribed by the RNA Polymerase III complex produce a precursor tRNA transcript that is cleaved by RNAse P and Z to produce a mature tRNA transcript. This is further stabilized by the Lupus Autoantigen (La) that prevents entering of tRNAs into RNA silencing pathways. Entering of unstable tRNAs into RNAi pathways leads to the production of tRFs, although tRFs from the trailer regions of the pre-tRNA can also be formed independent of Dicer activity. Mature and stable tRNAs are exported to the cytoplasm where they are methylated by Dnmt2 to prevent cleavage by angiogenin. Angiogenin cleavage produces two fragments from mature tRNAs, derived from the 5’ or 3’ ends of the mature tRNA and termed 5’ or 3’ tRNA halves.
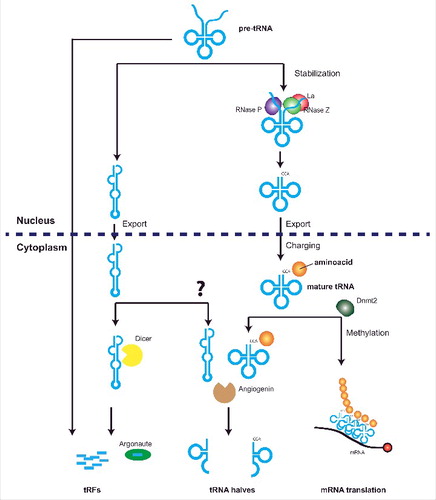
tRNA halves
In mammals, yeast and Drosophila, tRNA halves are fragments derived from a Dicer-independent but angiogenin/Rny1-dependent cleavage within the anticodon loop position of the tRNA mature molecule [Citation16]. This cleavage generates tRNA halves that can derive from both the 3’ and 5’ ends of mature tRNAs [Citation17]. Interestingly, the level of cytosine-5 methylation in the anticodon loop protects tRNAs against angiogenin cleavage and production of tRNA halves [Citation18-20]. The accumulation of tRNA halves correlates with reduced protein translation rates in both plants and mammals [Citation18,Citation20,Citation21]. Although close in length to piRNAs, tRNA halves interact directly with the ribosome [Citation22] so it seems unlikely that these fragments could be incorporated into argonaute (AGO) proteins and exert their functions through RNAi pathways.
tRFs
On the other hand, tRFs are integrated into the RNAi machinery. While different reports have showed different needs for Dicer in their biogenesis [Citation23-25], the majority of reports agree in their loading into AGO [Citation23,Citation26]. Three different classes of tRFs are produced from the different tRNA precursors and mature transcripts: tRF-5s and tRF-3s that derive from the 5’ or 3’ end of the mature tRNA molecule, and tRF-1s which derives from the 3’ trailer fragment of the precursor tRNA transcripts (reviewed in [Citation27]). The stability of the secondary structure of mature tRNAs plays an important role in the biogenesis of tRFs. Reduced levels of the Pol III transcript-stabilizing Lupus autoantigen (La) protein causes transport of immature and potentially mis-folded tRNA transcripts to the cytoplasm where they are processed by Dicer into tRFs and subsequently loaded into AGO [Citation28]. This suggests that tRF biogenesis could occur only when tRNA secondary structure is compromised, possibly explaining the apparent independence of Dicer for their biogenesis and their association with stress [Citation24,Citation25]. An alternative tRF biogenesis pathway, similar to that for tRNA halves, could involve angiogenin-mediated cleavage. Whether their biogenesis involves Dicer or not, tRFs are loaded into different AGO proteins [Citation23,Citation26,Citation28]. Loading into AGO could be indicative for a role of tRFs in posttranscriptional regulation, however, it could also mean that their ubiquitous presence under specific circumstances could saturate AGO and hijack RNAi pathways through impaired incorporation of miRNAs, siRNAs or piRNAs. Preliminary bioinformatic analysis indicated that in plants tRFs have sufficient sequence complementarity to endogenous mRNAs to potentially exert posttranscriptional regulation [Citation26,Citation29]. This has been later confirmed by data from cross-linking ligation and sequencing of hybrids (CLASH) and uncapped 5’-P RNA sequencing in both plants [Citation23] and mammals [Citation25,Citation30]. These techniques, previously used to identify miRNA-targeted mRNAs, have revealed that tRFs target numerous genic mRNAs in humans [Citation25], and that in plants their presence correlates with reduced transcript accumulation for some bioinformatically-predicted targets [Citation29]. Other reports have shown that a 19 nt tRF-5s, weakly associated with AGO [Citation31], and a 26 nt tRF-5s in the archaeon Haloferax volcanii are involved in the inhibition of translation [Citation32,Citation33], in a process that seems to be independent of the sequence complementarity between the tRF and the mRNA [Citation32]. It seems logical to envision that tRFs can have parallel roles inducing mRNA cleavage and inhibiting translation in a miRNA-like fashion [Citation34,Citation35]. Probably, in situations where tRFs are highly produced, canonical sRNAs (miRNAs, siRNAs and piRNAs) and tRFs compete for a limited amount of AGO proteins. Thus, an excess of tRFs present in the cytoplasm and unable to load into AGO, could be free (or more prone) to interact with the ribosome and interfere with translation. Interestingly, in the reproductive tissue of Drosophila, Aubergine-loaded piRNAs can sequester mRNAs without a perfect sequence complementarity in germ granules [Citation36]. The described roles of tRFs are summarized in .
Figure 2. Known and speculative activity of tRFs. A. tRFs are known to act at two different levels: First, tRFs loaded into argonaute (AGO) can induce RNAi on targets mRNAs and potentially induce the initiation of secondary sRNA production. At the same time, degradation of tRNAs to tRFs inhibits the translation of mRNAs through a pathway yet to be identified. Translation inhibition could also take place due to degradation of mature tRNAs into tRFs and potential lack of mature tRNAs. B. Interaction of tRFs with the replication cycle of retrotransposons. tRFs loaded into AGO proteins are known to target retrotransposons and induce the production of secondary sRNAs from their RNA transcripts. At the same time, lack of mature tRNAs can inhibit translation of retrotransposon proteins and prevent reverse transcription. C. Proposed mechanisms linking TE reactivation and tRF biogenesis. Left panel: During strong epigenetic reprogramming, erasure of H3K9me3 and H3K27me3 leads to TE reactivation and excessive tRNA production. Excessive tRNA pool could be incorporated into RNAi and induce the production of tRFs. Right panel: Spurious reactivation of TE transcription can lead to an anomalous usage of the tRNA pool that if sensed by the cell can lead to angiogenin delocalization from the nucleus to the cytoplasm. This could lead to increased degradation of tRNAs into tRNA halves or direct tRF production. * Seconday RNAi will only take place in organisms with RdRPs like plants, fungi and invertebrate animals.
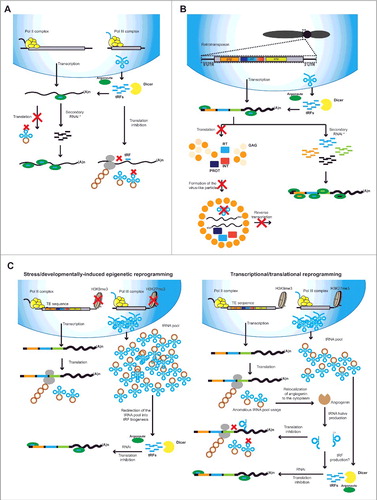
tRFs target transposable elements
Interestingly, two recent reports have shown that tRFs target TEs (and specifically retrotransposons) in both plants and mammals (). First, in Arabidopsis thaliana tRFs derived from the 5’ processing of mature tRNAs target TE transcripts (mainly elements of the Gypsy family of retrotransposons) [Citation23]. This specific class of tRF-5s accumulates naturally in the mature pollen grain, a tissue that also encloses a natural reactivation of TEs [Citation7]. Another recent study indicates that in mouse cells where TE reactivation takes place (SETB1-depleted embryonic and trophoblast stem cells) another class of tRFs, tRF-3s, regulate the transcription of retrotransposons by targeting their primer binding site (PBS) region [Citation30]. Retrotransposons, retroviruses and pararetroviruses harbor in their sequences a PBS sequence that is targeted through perfect complementarity by different tRNA molecules (including 5’ tRNA halves), but mainly by the 3’ region of certain mature tRNAs. Those mature tRNAs are captured from the cytoplasmic pool into retrotransposon viral-like structures and are used as primer molecules in their retrotranscription [Citation37,Citation38]. Schorn and colleagues (2017) describe the generation of two different tRF-3s targeting the PBS with different outcomes: 18 nt tRF-3s that inhibit transcription and 22 nt tRF-3s which induce RNAi of retrotransposons.
Both reports describe the accumulation of tRFs under situations where retrotransposon reactivation takes place (the pollen grain and mouse stem cells) pointing to a co-activation of transposon transcriptional activity and tRF biogenesis. tRNA and TE transcriptional activities are regulated by epigenetic marks that are, nevertheless, different in nature. While TEs are regulated by H3 lysine 9 trimethylation (H3K9me3) which is involved in the establishment of canonical heterochromatin, tRNAs are controlled by H3 lysine 27 trimethylation (H3K27me3) which is a heterochromatic mark dynamically regulated during development and controls the transcription of tRNAs with specific codons for the tRNA pool needs [Citation39]. Interestingly, anomalous tRNA processing in Drosophila leads to replication stress and indirect TE reactivation due to decreased H3K9me3 levels [Citation40]. Different scenarios could be envisioned about the intriguing concurrence of TE reactivation and tRF accumulation (). First, tRFs could derive as a side effect from the unused or excessive tRNA pool synthesized during heavy epigenetic reprogramming where both H3K9me3 and H3K27me3 are erased. Interestingly tRFs are produced during stress, which could be a consequence of a global re-organization of the tRNA pool [Citation39]. Stress is usually coupled to TE reactivation due to the reprogramming of epigenetic marks governing both stress-associated genes and TEs [Citation41,Citation42]. A second scenario, envisions that TE reactivation and translation of their coding sequences will cause impairing of the strongly regulated tRNA pool. Potentially, the cell could be able to sense these changes in codon usage and induce the cleavage of mature tRNAs in order to inhibit unwanted translation. Interestingly, during stress, angiogenin is relocated from the nucleus (where it stimulates rRNA transcription) to the cytoplasm where it mediates tRNA cleavage in stress granules [Citation43]. Potentially, the addition of TE coding sequences to the cytoplasm will add an extra need for rRNA transcription that could induce the cellular relocation of angiogenin.
Besides being produced during stress conditions, tRFs (and also tRNA-halves) accumulate in gametes and reproductive tissues [Citation23,Citation44]. Gametes and associated reproductive tissues experience a natural reactivation of TE expression as a consequence of epigenetic reprogramming. This epigenetic reprogramming involves a transcriptional reprogramming that could redirect the tRNA pool into the production of tRNA-derived sRNAs [Citation23,Citation44]. Interestingly tRF-5s and 5’ tRNA halves accumulating in the sperm have a transgenerational role and regulate the transcription of LTR-associated genes in the zygote by unknown mechanisms [Citation45,Citation46]. Gamete maturation and fertilization face the duality of requiring an extensive epigenetic and transcriptional reprogramming and ensuring the protection against epigenetically regulated TEs, which is basic for maintaining genome stability [Citation47-49]. The production of tRNA-derived sRNAs during reproduction could be a consequence of this duality, being an effect of epigenetic reprogramming and an active factor of TE control.
Speculations and future directions
In summary, taking together all the cases reported to date it is plausible to speculate that tRF production could be a buffer for TE transposition during extensive transcriptional and epigenetic reprogramming ensuring the transgenerational genome stability [Citation23]. Whether tRF biogenesis involves epigenetic or posttranscriptional regulation of the tRNA pool, using the degradation fragments from unused or excessive tRNAs is a cost-efficient way to regulate translation (through tRNA cleavage) and control in parallel the expression of TEs (through tRFs). Together with this, tRFs could have an alternative role as source of protective Pol III-dependent sRNAs when Pol II activity is impaired. For example, TE transcripts in plants are initially recognized by miRNAs, which direct the production of secondary easiRNAs [Citation50]. tRFs could take that role when Pol II activity is compromised and miRNAs cannot be produced.
The interaction between tRNAs and RNAi pathways is exciting and adds a new layer of regulatory power to tRNAs beyond their well-known role in regulating translation. Nevertheless, we have only uncovered the surface of the roles of tRNA-derived small RNAs. We do not yet completely understand why tRNA-derived sRNAs accumulate preferentially in gametes or under stress. It is furthermore unknown the potential role (if any) of tRNA halves as precursor molecules for tRF biogenesis. We also lack insights on how tRNAs adopted their role to regulate TE expression: have some tRNAs been selected during evolution due to their ability to produce tRNA halves or tRFs? Intriguingly tRNAs derive from the ancestral ligation of precursor tRNA halves [Citation51], raising the question if the ancestral role of tRNA precursors was the inhibition of translation rather than the promotion of it. The analysis of the role of tRFs is a relatively recent topic of research, however, it has changed our view of tRNAs as regulatory molecules. The ubiquitous presence of tRNAs in all organisms highlights the potential relevance that both tRFs and tRNA halves can have in the regulation of TE activity. From our previous perspective as passive RNAs to our current knowledge of their dynamic behavior, tRNAs are shaping our view on how functional RNAs can act at more than one layer of control.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Lisch D. How important are transposons for plant evolution? Nat Rev Genet. 2013;14:49–61. doi:10.1038/nrg3374
- Slotkin RK, Martienssen R. Transposable elements and the epigenetic regulation of the genome. Nat Rev Genet. 2007;8:272–85. doi:10.1038/nrg2072
- Martinez G, Slotkin RK. Developmental relaxation of transposable element silencing in plants: functional or byproduct? Curr Opin Plant Biol. 2012;15:496–502. doi:10.1016/j.pbi.2012.09.001
- Martienssen RA. Heterochromatin, small RNA and post-fertilization dysgenesis in allopolyploid and interploid hybrids of Arabidopsis. New Phytol. 2010;186:46–53. doi:10.1111/j.1469-8137.2010.03193.x
- Cavrak VV, Lettner N, Jamge S, et al. How a retrotransposon exploits the plant's heat stress response for its activation. PLOS Genetics. 2014;10:e1004115. doi:10.1371/journal.pgen.1004115
- Martinez G, Panda K, Kohler C, et al. Silencing in sperm cells is directed by RNA movement from the surrounding nurse cell. Nat Plants. 2016;2:16030. doi:10.1038/nplants.2016.30
- Slotkin RK, Vaughn M, Borges F, et al. Epigenetic reprogramming and small RNA silencing of transposable elements in pollen. Cell. 2009;136:461–72. doi:10.1016/j.cell.2008.12.038
- Ashe A, Sapetschnig A, Weick EM, et al. piRNAs can trigger a multigenerational epigenetic memory in the germline of C. elegans. Cell. 2012;150:88–99. doi:10.1016/j.cell.2012.06.018
- Malone CD, Brennecke J, Dus M, et al. Specialized piRNA pathways act in germline and somatic tissues of the Drosophila ovary. Cell. 2009;137:522–35. doi:10.1016/j.cell.2009.03.040
- Brennecke J, Malone CD, Aravin AA, et al. An epigenetic role for maternally inherited piRNAs in transposon silencing. Science. 2008;322:1387–92. doi:10.1126/science.1165171
- Hoagland MB, Stephenson ML, Scott JF, et al. A soluble ribonucleic acid intermediate in protein synthesis. J Biol Chem. 1958;231:241–57.
- Stoecklin G, Diederichs S. tRNAs: new tricks from old dogs. EMBO J. 2014;33:1981–3. doi:10.15252/embj.201489634
- Dhahbi JM. 5' tRNA Halves: The next generation of immune signaling molecules. Front Immunol. 2015;6:74. doi:10.3389/fimmu.2015.00074
- Pederson T. Regulatory RNAs derived from transfer RNA? RNA. 2010;16:1865–9. doi:10.1261/rna.2266510
- Wang Y, Li H, Sun Q, et al. Characterization of small RNAs derived from tRNAs, rRNAs and snoRNAs and their response to heat stress in wheat seedlings. PLoS One. 2016;11:e0150933. doi:10.1371/journal.pone.0150933
- Thompson DM, Parker R. Stressing out over tRNA cleavage. Cell. 2009;138:215–9. doi:10.1016/j.cell.2009.07.001
- Garcia-Silva MR, Cabrera-Cabrera F, G®πida MC, et al. Novel aspects of tRNA-derived small RNAs with potential impact in infectious diseases. Adv Biosci Biotechnol. 2013;4(5):9.
- Blanco S, Dietmann S, Flores JV, et al. Aberrant methylation of tRNAs links cellular stress to neuro-developmental disorders. EMBO J. 2014;33:2020–39. doi:10.15252/embj.201489282
- Schaefer M, Pollex T, Hanna K, et al. RNA methylation by Dnmt2 protects transfer RNAs against stress-induced cleavage. Genes Dev. 2010;24:1590–5. doi:10.1101/gad.586710
- Tuorto F, Liebers R, Musch T, et al. RNA cytosine methylation by Dnmt2 and NSun2 promotes tRNA stability and protein synthesis. Nat Struct Mol Biol. 2012;19:900–5. doi:10.1038/nsmb.2357
- Zhang S, Sun L, Kragler F. The phloem-delivered RNA pool contains small noncoding RNAs and interferes with translation. Plant Physiol. 2009;150:378–87. doi:10.1104/pp.108.134767
- Ivanov P, Emara MM, Villen J, et al. Angiogenin-induced tRNA fragments inhibit translation initiation. Mol Cell. 2011;43:613–23. doi:10.1016/j.molcel.2011.06.022
- Martinez G, Choudury SG, Slotkin RK. tRNA-derived small RNAs target transposable element transcripts. Nucleic Acids Res. 2017;45:5142–52. doi:10.1093/nar/gkx103
- Alves CS, Vicentini R, Duarte GT, et al. Genome-wide identification and characterization of tRNA-derived RNA fragments in land plants. Plant Mol Biol. 2016;93:35. doi:10.1007/s11103-016-0545-9.
- Kumar P, Anaya J, Mudunuri SB, et al. Meta-analysis of tRNA derived RNA fragments reveals that they are evolutionarily conserved and associate with AGO proteins to recognize specific RNA targets. BMC Biol. 2014;12:78. doi:10.1186/s12915-014-0078-0
- Loss-Morais G, Waterhouse PM, Margis R. Description of plant tRNA-derived RNA fragments (tRFs) associated with argonaute and identification of their putative targets. Biol Direct. 2013;8:6. doi:10.1186/1745-6150-8-6
- Kumar P, Kuscu C, Dutta A. Biogenesis and Function of Transfer RNA-Related Fragments (tRFs). Trends Biochem Sci. 2016;41:679–89. doi:10.1016/j.tibs.2016.05.004
- Hasler D, Lehmann G, Murakawa Y, et al. The Lupus Autoantigen La Prevents Mis-channeling of tRNA Fragments into the Human MicroRNA Pathway. Mol Cell. 2016;63:110–24. doi:10.1016/j.molcel.2016.05.026
- Wang Q, Li T, Xu K, et al. The tRNA-Derived Small RNAs Regulate Gene Expression through Triggering Sequence-Specific Degradation of Target Transcripts in the Oomycete Pathogen Phytophthora sojae. Front Plant Sci. 2016;7:1938. doi:10.3389/fpls.2016.01938
- Schorn AJ, Gutbrod MJ, LeBlanc C, et al. LTR-Retrotransposon Control by tRNA-Derived Small RNAs. Cell. 2017;170:61–71 e11. doi:10.1016/j.cell.2017.06.013
- Cole C, Sobala A, Lu C, et al. Filtering of deep sequencing data reveals the existence of abundant Dicer-dependent small RNAs derived from tRNAs. RNA. 2009;15:2147–60. doi:10.1261/rna.1738409
- Sobala A, Hutvagner G. Small RNAs derived from the 5' end of tRNA can inhibit protein translation in human cells. RNA Biol. 2013;10:553–63. doi:10.4161/rna.24285
- Gebetsberger J, Wyss L, Mleczko AM, et al. A tRNA-derived fragment competes with mRNA for ribosome binding and regulates translation during stress. RNA Biol. 2016:1–10.
- Li S, Liu L, Zhuang X, et al. MicroRNAs inhibit the translation of target mRNAs on the endoplasmic reticulum in Arabidopsis. Cell. 2013;153:562–74. doi:10.1016/j.cell.2013.04.005
- Llave C, Xie Z, Kasschau KD, et al. Cleavage of Scarecrow-like mRNA targets directed by a class of Arabidopsis miRNA. Science. 2002;297:2053–6. doi:10.1126/science.1076311
- Vourekas A, Alexiou P, Vrettos N, et al. Sequence-dependent but not sequence-specific piRNA adhesion traps mRNAs to the germ plasm. Nature. 2016;531:390–4. doi:10.1038/nature17150
- Marquet R, Isel C, Ehresmann C, et al. tRNAs as primer of reverse transcriptases. Biochimie. 1995;77:113–24. doi:10.1016/0300-9084(96)88114-4
- Mak J, Kleiman L. Primer tRNAs for reverse transcription. Journal of Virology. 1997;71:8087–95.
- Gingold H, Tehler D, Christoffersen NR, et al. A dual program for translation regulation in cellular proliferation and differentiation. Cell. 2014;158:1281–92. doi:10.1016/j.cell.2014.08.011
- Molla-Herman A, Valles AM, Ganem-Elbaz C, et al. tRNA processing defects induce replication stress and Chk2-dependent disruption of piRNA transcription. EMBO J. 2015;34:3009–27. doi:10.15252/embj.201591006
- Hunter RG, Gagnidze K, McEwen BS, et al. Stress and the dynamic genome: Steroids, epigenetics, and the transposome. Proc Natl Acad Sci U S A. 2015;112:6828–33. doi:10.1073/pnas.1411260111
- Probst AV, Mittelsten Scheid O. Stress-induced structural changes in plant chromatin. Curr Opin Plant Biol. 2015;27:8–16. doi:10.1016/j.pbi.2015.05.011
- Pizzo E, Sarcinelli C, Sheng J, et al. Ribonuclease/angiogenin inhibitor 1 regulates stress-induced subcellular localization of angiogenin to control growth and survival. J Cell Sci. 2013;126:4308–19. doi:10.1242/jcs.134551
- Garcia-Lopez J, Hourcade Jde D, Alonso L, et al. Global characterization and target identification of piRNAs and endo-siRNAs in mouse gametes and zygotes. Biochim Biophys Acta. 2014;1839:463–75. doi:10.1016/j.bbagrm.2014.04.006
- Sharma U, Conine CC, Shea JM, et al. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science. 2016;351:391–6. doi:10.1126/science.aad6780
- Chen Q, Yan M, Cao Z, et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science. 2016;351:397–400. doi:10.1126/science.aad7977
- Kawashima T, Berger F. Epigenetic reprogramming in plant sexual reproduction. Nat Rev Genet. 2014;15:613–24. doi:10.1038/nrg3685
- Seisenberger S, Peat JR, Hore TA, et al. Reprogramming DNA methylation in the mammalian life cycle: building and breaking epigenetic barriers. Philos Trans R Soc Lond B Biol Sci. 2013;368:20110330. doi:10.1098/rstb.2011.0330
- Feng S, Jacobsen SE, Reik W. Epigenetic reprogramming in plant and animal development. Science. 2010;330:622–7. doi:10.1126/science.1190614
- Creasey KM, Zhai J, Borges F, et al. miRNAs trigger widespread epigenetically activated siRNAs from transposons in Arabidopsis. Nature. 2014;508:411–5. doi:10.1038/nature13069
- Di Giulio M. The origin of the tRNA molecule: Independent data favor a specific model of its evolution. Biochimie. 2012;94:1464–6. doi:10.1016/j.biochi.2012.01.014
