ABSTRACT
Exportin 5 (Exp5, XPO5) is a nuclear export factor that functions in the microRNA (miRNA) biogenesis pathway to transport precursor miRNAs (pre-miRNAs) from the nucleus to the cytoplasm. Most of our current understanding of the Exp5 and pre-miRNA interaction is based on the investigation of how Exp5 binds to human pre-miR-30a (pre-miR-30 for short). As there are hundreds of human miRNA genes, how representative pre-miR-30 is, whether or how Exp5 interacts with distinct cargoes differentially, or whether Exp5 regulates miRNA expression, is unknown. Here we examined and compared the interactions between Exp5 and 157 human pre-miRNAs. We found that Exp5 binds distinct pre-miRNAs with modest variations in efficiencies, with the 3ʹ overhang and the apical loop in pre-miRNAs being the two major discriminating factors. Exp5 binding efficiencies do not significantly correlate with endogenous miRNA expression, suggesting that Exp5 activity does not contribute to differential miRNA expression in vivo. Nonetheless, in human cells with reduced Exp5 levels, preferential Exp5 binding correlated with miRNA expression changes. Thus, our study provides a global picture of how Exp5 interacts with human pre-miRNAs and its role in regulating miRNA expression.
Abbreviations: Exp5: Exportin 5; miRNA: microRNA; pri-miRNA: primary microRNA; pre-miRNA: precursor microRNA; nt: nucleotide
KEYWORDS:
1. Introduction
miRNAs are a class of approximately 22 nucleotide (nt) long RNAs that are widely distributed in metazoans and perform critical gene regulatory functions [Citation1]. miRNAs are generated through a step-wise process that starts with the transcription of primary miRNA transcripts (pri-miRNAs) and proceeds with the production of pre-miRNAs in the nucleus, and miRNA duplex intermediates and mature miRNAs in the cytoplasm [Citation1–10]. Exp5 binds many cellular RNAs, and as a member of the karyopherin family of nucleocytoplasmic transport factors, mediates the nuclear export of pre-miRNAs and potentially other minihelix-containing RNAs such as the human Y1 RNA, adenovirus VA1 RNA, and transfer RNAs [Citation11–17]. Exp5 acts in concert with its Ran cofactor. In the nucleus Ran is associated with the GTP molecule, and the Exp5:RanGTP complex binds its cargoes such as pre-miRNAs to escort the latter out of the nucleus. Once in the cytoplasm GTP is hydrolysed to GDP, leading to the release of pre-miRNAs from Exp5:RanGDP, allowing the downstream processing of pre-miRNAs.
The most physiological relevant class of Exp5 cargoes are the pre-miRNAs. Previous studies had examined how Exp5 binds to human pre-miR-30 and shown that Exp5 prefers an RNA stem of at least 16 basepairs but disfavours a 5ʹ overhang, and that Exp5 association additionally protects pre-miR-30 in the process [Citation18]. A solved crystal structure of the Exp5:Ran:pre-miR-30 complex provided a mechanistic explanation of those observations [Citation19]. Nonetheless, the human genome encodes hundreds of (pre-)miRNAs with different sequences and predicted secondary structures, and how Exp5 interacts with these diverse cargoes has not been explored, and whether Exp5 plays a regulatory role in global miRNA expression is unknown. The latter function has been proposed for Drosha, the enzyme that cleaves pri-miRNAs to produce pre-miRNAs [Citation6–10]. Human Drosha cleaves hundreds of its pri-miRNA substrates with different efficiencies, which correlates with mature miRNA expression in vivo, indicating that Drosha, a common miRNA processing factor just like Exp5, further contributes to differential miRNA expression [Citation20]. Does Exp5 analogously and preferentially bind to its pre-miRNA cargoes, and does Exp5 regulate miRNA expression at the genome-wide scale? To answer these questions, in this study we performed gel mobility shift assays to examine and compare how Exp5 interacted with 157 representative human pre-miRNAs, what RNA features modulate the interactions, and the potential implications on miRNA expression in vivo.
2. Materials and methods
2.1. Molecular cloning
The original plasmids expressing human Exp5 and Ran(Q69L), pKMyc-Exp5 and pQE60-Ran(Q69L), respectively, were from Addgene (Watertown, MA, USA) [Citation11]. Ran(Q69L) is a Ran mutant locked in a GTP-binding conformation, which facilitates the studies of Exp5 and RNA binding in vitro. To express proteins used in this study, we made pET-28a-Exp5 and pET-28a-Ran(Q69L). pET-28a-Exp5 was constructed by amplifying Exp5 sequence using Phusion DNA Polymerase (New England BioLabs, Ipswich, MA, USA) with primers: 5ʹ- CATGCCATGGCGATGGATCAAGTAAACGC-3ʹ and 5ʹ- ATTCGCGGCCGCGGGTTCAAAGATGGTGGCCAG-3ʹ, digesting the PCR product with NcoI and NotI (New England BioLabs) and inserting it into pET-28a(+) (Merck, Darmstadt, Germany). pET-28a-Ran(Q69L) was constructed by amplifying the Ran(Q69L) sequence with primers: 5ʹ- ACGCGTCGACGCATGGCTGCGCAGGGAG-3ʹ and 5ʹ- ATTCGCGGCCGCTCACAGGTCATCATCCTCATCCG-3ʹ, digesting the PCR product with SalI and NotI (New England BioLabs) and inserting it into pET-28a(+). Constructs were verified by Sanger sequencing (Sangon, Shanghai, China).
2.2. Protein purification
BL21 cells (Sangon) transformed with pET-28a-Exp5 or pET-28a-Ran(Q69L) were induced, lysed, and His-tagged proteins isolated using the Ni-NTA beads, per manufacture’s instructions (Qiagen, Hilden, Germany). Eluted fractions were dialysed against 20 mM Tris-HCl pH 7.5, 1 mM EDTA, 1 mM DTT, and 10% glycerol at 4°C overnight. Concentrations of Exp5 and Ran(Q69L) were estimated by comparison to known amounts of bovine serum albumin (BSA; Sangon) after gel electrophoresis and Coomassie staining. Proteins were stored at −20°C until use.
2.3. Pre-miRNA production
miRNA genomic sequence information was obtained from miRBase [Citation21]. Altogether this study examined 157 (pre-)miRNAs, representing most human miRNA families that we had previously tested in Drosha and Dicer processing experiments [Citation20,Citation22]. List of the pre-miRNAs are provided in Supplementary Table S1. To prepare templates for pre-miRNA production, the T7 promoter primer and a miRNA-specific 3ʹ primer were used to amplify the previously prepared DNA templates of pre-miRNAs [Citation22], or overlapping primers were designed, and DNA synthesized by PCR [Citation22], and then treated with the Klenow fragment to produce blunt ended DNA (New England BioLabs). Because the T7 RNA polymerase transcribes from a G residue, if the 5ʹ end of a pre-miRNA is not G, we would change it to G and modify the corresponding residue near the 3ʹ end, to maintain the predicted secondary structure [Citation22]. Pre-miRNAs were designed based on existing miRBase information, with additional variants constructed later similarly. All primer sequences are provided in Supplementary Table S1.
Pre-miRNAs were then synthesized by in vitro transcription using the T7 RiboMAX™ RNA Production System (Promega, Madison, WI, USA). After DNase digestion and Trizol purification (Invitrogen, Carlsbad, CA, USA), RNA was dissolved in water, quantified using Nanodrop (ThermoFisher, Waltham, MA, U.S) and denaturing gel electrophoresis, and stored at −20°C until use. For the radioactive probe used in gel shift assays, pre-miR-30 was in vitro transcribed (Promega) in the presence of [α-32P] CTP (PerkinElmer, Waltham, MA, USA) [Citation18].
2.4. Gel mobility shift assay
Gel shift assays were performed according to Zeng and Cullen [Citation18] with modifications. A typical binding reaction contained approximately 70–100 nM of Exp5, 1 αM of Ran(Q69L), and 0.5–1 nM of 32P-labled pre-miR-30 and was incubated at room temperature for 30–40 min. When an unlabelled (cold) pre-miRNA was included for competition, it was usually used at 200 nM and incubated with Exp5 and Ran(Q69L) for 15 min before 32P-labelled pre-miR-30 was added. To compare results from experiments performed on separate days, a sample with the cold pre-miR-30 competitor was used in every binding experiment as a normalization control. Reactions were resolved by electrophoresis on 8% native polyacrylamide gels. Afterwards gels were fixed, and data analysed by phosphorimaging (GE Healthcare, Chicago, IL, USA). For each pre-miRNA, its raw competition efficiency was calculated as the ratio of free 32P-pre-miR-30 in the presence of the cold pre-miRNA. Relative competition or binding efficiency was then calculated as the raw competition efficiency dividing that of the cold pre-miR-30 in the same experiment, which was set at 1. Each pre-miRNA was tested at least twice to obtain a consiste0nt result.
2.5. Statistics and secondary structure prediction
GraphPad Prism 5.0 (GraphPad Software, San Diego, CA, USA) was used for Spearman rank correlation, Student’s t-test, paired t-test, and Mann-Whitney test (two-tailed). miRNA genomic sequence and family information was retrieved from miRbase [21]. miRNA expression data in 20 human tissues were acquired from the National Center for Biotechnology Information’s Gene Expression Omnibus datasets (Supplementary Table S1). The expression level of a miRNA was calculated as the sum of the sequence reads or microarray signals of the corresponding miRNA-5p and miRNA-3p [Citation20]. Normalized expression values of individual miRNAs from diverse human tissues were combined to reduce cell-specific effects. For secondary structure prediction, the actual pre-miRNA cargoes (Supplementary Table S1) were folded using Mfold, under default conditions [Citation23,Citation24]. The most stable conformations were adopted for further computation. Gibbs energy (∆G) of the terminal loop region was calculated from the pre-miRNA structural prediction, with the terminal loop region starts from the first nucleotide after the 3ʹ end of the actual or predicted miRNA-5p and ends at its corresponding nucleotide at the 3ʹ arm [Citation20]. ∆G of the miRNA duplex region was ∆G of the pre-miRNA subtracted by that of the terminal loop region. ∆G of the pre-miRNA extended stems and apical loops were calculated analogously.
2.6. Cell cultures and miRNA expression analyses
Human 293 T cells (American Type Culture Collection, Manassas, VA, USA) were maintained at 37°C and 5% CO2 in Dulbecco’s modified Eagle’s medium supplemented with 10% foetal bovine serum and 2 mM L-glutamine (Invitrogen). RNA interference (RNAi) experiments were carried out by transfection of small interfering RNAs using Lipofectamine 2000 (Invitrogen) and analysed 5 days later [Citation13]. Exp5 and the loading control α-actin proteins were detected with primary antibodies against Exp5 and actin (Abcam, Cambridge, MA, USA), respectively, by Western blotting [Citation25]. Total RNA was isolated from 293 T cells using Trizol, and genome-wide miRNA expression was profiled using a custom microarray platform [Citation25]. Three biological replicate RNAi experiments were performed, raw data from microarrays were background subtracted and normalized, and averages of miRNA expression changes were correlated to relative Exp5 binding efficiencies. miRNA expression data have been deposited to Gene Expression Omnibus under the accession number GSE174061, and the raw and normalized data included in Supplementary Table S1.
3. Results
3.1. Preparation and analysis of recombinant Exp5 and Ran(Q69L) proteins
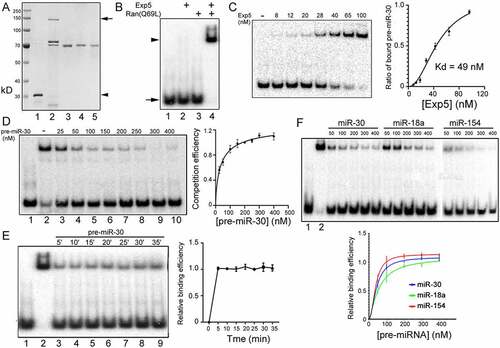
We isolated recombinant Ran(Q69L) and Exp5 proteins separately from overexpressing bacteria (), lanes 1 and 2, respectively) and confirmed that Exp5 required Ran(Q69L) to bind pre-miR-30 in a gel shift assay (), compare lanes 2, 3, and 4). Below we would use Exp5 to denote Exp5:Ran(Q69L), unless indicated otherwise. Dose response experiments indicated that approximately 70% 32P-labelled pre-miR-30 binding was achieved with 65 nM of Exp5 ()). When cold pre-miR-30 was added, 32P-labelled pre-miR-30 binding to Exp5 was reduced, e.g. by approximately 90% with 200 nM of pre-miR-30 ()). The length of incubation time with 32P-labelled pre-miR-30 had little effect on Exp5 interaction, suggesting a slow turnover of Exp5:pre-miRNA complexes, or that our experimental design provided a stable condition to measure competition ()). Lastly, we examined how varying amounts of three separate pre-miRNAs, pre-miR-30, pre-miR-18a, and pre-miR-154, interfered with Exp5 binding to the 32P-labelled pre-miR-30. There was no crossover in the competition/binding curves in the same experiments, suggesting that poor cargoes would never compete better than good cargoes for Exp5 binding under these experimental conditions ()). Results from these studies enabled us to establish the criteria for using end-point gel shift assays to investigate how Exp5 interacted with a large number of human pre-miRNAs in vitro.
Figure 1. Preparation of Exp5 and Ran(Q69L) proteins and gel shift assays. (a) Image of a Coomassie blue stained gel containing purified recombinant proteins and known amounts of BSA. Protein markers in kilodaltons (kD) are listed on the left, and the arrow and arrowhead point to Exp5 and Ran(Q69L), respectively. Lane 1: Ran(Q69L); lane 2: Exp5; lanes 3–5: 4, 2, and 1 αg of BSA. (b-f) Gel shift assay results. (b) Gel shift assay testing the binding of a 32P-labelled pre-miR-30 probe to recombinant Exp5 and Ran(Q69L) proteins. Lane 1: pre-miR-30 probe only; lane 2: with Exp5 added; lane 3: with Ran(Q69L) added; lane 4: with both Exp5 and Ran(Q69L) added. The arrow and arrowhead point to the free pre-miR-30 and Exp5-bound pre-miR-30, respectively. (c) Gel shift assays using varying amounts of Exp5 (concentrations indicated on top), with 1 αM Ran(Q69L). The graph on right quantifies the data, with averages, standard deviations, and estimated Kd shown. (d) Competition assays using constant Exp5 but varying amounts of cold pre-miR-30 competitor (concentrations indicated on top). Lane 1: probe only; lanes 2–10: with Exp5 added; lane 2: no competitive RNA; lanes 3–10: with different amounts of competitive RNAs. The graph on right quantifies the data, showing averages and standard deviations of raw Exp5 competition efficiencies (y-axis). (e) Time-dependent influence on Exp5:pre-miR-30 interaction. Exp5 was mixed with 200 nM of cold pre-miR-30 for 15 min, then a 32P-labelled pre-miR-30 was added and incubated for the indicated times. Lane 1: probe only; lanes 2–9: with Exp5 added; lane 2: no competitive RNA; lanes 3–9: with the cold RNA competitor but varying times of incubation with the 32P-labelled pre-miR-30. The graph on right quantifies the data showing the averages and standard deviations of the relative Exp5 binding or competition efficiencies (y-aixs). (f) Competition assays using varying amounts of three different cold pre-miRNAs (concentrations shown on top in nM). Lane 1: probe only; lane 2: with Exp5 added. The graph on right quantifies the data, with averages and standard deviations of the relative binding efficiencies (y-aixs)
3.2. Comparing the binding by Exp5 to a large number of pre-miRNAs
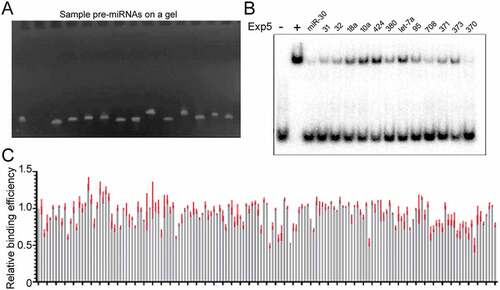
Only the interaction between Exp5 and pre-miR-30 had been evaluated in detail [Citation18,Citation19]. As there are hundreds of pre-miRNAs in humans, it is intuitive to assume that Exp5 does not bind them equally, but there had been no relevant reports, nor is it known whether or how well the conclusion from the pre-miR-30 work applied to other pre-miRNAs. So in this study we evaluated 157 pre-miRNAs (including pre-miR-30), representing most of the miRNA families commonly expressed in human cells. First we synthesized the pre-miRNAs by in vitro transcription ()). We then used competitive gel shift assays to measure and compare how different cold pre-miRNAs reduced Exp5 binding to a 32P-labelled pre-miR-30 probe: the stronger the reduction, the tighter the Exp5 binding to a pre-miRNA. As ) shows, all pre-miRNA competitors reduced 32P-pre-miR-30 binding by Exp5. Furthermore, some pre-miRNAs, e.g. pre-miR-31, competed better than others, e.g. pre-miR-424. ) summarizes relative Exp5 binding efficiency data for the 157 pre-miRNAs, which are also presented in Supplementary Table S1.
Figure 2. Large scale Exp5 binding experiments. (a) A representative image of pre-miRNAs used in competitive gel shift assays. Pre-miRNAs were synthesized by in vitro transcription, purified, examined by running an aliquot on a denaturing gel, and visualized after ethidium bromide staining. Each lane shows a different pre-miRNA. (b) Representative results of competitive gel shift assays. Cold pre-miRNAs tested are indicated on top of the image. (c) Summary of the relative Exp5 binding efficiencies (y-axis) of 157 pre-miRNAs (x-axis). Averages and standard deviations (in red) are shown
3.3. Determinants of the efficiencies of Exp5:pre-miRNA binding
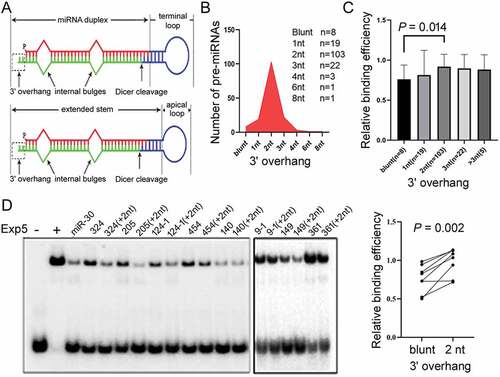
As Exp5 does not bind all pre-miRNAs equally, how does it discriminate its cargoes? A pre-miRNA contains two main structural features separated by Dicer cleavage sites: the miRNA duplex and the terminal loop region, which can be further characterized by a short stem and an apical loop when predicted by RNA folding algorithms ()). Earlier studies testing pre-miR-30 indicated that Exp5 binding was inhibited by a 5ʹ overhang 2 nt or longer but was able to accommodate a blunt end in an RNA haripin [Citation18]. Designed according to the miRNA-5p and miRNA-3p information in miRBase, pre-miRNAs used in this study lacked a long 5ʹ overhang but varied in the 3ʹ overhang (), Supplementary Table S1). For example, 19, 103, 22 pre-miRNAs were predicted to contain a 1 nt, 2 nt, and 3 nt overhang, respectively, but eight were predicted to have a blunt end structure ()). Those eight pre-miRNAs, pre-miR-9-1, pre-miR-124-1, pre-miR-149, pre-miR-140, pre-miR-205, pre-miR-324, pre-miR-361, and pre-miR-454, bound Exp5 poorer than pre-miRNAs with a 2 nt overhang ()). Because Drosha canonically produces 2 nt 3ʹ overhangs in its products, these blunt-ended pre-miRNAs might have been designed incorrectly based on miRBase information. For these eight RNAs, therefore, we designed updated pre-miRNAs containing a 2 nt 3ʹ overhang (Supplementary Table S1) and repeated gel shift experiments. ) revealed that a 2 nt 3ʹ overhang enhanced pre-miRNA binding by Exp5. We would include data from these eight new pre-miRNAs in subsequent analyses to investigate differential Exp5 binding.
Figure 3. Effects of 3ʹoverhangs on Exp5 binding. (a) Schematics of pre-miRNA sub-structures. Dicer cleavage generates the terminal loop sequence depicted in blue and the miRNA duplex. (b) Length distribution of the predicted 3ʹ overhangs in the 157 pre-miRNAs. (c) Relative Exp5 binding efficiencies (y-axis) of pre-miRNAs with different 3ʹ overhangs. Averages, standard deviations, and P value (Mann-Whitney test) are shown. (d) Competitive gel shift assays with pre-miRNAs that were blunt-ended or contained 2 nt 3ʹ overhangs. Data are summarized in the graph on the right, P value from the paired t-test
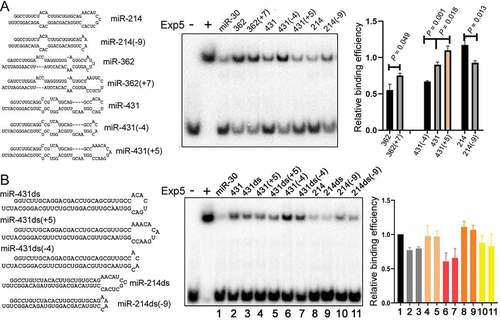
First we showed that sequences of the 2 nt 3ʹ overhangs had no effect on Exp5 binding efficiencies, as expected ()). We then predicted the secondary structures of pre-miRNAs, the duplex moiety, and the terminal loop region (), top panel), and correlated their ∆G to relative Exp5 binding efficiencies. There was no significant correlation between Exp5 binding and any of these structures () and (c); Supplementary Table S1). But if we considered only the apical loop, part of the terminal loop region (), bottom panel), a significant, positive correlation was observed between its ∆G or size (in nt) and Exp5 binding (); Supplementary Table S1), indicating that Exp5 prefers a looser structure at this end. To confirm this result, we constructed mutants of pre-miR-214, pre-miR-362, and pre-miR-431 varying the size of their apical loop, and found that enlarging the loop allowed for stronger competition, while minimizing the loop weakened competition ()). We further tested if improving the double-stranded RNA content in pre-miRNAs enhanced Exp5 binding; it did not, as shown in ), suggesting that that stem feature in natural pre-miRNAs is essentially optimal in terms of interacting with Exp5.
Figure 4. Pre-miRNA features underlying the interactions with Exp5. (a) Impacts of 3ʹ overhang sequences (x-axis) on relative Exp5 binding efficiencies (y-axis). Pre-miRNAs with a 2 nt 3ʹ overhang were examined. (b) Correlation between ∆G of pre-miRNAs (y-axis) with relative Exp5 binding efficiencies (x-axis). Sample size (N), Spearman correlation coefficient (ρ), and P values are listed. (c) Correlation between relative Exp5 binding efficiencies (x-axis) and ∆G of the miRNA duplex, terminal loop region and size of the terminal loop region (y-axes). See ) for pre-miRNA sub-structures. Sample size (n), Spearman correlation coefficient (ρ), and P values are listed. (d) Correlation between relative Exp5 binding efficiencies (x-axis) and ∆G of the extended miRNA duplex, apical loop and size of the apical loop (y-axes). See ) for pre-miRNA sub-structures. Sample size (n), Spearman correlation coefficient (ρ), and P values are listed
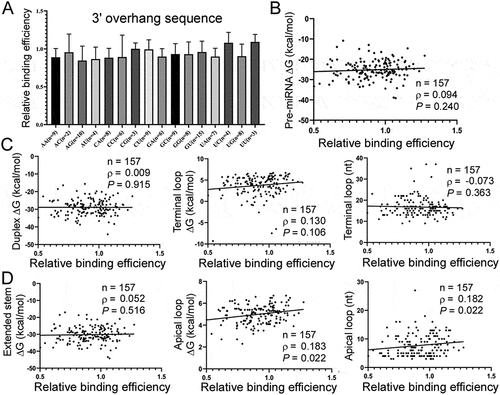
Figure 5. Effects of pre-miRNA mutations on Exp5 binding. (a) Competitive gel shift assays with pre-miRNAs containing a bigger or smaller apical loop. Pre-miRNA sequences and predicted secondary structures are shown on the left, and the graph on the right summarizes the data. Averages, standard deviations, and P values from t-tests are indicated in the graph. (b) Competitive gel shift assays with pre-miR-431 and pre-miR-214 variants with or without internal bulges. RNA sequences and predicted secondary structures are listed on the left as well as in (A), and data summarized on right, with averages and standard deviations shown in the graph. Lanes (samples) in the gel image correspond to those in the graph
3.4. Relationship between Exp5 binding and miRNA expression
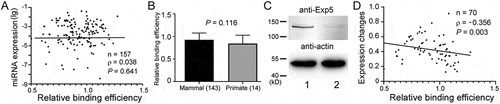
Is there a physiological consequence of Exp5 selectivity towards pre-miRNAs? To answer this question, we compared the relative Exp5 binding efficiencies to genome-wide miRNA expression in humans, using the publicly available, miRNA sequencing datasets. Relative Exp5 binding of pre-miRNAs did not correlate with human miRNA expression in vivo (); Supplementary Table S1). The differences in Exp5 binding efficiencies of pre-miRNAs that are conserved in mammals and those conserved in only primates are not significant (); Supplementary Table S1). These results contrasted with those obtained for human Drosha [Citation20]. To further test whether changes in Exp5 expression could still affect miRNA biogenesis, we knocked down Exp5 levels by RNAi (), lanes 1 and 2) and profiled miRNA expression by microarrays. We found that there was a negative and significant correlation between relative Exp5 binding efficiencies and changes in mature miRNA levels (); Supplementary Table S1). These results suggest that fluctuation in Exp5 levels may impact miRNA biogenesis differentially according to how well individual pre-miRNAs bind to Exp5.
Figure 6. Relation between miRNA expression and pre-miRNA:Exp5 interaction. (a) Correlation between endogenous human miRNA expression (y-axis) and relative Exp5 binding efficiencies (x-axis). Sample size (N), Spearman correlation coefficient (ρ), and P values are indicated. (b) Comparison of the relative Exp5 binding efficiencies (y-axis) of human pre-miRNAs that are conserved in primates only and pre-miRNAs that are conserved in other mammals as well. Numbers of miRNAs in the categories are in the parentheses, and P value from the Mann-Whitney test is indicated. (c) Western blot analyses of Exp5 knockdown by RNAi in 293 T cells. Lane 1: control transfected cells; lane 2: cells transfected with an Exp5 small interfering RNA. Positions of protein markers are indicated in the left. (d) Correlation between miRNA expression changes (y-axis) and relative Exp5 binding efficiencies (x-axis) under the Exp5 RNAi condition. Sample size (n), Spearman correlation coefficient (ρ), and P value are shown
4. Discussion
In this study we examined the interactions between Exp5 and 157 representative human pre-miRNAs. We showed that Exp5 binds all the pre-miRNAs, even though the affinities vary modestly. We identified a 3ʹ overhang and a sizable apical loop in pre-miRNAs as characteristics preferred by Exp5. While relative Exp5 binding efficiencies do not correlate with mature miRNA levels in vivo, they may nonetheless impact miRNA production when Exp5 expression is perturbed.
Previous studies using VA1 RNA and pre-miR-30 as cargoes revealed that Exp5 prefers RNAs with at least a 16-basepair stem feature and a 3ʹ overhang [Citation12,Citation18]. Our current study examined a large number of predicted human pre-miRNAs, which all satisfy the stem requirement, and ∆G of the stem regions in pre-miRNAs do not correlate with Exp5 binding efficiencies (). Thus, the duplex regions in human pre-miRNAs are sufficiently long and stable for Exp5 interaction ()). Many miRNAs have isoforms [Citation1], and miRBase depicts only the 5ʹ and 3ʹ boundaries of all the miRNA isoforms [Citation21]. Consequently, while most of our initial pre-miRNA designs contained the predicted, 2 nt 3ʹ overhangs, some had 1 nt, 3nt, or longer 3ʹ overhangs, and eight even had a blunt end ()). Those eight pre-miRNAs bound Exp5 weaker than other pre-miRNAs, but restoring a 2 nt 3ʹ overhang remedied the deficiency () and (d)). These results agree with our current knowledge of the interaction between Exp5 and RNAs [Citation12,Citation18,Citation19], but with a much larger dataset of genuine human pre-miRNA cargoes. An unexpected finding, however, is that the apical loop in pre-miRNAs impacts Exp5 binding, which increases with the ∆G and size of the loop () and ). The mechanism is unknown, as the solved crystal structure of Exp5:Ran:pre-miR-30 did not reveal how Exp5 interacts with the loop region in pre-miR-30 [Citation19]. A possible explanation is that an apical loop that is too small might introduce and propagate strain in the stem region that hinders Exp5 binding to the rest of the hairpin.
We have proposed that Drosha, an essential ribonuclease in the production of canonical animal miRNAs, by differentially cleaving its hundreds of substrates, further regulates global miRNA expression [Citation20]. Conserved miRNAs are also better Drosha substrates, which likely facilitates their expression in vivo [Citation20]. Interestingly, such a regulatory role is not ascribed to Dicer, another enzyme that also cleaves pre-miRNA substrates selectively [Citation22]. As the cleavage of pri-miRNAs initiates the irreversible step in miRNA biogenesis, it makes sense that the regulatory function of Drosha upstream would dominate. This study further showed that although Exp5 likewise exhibits preferences for certain pre-miRNAs over others, Exp5 binding selectivity does not correlate with human miRNA expression either, just like in the case of Dicer ()). Nonetheless, in human 293 T cells with decreased Exp5, changes in individual miRNA expression correlate with relative Exp5 binding efficiencies ()). It is not straightforward to predict a priori how miRNAs should behave when Exp5 levels drop, but we found that the more strongly a pre-miRNA binds Exp5, the more significantly the corresponding miRNA reduces its expression ()), suggesting that better Exp5 cargoes might be more sensitive to and dependent on Exp5, e.g. for nuclear export and/or protection. Future studies will address the mechanism in detail. That Exp5 association protects a pre-miRNA from nuclease degradation has been reported [Citation18].
In summary, our work characterized how Exp5 interacts with a large number of human pre-miRNAs, confirmed and identified RNA features important for the interactions, and further revealed the role of preferential pre-miRNA binding by Exp5 in the regulation of global miRNA expression.
Supplemental Material
Download MS Excel (435 KB)Acknowledgments
We thank Wenjing Li, Min Hu, and Qiuhuan Tian for technical assistance.
Disclosure statement
The authors declare no conflict of interest.
Data availability
The datasets generated during the current study are available in the Gene Expression Omnibus database under the accession number GSE174061.
Supplementary material
Supplemental data for this article can be accessed here
Additional information
Funding
References
- Bartel DP. Metazoan microRNAs. Cell. 2018;173:20–51.
- Billy E, Brondani V, Zhang H, et al. Specific interference with gene expression induced by long double-stranded RNA in mouse embryonal teratocarcinoma cell lines. Proc Natl Acad Sci USA. 2001;98:14428–14433.
- Grishok A, Pasquinelli AE, Conte D, et al. Genes and mechanisms related to RNA interference regulate expression of the small temporal RNAs that control C elegans developmental timing. Cell. 2001;106:23–34.
- Hutvágner G, McLachlan J, Pasquinelli AE, et al. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science. 2001;293:834–838.
- Ketting RF, Haverkamp TH, Van Luenen HG, et al. Dicer functions in RNA interference and in synthesis of small RNA involved in developmental timing in C elegans. Genes Dev. 2001;15:2654–2659.
- Lee Y, Ahn C, Han J, et al. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425:415–419.
- Denli AM, Tops B, Plasterk RHA, et al. Processing of pri-microRNAs by the microprocessor complex. Nature. 2004;432:231–235.
- Gregory RI, Yan KP, Amuthan G, et al. The microprocessor complex mediates the genesis of microRNAs. Nature. 2004;432:235–240.
- Han J, Lee Y, Yeom KH, et al. The Drosha-DGCR8 complex in primary microRNA processing. Genes Dev. 2004;18(7):3016–3302.
- Landthaler M, Yalcin A, Tuschl T. The human DiGeorge syndrome critical region gene 8 and its D melanogaster homolog are required for miRNA biogenesis. Curr Biol. 2004;14:2162–2167.
- Brownawell AM, Macara IG. Exportin-5, a novel karyopherin, mediates nuclear export of double-stranded RNA binding proteins. J Cell Biol. 2002;156:53–64.
- Gwizdek C, Ossareh-Nazari B, Brownawell AM, et al. Exportin-5 mediates nuclear export of minihelix-containing RNAs. J Biol Chem. 2003;278(8):5505–5550.
- Yi R, Qin Y, Macara IG, et al. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003;17:3011–3016.
- Bohnsack MT, Czaplinski K, Gorlich D. Exportin 5 is a RanGTP-dependent dsRNA-binding protein that mediates nuclear export of pre-miRNAs. RNA. 2004;10:185–191.
- Lund E, Guttinger S, Calado A, et al. Nuclear export of microRNA precursors. Science. 2004;303:95–98.
- Kim YK, Kim B, Kim VN. Re-evaluation of the roles of DROSHA, Exportin 5, and DICER in microRNA biogenesis. Proc Natl Acad Sci USA. 2016;113:E1881–E1889.
- Wang J, Lee JE, Riemondy K, et al. XPO5 promotes primary miRNA processing independently of RanGTP. Nat Commun. 2020;11:1845.
- Zeng Y, Cullen BR. Structural requirements for pre-microRNA binding and nuclear export by Exportin 5. Nucl Acids Res. 2004;32:4776–4785.
- Okada C, Yamashita E, Lee SJ, et al. A high-resolution structure of the pre-microRNA nuclear export machinery. Science. 2009;326:1275–1279.
- Feng Y, Zhang X, Song Q, et al. Drosha processing controls the specificity and efficiency of global microRNA expression. Biochim Biophy Acta-Gene Reg Mech. 2011;1809:700–707.
- Kozomara A, Birgaoanu M, Griffiths-Jones S. miRBase, from microRNA sequences to function. Nucl Acids Res. 2019;47:D155–D162.
- Feng Y, Zhang X, Graves P, et al. A comprehensive analysis of precursor microRNA cleavage by human dicer. RNA. 2012;18:2083–2092.
- Mathews DH, Sabina J, Zuker M, et al. Expanded sequence dependence of thermodynamic parameters improves prediction of RNA secondary structure. J Mol Biol. 1999;288:911–940.
- Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucl Acids Res. 2003;31:3406–3415.
- Zhang X, Graves PR, Zeng Y. Stable argonaute2 overexpression differentially regulates microRNA production. Biochim Biophys Acta-Gene Reg Mech. 2009;1789:153–159.
