ABSTRACT
Phage infection is one of the major threats to prokaryotic survival, and prokaryotes in turn have evolved multiple protection approaches to fight against this challenge. Various delicate mechanisms have been discovered from this eternal arms race, among which the CRISPR-Cas systems are the prokaryotic adaptive immune systems and phages evolve diverse anti-CRISPR (Acr) proteins to evade this immunity. Until now, about 90 families of Acr proteins have been identified, out of which 24 families were verified to fight against subtype I-F CRISPR-Cas systems. Here, we review the structural and biochemical mechanisms of the characterized type I-F Acr proteins, classify their inhibition mechanisms into two major groups and provide insights for future studies of other Acr proteins. Understanding Acr proteins in this context will lead to a variety of practical applications in genome editing and also provide exciting insights into the molecular arms race between prokaryotes and phages.
Introduction
Co-evolution between prokaryotes and viruses leaves us many exciting phenomena to explore. For bacteria and archaea, viruses infecting them are known as phages which possess a constant threat to their survival. Under such a tremendous selective pressure, bacteria and archaea have evolved multiple immune systems to fight against the phage infection. These include adaptive immune systems such as the CRISPR (clustered regularly interspaced short palindromic repeats)-Cas system, innate immune systems such as restriction-modification (RM) and bacteriophage exclusion (BREX) systems, abortive infection (Abi) systems that resist infection by induction of cell dormancy or death, and additional systems whose mechanisms have not yet been elucidated [Citation1]. Among these antiviral defence systems, CRISPR-Cas systems are the only known prokaryotic adaptive systems and are now extensively utilized as a genome editing tool.
A typical CRISPR-Cas system is composed of two indispensable components, that is, CRISPR sequences and CRISPR-associated (Cas) proteins. Based on the composition of the system, CRISPR-Cas systems are divided into two classes: class 1 which employs multi-subunit surveillance complexes and class 2 which functions through a single multi-domain Cas effector. The two classes are further classified into six types (I–VI) based upon their signature Cas proteins, and each type comprises multiple subtypes [Citation2]. Class 1 CRISPR-Cas systems include types I, III, and IV, in which type I system is the most ubiquitous one and further divided into seven subtypes: I-A through I-F and I-U [Citation3]. Class 2 systems include types II, V and VI, out of which plenty of subtypes of type V have sprung up in the recent few years [Citation2]. Despite of the diversity, all the CRISPR-Cas subtypes are exclusively guided by the CRISPR RNA (crRNA), driven by Cas proteins, and function in three distinct stages: the integration of foreign nucleic acid segments into the CRISPR array (adaptation), the transcription and maturation of crRNAs (biogenesis), and the execution of the crRNA-guided target DNA/RNA detection and destruction (interference) [Citation4].
However, phages have evolved multiple ways to evade or neutralize the CRISPR-Cas immunity. In 2013, five distinct genes from phages were verified to resist the type I-F CRISPR-Cas systems, and thus the proteins encoded by these genes to inhibit CRISPR-Cas systems were named as anti-CRISPR (Acr) proteins, AcrIF1-5 [Citation5]. Since then, numerous studies have been conducted on the identification and characterization of Acr proteins. To date, about 90 families of Acr proteins have been identified [Citation6] and summarized in an updating document [Citation7]. Meanwhile, multiple reviews have addressed the discovery, classification and evolution, biochemical mechanisms and structures of Acr proteins [Citation8–14]. Now it seems that Acr proteins own a comparative diversity with the CRISPR-Cas systems, as Acr proteins have been identified in multiple subtypes of CRISPR-Cas systems () [Citation13]. Type I-F CRISPR-Cas system is one of the best characterized subtype and also the first subtype with identified Acr proteins [Citation5]. Here, we review the exciting advances in the structural and biochemical mechanisms of Acr proteins against type I-F CRISPR-Cas systems, which gives an interesting perspective of the ongoing arms race between prokaryotes and their predators.
Table 1. Numbers of identified and structure-solved Acr proteins
Type I-F CRISPR-Cas system
Due to the simplicity and programmability, until now, most CRISPR-Cas genome editing tools have been developed based on class 2 systems, especially type II and type V CRISPR-Cas systems [Citation15,Citation16]. However, genome manipulation based on class 1 CRISPR-Cas systems such as type I systems was recently reported to exhibit unique advantages, especially in long-range genome manipulation and deletion screen [Citation17–19]. Among these, the type I-F CRISPR system has also gradually emerged as a powerful tool for genome editing [Citation20–24].
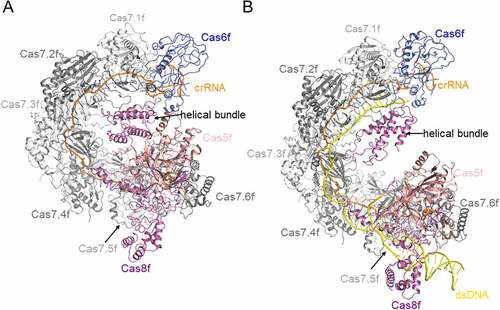
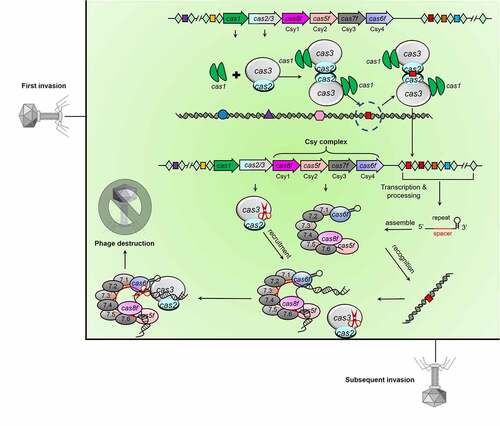
Type I CRISPR-Cas systems encode Cascade (CRISPR-associated complex for antiviral defence) complexes to recognize target DNA, and subsequently recruit the nuclease-helicase Cas3 to execute DNA cleavage [Citation4]. Out of type I CRISPR-Cas systems, the type I-F system comprises six Cas components (), that is, Cas1, Cas2/3, Cas5f (formerly Csy2), Cas6f (formerly Csy4), Cas7f (formerly Csy3), and Cas8f (formerly Csy1). Cas1 and Cas2 are universal across most CRISPR-Cas types for spacer adaptation [Citation3]. Uniquely, the trans-acting nuclease-helicase in type I-F systems is encoded as a Cas2-Cas3 fusion, that is, Cas2/3. The Cascade (or named as Csy) complex is assembled guided by the crRNA, comprising one Cas5f, one Cas6f, one Cas8f, and six Cas7f proteins. The Csy complex first interacts with target dsDNA (double-stranded DNA) through PAM (protospacer adjacent motif) recognition and then hybridization between the target DNA strand and the crRNA guide triggers an R-loop to displace the non-target strand of dsDNA [Citation25,Citation26]. Finally, the Cas2/3 nuclease-helicase is recruited to the Csy complex to fulfil the degradation process ().
Figure 1. Working mechanisms of type I-F CRISPR-Cas system
Three-dimensional structures have been solved for all the Cas components in type I-F system [Citation27–34]. Among these, Chowdhury et al. provided the first glimpse into the structural architecture of the Csy complex through the determination of a 3.4-Å-resolution cryo-EM structure ()) [Citation31]. The Csy complex folds into an asymmetric spiral in a seahorse-like shape, in which the Cas6f subunit is stably associated with the 3ʹ end of the crRNA in the head region. In the tail region, Cas5f and Cas8f form a stable 1:1 heterodimer in which the 5ʹ handle of the crRNA is anchored. The core crRNA and six extensively crRNA-interacting Cas7f subunits form the spiral backbone. Subsequently, Guo et al. determined the structures of the Csy complex before and after target DNA binding using a partially duplexed DNA, to discern the conformational change caused by target DNA binding [Citation32]. In the structures reported in that study, the most prominent conformational change induced by DNA binding is a dramatic, ~20 Å elongation of the Csy spiral [Citation32]. Afterwards, Rollins et al. determined a 3.2-Å-resolution structure of the Csy complex bound to an 80-bp fully duplexed dsDNA ()) [Citation35], which reveals other dramatic conformational changes not observed in the previously determined Csy-DNA structure. Typically, a 180-degree rotation of the C-terminal helical bundle of the Cas8f subunit is observed, which is driven by R-loop formation. This conformational change further exposes a ‘nuclease recruitment helix’ in the helical bundle of Cas8f, which is buried in the apo Csy structure, to mediate Cas2/3 binding.
Anti-CRISPR proteins against type I-F CRISPR-Cas systems
In 2013, Bondy-Denomy et al. [Citation5] first identified five distinct ‘anti-CRISPR’ genes in the genomes of bacteriophages infecting P. aeruginosa, which enable phages to evade type I-F CRISPR-Cas systems. To date, a total of 24 families of Acr proteins have been verified to inactivate type I-F systems () [Citation36–38]. More importantly, the structures of 11 of these Acr proteins, alone or in combination with the Csy complex or Cas2/3, have been reported for the elucidation of their working mechanisms. Here, we tentatively classify these 11 Acr proteins into two major groups and five subgroups, according to their distinct biochemical mechanisms, which will be reviewed below.
Table 2. Summary of detailed information about Acr proteins against type I-F CRISPR-Cas system
Group 1: Inhibition of target DNA binding
Inhibition of hybridization between target DNA and crRNA
AcrIF1 is a 78-residue protein from the JBD30 phage. Being one of the first identified Acr proteins, extensive literatures have addressed its structural and biochemical mechanisms [Citation31,Citation32,Citation39–41]. First, it was described to interact with the Csy complex to block the binding between the Csy complex and its target DNA through biochemical assays [Citation39]. Shortly afterwards, Maxwell et al. solved the solution structure of AcrIF1 by nuclear magnetic resonance (NMR), and identified the key residues of AcrIF1 in its interaction with the Csy complex by structure-guided mutational studies [Citation40]. AcrIF1 comprises four anti-parallel β-strands, followed by two α-helices (β1β2β3β4α1α2). Later, cryo-EM structures of the Csy-AcrIF1 complex were reported by three groups, respectively [Citation31,Citation32,Citation41]. All the three studies revealed that AcrIF1 engages the Cas7f backbone of the Csy complex to prevent the hybridization between target DNA and the crRNA ()). However, while Chowdhury et al. and Guo et al. both showed that two copies of AcrIF1 bind to the interface between Cas7.5f and Cas7.6f, and the interface between Cas7.3f and Cas7.4f, respectively [Citation22,Citation23], Peng et al. reported another binding mode in which three AcrIF1 molecules engage the interfaces formed by Cas7.4f-Cas7.5f and Cas7.2f-Cas7.3f, and a single Cas7.6f, respectively [Citation41]. According to the Coomassie staining estimation reported by Bondy-Denomy et al., the stoichiometry of AcrIF1 is estimated to be 2.6 ± 0.3 proteins per Csy complex [Citation39]. We speculate that the complex mode of AcrIF12:Csy1 and AcrIF13:Csy1 may both exist, dependent on the local concentrations of the two proteins and whether there are other Acr proteins bound simultaneously on the Csy complex. However, it remains unknown how many AcrIF1 copies are sufficient to exert a full inhibition.
Figure 3. Overall structures of Csy complexed with AcrIF1, AcrIF9 and AcrIF14
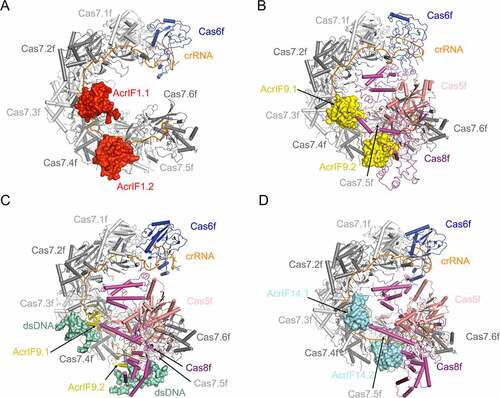
AcrIF9 is a 68-residue protein and comprises a pair of antiparallel β-sheets connected by an α-helix in the middle (β1β2α1β3β4) [Citation42–44]. First, Zhang et al. [Citation44] published a structure of Csy-AcrIF9 complex, which showed that two copies of AcrIF9 interact with the Csy complex through engaging the thumb domains of the Cas7.4f and Cas7.6f subunits, respectively ()). Structural comparison between the Csy-AcrIF9 and Csy-dsDNA structures suggests that AcrIF9 competes with target DNA to bind the Csy complex. Therefore, AcrIF9 was predicted to prevent target DNA binding. Later, however, Hirschi et al. [Citation43] reported that in EMSA (electrophoretic mobility shift assay) experiments AcrIF9 does not abrogate dsDNA binding of the Csy complex, but a super-shifted band occurs with slower mobility compared to the band of Csy-dsDNA. Combined with the cryo-EM structure of Csy-AcrIF9-dsDNA ()), their study uncovered that apart from inhibiting the hybridization between crRNA and target DNA, AcrIF9 exhibits another layer of inhibition that it renders the Csy complex with the ability to bind non-sequence-specific DNA molecules. Interestingly, AcrIF9 alone, under the same concentrations, does not bind the corresponding DNA in the EMSA assay. Shortly afterwards, Kim et al. showed that AcrIF9 interacts with DNA when it is added with a high concentration (>20 μM), suggesting a weak DNA binding ability of AcrIF9 [Citation42]. Furthermore, the study by Lu et al. [Citation45] revealed that the activity of inducing the non-sequence-specific DNA binding depends on a conserved positive patch on AcrIF9 and a positive patch on the Cas8f subunit of the Csy complex. Notably, the ability of inducing non-sequence-specific DNA binding also contributes to the inhibition capacity of AcrIF9 in vivo [Citation45].
AcrIF14 is a 124-residue two-domain protein from the Mcat5 phage. According to its structure reported by Gabel et al., AcrIF14 is divided into two domains: the N-terminal domain (NTD, residues 1–79) and the C-terminal domain (CTD, residues 80–124) ()) [Citation46]. In the cryo-EM structure of Csy-AcrIF14 ()), while the AcrIF14CTD interacts with the Csy complex and well-ordered, AcrIF14NTD shows no direct interactions with the Csy complex and exhibits a poor cryo-EM density. The AcrIF14CTD comprises three anti-parallel β-strands followed by an α-helix. The AcrIF14NTD is composed exclusively of α helices and not included in the final structure due to the limitations of the density. Two copies of AcrIF14 mainly form polar interactions with the thumb domains of Cas7.4f and Cas7.6f, respectively ()), reminiscent of AcrIF9. Meanwhile, each AcrIF14 molecule also engages the nucleobases of two nucleotides of the crRNA. Structural comparison between the Csy-AcrIF14 and Csy-dsDNA structures shows that AcrIF14 also occupies the binding sites in thumb domains of Cas7.4f/Cas7.6f and the crRNA of the target DNA strand. Therefore, AcrIF14 was also predicted to inhibit the hybridization between crRNA and target DNA. However, it still remains unknown whether AcrIF14 is able to induce non-sequence-specific DNA binding when complexed with the Csy complex as AcrIF9 does.
dsDNA mimic proteins
AcrIF2 is a 90-residue protein from P. aeruginosa phage D3112. It is an acidic protein with a theoretical isoelectric point (pI) of 3.67 (). The overall structure of AcrIF2 is composed of four antiparallel β-strands flanked by a pair of antiparallel α helices at both sides (α1α2β1β2β3β4α3α4) [Citation31,Citation32,Citation41,Citation47]. Notably, the topological structure of AcrIF2 is very similar with that of AcrIF1 (β1β2β3β4α1α2), with two additional N-terminal antiparallel α helices. Distinct from AcrIF1, AcrIF9 and AcrIF14, AcrIF2 interacts with the positively charged residues in the N-terminal hook domain of Cas8f and the thumb of Cas7.6f, which together form a vise-like structure and crucial for DNA binding ()). Moreover, AcrIF2 exhibits a pseudo-helical distribution of acidic residues on its surface, similar as the charge distribution on the helical backbone of a dsDNA molecule. Therefore, AcrIF2 was proposed to be a DNA mimic protein in the study of Chowdhury et al [Citation31]. Afterwards, Guo et al. solved the structures of both Csy-AcrIF2 and Csy-dsDNA complexes, which showed that the positions of the hook domain of Cas8f in the two structures are different and the binding site of AcrIF2 only partially overlaps with that of the DNA duplex [Citation32]. Taken together, AcrIF2 sterically prevents the binding of DNA duplex through both interacting with the ‘DNA vise’ of the Csy complex, and pushing the hook domain away from Cas7.6f.
Figure 4. Overall structures of Csy complexed with AcrIF2, AcrIF6, AcrIF7, AcrIF8 and AcrIF10
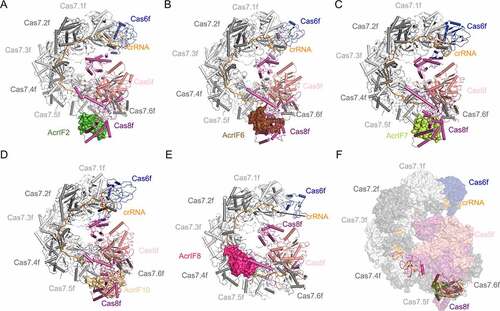
AcrIF6 is also a small acidic protein with 100 residues from a Pseudomonas prophage. Distinct from the above Acr proteins, AcrIF6 is composed exclusively of α helices [Citation44]. The complex structure of Csy-AcrIF6 showed that AcrIF6 also targets the Cas5f-Cas8f tail and Cas7.6f via extensive contacts ()) [Citation44]. A close view of the interface revealed that K247 and T246 are the major residues of Cas8f involved in the interactions. Mutations of the AcrIF6 residues that interact with K247 and T246 largely impaired the inhibition activity of AcrIF6 [Citation44]. Notably, both K247 and T246 belong to the lysine-containing wedge (or K-wedge), which sterically blocks the base pairing of target dsDNA, thus playing important roles in target DNA recognition and crRNA-DNA hybridization [Citation35]. Importantly, besides type I-F CRISPR-Cas system, AcrIF6 also shows inhibition towards type I-E system. Based on the resemblance of the Cascade complex between the type I-F and type I-E systems, we speculate that AcrIF6 might accomplish the inhibition of the two systems similarly by blocking the unwinding and binding of the target DNA duplex.
AcrIF7 (87 residues) is another acidic protein from P. aeruginosa phage LPB1, which interacts with the tail region of the Csy complex. Its solution structure was first determined by Kim et al. through NMR approaches [Citation48]. AcrIF7 adopts a novel β1β2α1α2β3 fold, with multiple clusters of negatively charged residues on its surface. Their study revealed that AcrIF7 competes with AcrIF2 to bind the Cas8f-Cas5f subcomplex, which is dependent on the surface negatively charged residues of AcrIF7. Afterwards, the structure of Csy-AcrIF7 complex was reported by Gabel et al. ()) [Citation46]. Similar with AcrIF2 and AcrIF6, AcrIF7 also targets the ‘DNA vise’ located in the Cas8f-Cas5f tail, and engages N250 and N111 of the Cas8f subunit, which are also reported to recognize the PAM duplex on the target DNA [Citation44]. Comparison of the structures of Csy-AcrIF7 and Csy-dsDNA shows that AcrIF7 competes with target dsDNA to bind the Cas8f-Cas5f tail. Therefore, AcrIF7 is another DNA mimic Acr protein.
AcrIF10 is a 97-residue protein from S. xiamenensis prophage also with negatively charged surfaces. According to the Csy-AcrIF10 structure reported by Guo et al. ()), AcrIF10 occupies a region of the Csy complex where some residues (e.g. Cas8fK77, Cas8fR78, Cas5fR90 and Cas7.6fK299) are involved in DNA binding [Citation32]. Hence, the interaction between the Csy complex, especially the Cas8f hook domain and AcrIF10 decreases the probability of DNA binding. Consistent with the function of AcrIF10 as a DNA mimic, upon AcrIF10 binding, the tail of the Csy complex undergoes a structural change with the hook domain of Cas8f swinging towards Cas7.6f, which also happens after dsDNA binding.
AcrIF8 is a 92-residue protein from P. carotovorum phage ZF40. In the cryo-EM structure of AcrIF8-Csy complex, AcrIF8 is located at the cavity surrounded by Cas7.4–7.6f, Cas5f and Cas8f with a single copy ()) [Citation44]. Specifically, the α-helix of AcrIF8 inserts into a cavity formed by Cas5f-Cas8f and Cas7.6f, interacting with Cas5fK76, Cas7.6fK257 and Cas8fR210 and Cas8fK216. T29, I31, A32 and N33 of AcrIF8 also form potential interactions with the nucleobases of three nucleotides of the crRNA (U21, U22 and G23), which should base pair with the corresponding nucleotides of the target DNA strand in the Csy-dsDNA structure. Taken together, AcrIF8 perturbs DNA-crRNA hybridization by interacting with the Cas5f, Cas7.4–7.6f, and Cas8f subunits and three nucleotides of the crRNA. Therefore, although AcrIF8 engages a different cavity from that of AcrIF2/6/7/10 ()), we classify it as a DNA mimic protein here.
Acr protein with enzymatic activity
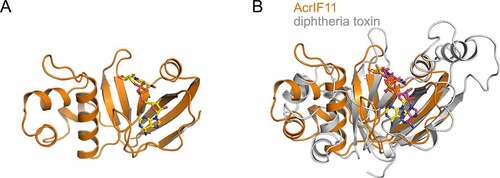
AcrIF11 is a 132-residue protein whose inhibition ability against the type I-F system is so potent that it was employed as a positive control for strong inhibition of the type I-F system in vivo [Citation38]. Unlike the above mechanisms, AcrIF11 does not stably interact with the Csy complex or Cas2/3 upon incubation in vitro. Attracted by this unusual phenomenon, our group solved the crystal structure of AcrIF11 to explore its mechanism ()) [Citation49]. During the process of crystal optimization, it was found that β-NAD+ improved the diffraction of AcrIF11 as an additive and was also found in the finally refined AcrIF11 structure. Structural alignment using the Dali server revealed that the catalytic domain of diphtheria toxin (PDB: 1TOX) is the closest structural homolog of AcrIF11 ()). Similar as diphtheria toxin which possesses ADP-ribosylation activity, AcrIF11 was found to ADP-ribosylate the Csy complex at the N250 residue in the ‘K-wedge’ of Cas8f subunit, a residue required for PAM recognition. ADP-ribosylation of the Csy complex resulted in abolishment of its interaction with target DNA [Citation49]. Regarding the interacting sites of the Csy complex, besides Cas8f, we found that K58 and K60 residues of the Cas7.6f subunit are also engaged by AcrIF11. Taken together, AcrIF11 is the first identified Acr protein with enzymatic activity of the type I-F, and even class 1 CRISPR-Cas systems.
Figure 5. Overall structure of AcrIF11 and its alignment with diphtheria toxin
Group 2: Inhibition of the recruitment of Cas2/3
Mimic of the C-terminal helical bundle of Cas8f
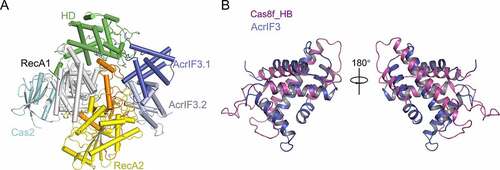
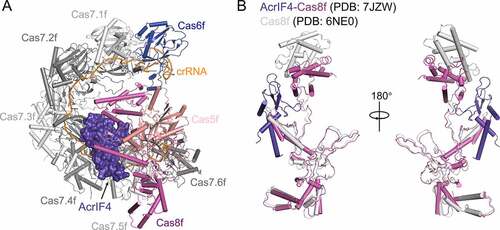
AcrIF3 is a 100-residue protein from P. aeruginosa phage JBD88a. In 2015, biochemical assays showed that AcrIF3 does not bind the Csy complex, but interacts with Cas2/3 [Citation39]. Afterwards, the crystal and cryo-EM structures of the PaCas2/3-AcrIF3 complex were solved by two different groups to further elucidate its working mechanism [Citation28,Citation29]. AcrIF3 exists as a homodimer in solution, and the PaCas2/3-AcrIF3 complex is composed of an AcrIF3 homodimer and a PaCas2/3 monomer ()). The AcrIF3 protomer consists of six α-helices, and its dimerization is mediated by the α1 helices in both monomers. Mutational studies indicated that the dimerization of AcrIF3 is essential for its inhibition of the nuclease activity of Cas2/3. In their study, AcrIF3 was proposed to inhibit the activity of PaCas2/3 through preventing its access to the substrate DNA, blocking its recognition by Cas8f, and locking PaCas2/3 in an ADP-bound inactive form. Notably, through structural comparison, Rollins et al. [Citation35]suggested that AcrIF3 actually mimics the Cas2/3 docking site of the Csy complex, that is, the C-terminal helical bundle of Cas8f ()). This raises a very interesting possibility that some of the key structural elements of the Cas proteins may be adopted by phage proteins in the continuing arms race between phages and prokaryotes.
Restriction of conformational change of the Csy complex?
AcrIF4 is a 100-residue protein from the phage JBD24, which was also reported to bind the Csy complex by Bondy-Denomy et al. in 2015 [Citation39]. AcrIF4 folds in a two-domain structure, with an α-helical domain and a β-strand domain [Citation46]. The α-helical domain comprises of the N-terminal α1 and the C-terminal α2 and α3, and the β-strand domain is composed of two pairs of anti-parallel β-sheets (β1-β2 and β3-β4) flanked by α1 and α2/3. In the Csy-AcrIF4 structure ()), AcrIF4 binds at a position different from those of the above mentioned Acr proteins. Clamped between the Cas8f-Cas5f tail and the Cas7f backbone of the Csy complex, AcrIF4 mainly interacts with the middle and C-terminal helical bundle domains of Cas8f through a negatively charged surface, and forms additional contacts with Cas7.4f-7.6f and Cas5f [Citation46] ()). Since the C-terminal helical bundle domain of Cas8f will undergo a 180-degree rotation upon target DNA binding ()), AcrIF4 was proposed to inhibit this conformational change through its interactions with the helical bundle domain of Cas8f ()). Structural comparison between the Csy-AcrIF4 and Csy-dsDNA complexes suggested that AcrIF4 does not compete with target DNA to bind the Csy complex [Citation46]. This was further supported by the EMSA assays, which showed that addition of AcrIF4 to the Csy complex does not affect its binding to the target DNA. Therefore, AcrIF4 was predicted to lock the Csy complex in an inactive state and prevent the recruitment of Cas2/3 after target dsDNA binding. However, a previous in vivo assay suggested that AcrIF4 prevents the Csy complex to interact with its target DNA [Citation39]. Taken together, it still remains unknown how AcrIF4 exerts its inhibition capacity in vivo.
Figure 7. Overall structure of the Csy-AcrIF4 complex
Strategy similarities and future perspectives of type I-F Acr proteins
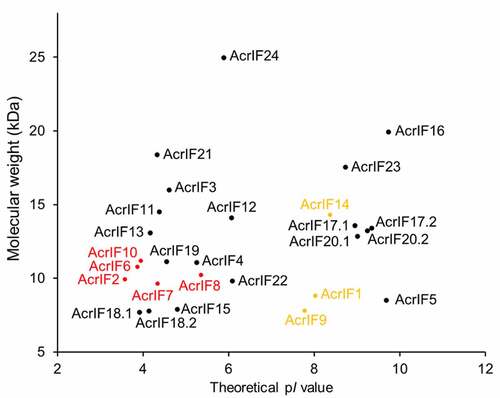
As described above, till now, structures and inhibition mechanisms of 11 Acr proteins against type I-F CRISPR system have been determined (). Out of these Acr proteins, five Acr proteins (AcrIF2/6/7/8/10) are classified as DNA mimic proteins. Low theoretical isoelectric point is one of the most important features of DNA mimic proteins, that is, AcrIF2 (pI 3.58), AcrIF6 (pI 3.87), AcrIF7 (pI 4.35), AcrIF8 (pI 5.36) and AcrIF10 (pI 3.95). A plot of the 24 known-type I-F Acr proteins with their molecular weights and theoretical pI values shows that these five proteins are located in a region together with AcrIF3/4/11/13/15/18/19/21 (). Out of these, AcrIF3 and AcrIF4 have been shown to bind Cas2/3 ()) and the middle domain/C-terminal helical bundle of Cas8f ()), respectively. Interestingly, despite lack of a complex structure, we have identified the ‘K-wedge’ of Cas8f as an important binding module for AcrIF11, reminiscent of AcrIF6 [Citation49]. Moreover, we also showed that AcrIF13 stably interacts with the Csy complex in the same study. Therefore, we predict that the remaining structurally uncharacterized Acr proteins, AcrIF13/15/18/19/21 would probably also be DNA mimic proteins by interacting with the tail region or the backbone of the Csy complex, if they have been confirmed to bind the Csy complex.
Figure 8. A summary of the inhibition mechanisms of the 11 AcrIF proteins reviewed in this study
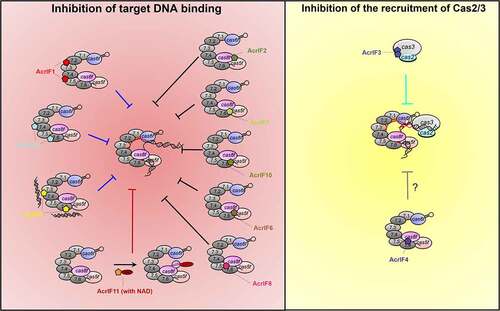
Figure 9. A plot of the 24 type I-F Acr proteins with their molecular weight and theoretical pI values
Before the discovery of Gabel et al. [Citation46], all identified type I-F Acr proteins that stably interact with the Csy complex fulfill their inhibition by blocking dsDNA binding. Therefore, AcrIF4 may conduct the inhibition with a totally distinct mechanism. Very recently, AcrIE2 was also found to bind the type I-E Cascade complex without preventing its target DNA binding [Citation50]. Surprisingly, binding of the Cascade:AcrIE2 complex led to either transcriptional activation or repression based on the binding sites in the in vivo transcriptional assay [Citation50]. It raised the question whether AcrIF4 could also affect the transcription when complexed with the Csy complex. Further information is needed for the biological role and the detailed inhibition mechanism of these two Acr proteins, although both AcrIF4 and AcrIE2 were now predicted to block the recruitment of the Cas3 nuclease [Citation46,Citation50].
AcrIF9 shows that two different mechanisms could coexist in one Acr. Although AcrIF9 is able to inhibit the CRISPR-Cas system through competing with target DNA to bind the Csy complex [Citation44], mutations of the positive patch of AcrIF9 does decrease its inhibition in the in vivo assay, suggesting that the induction of non-sequence-specific DNA binding by AcrIF9 also works in vivo [Citation43]. Since the binding position of AcrIF9 have been (AcrIF14) and may also be adopted by other Acr proteins, it is not surprising that other Acr proteins might also possess AcrIF9-like activity.
Stable, direct interactions with the Cas effectors have been a canonical approach utilized by Acr proteins, however, researchers have already suggested that there could be enzymatic Acr proteins with stronger inhibition ability in 2017 [Citation8]. AcrIF11 is the first discovered Acr protein with enzymatic activity towards class 1 CRISPR-Cas systems, and also the first Acr protein with ADP-ribosyl transferase activity. In our previous study, AcrIF11 was able to accomplish the inhibition with a concentration 100 times lower than that of AcrIF2/6/10 in the in vitro assays [Citation49]. AcrVA5 is another characterized Acr with enzymatic activity, which functions as an acetyltransferase to modify Lys635 of the Cas12a protein from Moraxella bovoculi, a residue also required for PAM recognition [Citation51,Citation52]. The examples of these two Acr proteins suggest that modification of the PAM recognition motif of Cas effectors may be one of the most effective strategies selected by evolution. Interestingly, homologs of AcrIF11 are found in more than 50 species of diverse Proteobacteria [Citation37], but type I-F CRISPR-Cas systems are only found in a portion of these species [Citation49]. This suggests that certain Cas components in other subtypes might also be substrates of AcrIF11 homologs, that is, AcrIF11 may mediate the inhibition of other types of CRISPR-Cas systems. Another issue about AcrIF11 is whether prokaryotes would produce ‘eraser’ enzymes to remove the modifications on the Csy complex added by AcrIF11, which could function as ‘anti-anti-CRISPRs’. Future studies should be conducted to investigate this intriguing point.
Although great attentions have been paid to the discovery of Acr proteins with novel inhibition mechanisms, most characterized Acrs were found to adopt canonical inhibition strategies, such as DNA mimics. One reason for this might be the fact that Acr proteins with canonical strategies, for example, DNA mimics are more prevalent than those with unusual mechanisms such as enzymatic modification in the course of evolution. On the other hand, characterization of an Acr protein with a non-canonical inhibition mechanism is more challenging than those with common mechanisms. Acrs with common mechanisms such as DNA mimics usually stably interact with Cas effectors and exhibit robust inhibition in the in vitro cleavage assay of the CRISPR-Cas systems. Therefore, Acrs that do not show inhibition in the in vitro cleavage assay might have a novel inhibition strategy. For example, AcrIF11 exhibits extremely weak interaction with the Csy complex and does not inhibit the in vitro DNA cleavage activity of the type I-F CRISPR-Cas system unless NAD is included into the reaction buffer. In the light of the low sequence identity between AcrIF11 and known ADP-ribosyl transferases, it is unlikely to characterize its enzymatic activity without its protein structural information. Therefore, protein structural information is extremely important in characterizing Acrs with novel inhibition mechanisms, which might be accelerated by the fast development of protein structure predictions [Citation51]. Till now, there are still 13 type I-F Acr proteins whose structures and detailed mechanisms remain unclear. In our previous study, we found that AcrIF5, AcrIF11 and AcrIF12 do not stably interact the Csy complex or the Cas2/3, which suggested that they might show non-canonical mechanisms [Citation49]. With AcrIF11 characterized as an ADP-ribosyl transferase, future studies into AcrIF5 and AcrIF12 might also uncover novel inhibition mechanisms.
In future studies, firstly, we anticipate that the great progress in protein structure prediction would facilitate the characterization of Acr proteins, especially those with novel mechanisms [Citation51,Citation53]. Secondly, almost all Acr proteins characterized thus far exclusively inhibit the target interference stage of the CRISPR-Cas systems. It is not surprising that Acr proteins that target the adaptation or biogenesis stages would also be characterized in the future, and testing of the interactions between Acrs and the components involved in these two stages might be a possible attempt in characterizing these Acrs. Thirdly, it is also interesting to examine whether CRISPR-Cas systems would crosstalk with other anti-phage systems from prokaryotes, and whether Acr proteins would counteract anti-phage systems other than CRISPR-Cas systems. Last but not least, with the application of type I-F CRISPR-Cas system in genome editing, exploitation of AcrIF proteins as regulatory tools would also attract more attention.
Till now, many progresses have been made in using type I-F CRISPR-Cas system as a genome editing tool. For example, Yan and colleagues have developed a single-plasmid-based method to use endogenous-type I-F system in P. aeruginosa for the characterization of its drug-resistance mechanism [Citation20]. Similarly, Zheng et al. also repurposed the endogenous-type I-F system in Zymomonas mobilis for genome editing [Citation21]. For heterologous utilization, type I-F Cas proteins within the Csy complex have been fused to transcription activation domains and developed as a transcriptional activator in human cell [Citation22]. Very recently, Yan and colleagues also successfully exploited type I-F CRISPR-Cas system as a transferrable tool in genome deletion and transcriptional regulation [Citation24]. Moreover, it has been reported that a large number of Tn7-like transposons are associated with type I-F CRISPR-Cas system variants lacking a Cas2-3 component [Citation54]. A type I-F Csy complex from Vibrio cholerae was reported to direct an accompanying transposase to integrate DNA downstream of a genomic target site complementary to guide RNA, representing the discovery of a programmable integrase system [Citation55]. Taken together, application of type I-F system in genome editing is of broad interest and rapidly developing, and also call the demand of type I-F Acrs in regulating the system. While no type I-F Acr proteins have been tested in regulating the genome editing activity of type I-F CRISPR-Cas system thus far, a large number of type II Acr proteins have already been verified to reduce off-target editing (AcrIIA4) [Citation56], cell cytotoxicity caused by extended Cas9 expression (AcrIIA2/4) [Citation57], and restrict editing to desired tissues (AcrIIC3) [Citation58], et al. In the near future, type I-F Acrs would also be utilized as regulatory tools of genome manipulation system based on type I-F CRISPR-Cas system.
Authors’ contributions
L. Y. and P. Y. wrote the original manuscript. Y. F. and Y. Z. revised the manuscript. All the authors read and agreed on the final manuscript.
Acknowledgments
We thank the lab members from Yue Feng’s lab for their helpful comments. We apologize for those whose work were not cited in this study due to space limitation.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bernheim A, Sorek R. The pan-immune system of bacteria: antiviral defence as a community resource. Nat Rev Microbiol. 2020;18:113–119.
- Makarova KS, Wolf YI, Iranzo J, et al. Evolutionary classification of CRISPR-Cas systems: a burst of class 2 and derived variants. Nat Rev Microbiol. 2020;18:67–83.
- Makarova KS, Wolf YI, Alkhnbashi OS, et al. An updated evolutionary classification of CRISPR-Cas systems. Nat Rev Microbiol. 2015;13:722–736.
- Brouns SJ, Jore MM, Lundgren M, et al. Small CRISPR RNAs guide antiviral defense in prokaryotes. Science. 2008;321:960–964.
- Bondy-Denomy J, Pawluk A, Maxwell KL, et al. Bacteriophage genes that inactivate the CRISPR/Cas bacterial immune system. Nature. 2013;493:429–432.
- Huang L, Yang B, Yi H, et al. AcrDB: a database of anti-CRISPR operons in prokaryotes and viruses. Nucleic Acids Res. 2021;49:D622–D9.
- Bondy-Denomy J, Davidson AR, Doudna JA, et al. A unified resource for tracking Anti-CRISPR names. CRISPR J. 2018;1:304–305.
- Borges AL, Davidson AR, Bondy-Denomy J. The discovery, mechanisms, and evolutionary impact of Anti-CRISPRs. Annu Rev Virol. 2017;4:37–59.
- Pawluk A, Davidson AR, Maxwell KL. Anti-CRISPR: discovery, mechanism and function. Nature Rev Microbiol. 2018;16:12–17.
- Stanley SY, Maxwell KL. Phage-encoded Anti-CRISPR defenses. Annu Rev Genet. 2018;52:445–464.
- Zhu Y, Zhang F, Huang Z. Structural insights into the inactivation of CRISPR-Cas systems by diverse anti-CRISPR proteins. BMC Biol. 2018;16:32.
- Davidson AR, Lu WT, Stanley SY, et al. Anti-CRISPRs: protein Inhibitors of CRISPR-Cas systems. Annu Rev Biochem. 2020;89:309–332.
- Li Y, Bondy-Denomy J. Anti-CRISPRs go viral: the infection biology of CRISPR-Cas inhibitors. Cell Host Microbe. 2020;29:704–714.
- Wiegand T, Karambelkar S, Bondy-Denomy J, et al. Structures and strategies of anti-CRISPR-mediated immune suppression. Annu Rev Microbiol. 2020;74:21–37.
- Cong L, Ran FA, Cox D, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823.
- Zetsche B, Heidenreich M, Mohanraju P, et al. Multiplex gene editing by CRISPR-Cpf1 using a single crRNA array. Nat Biotechnol. 2017;35:31–34.
- Dolan AE, Hou Z, Xiao Y, et al. Introducing a spectrum of long-range genomic deletions in human embryonic stem cells using type I CRISPR-Cas. Mol Cell. 2019;74:936–50.e5.
- Morisaka H, Yoshimi K, Okuzaki Y, et al. CRISPR-Cas3 induces broad and unidirectional genome editing in human cells. Nat Commun. 2019;10:5302.
- Csörgő B, León LM, Chau-Ly IJ, et al. A compact Cascade–Cas3 system for targeted genome engineering. Nat Methods. 2020;17:1183–1190.
- Xu Z, Li M, Li Y, et al. Native CRISPR-Cas-mediated genome editing enables dissecting and sensitizing clinical multidrug-resistant P. aeruginosa. Cell Rep. 2019;29:1707–17 e3.
- Zheng Y, Han J, Wang B, et al. Characterization and repurposing of the endogenous Type I-F CRISPR-Cas system of Zymomonas mobilis for genome engineering. Nucleic Acids Res. 2019;47:11461–11475.
- Chen Y, Liu J, Zhi S, et al. Repurposing type I-F CRISPR-Cas system as a transcriptional activation tool in human cells. Nat Commun. 2020;11:3136.
- Vo PLH, Ronda C, Klompe SE, et al. CRISPR RNA-guided integrases for high-efficiency, multiplexed bacterial genome engineering. Nat Biotechnol. 2020;39:480–489.
- Xu Z, Li Y, Cao H, et al. A transferrable and integrative type I-F Cascade for heterologous genome editing and transcription modulation. Nucleic Acids Res. 2021;gkab521. DOI:10.1093/nar/gkab521.
- Szczelkun MD, Tikhomirova MS, Sinkunas T, et al. Direct observation of R-loop formation by single RNA-guided Cas9 and Cascade effector complexes. Proc Natl Acad Sci U S A. 2014;111:9798–9803.
- Rutkauskas M, Sinkunas T, Songailiene I, et al. Directional R-Loop formation by the CRISPR-Cas surveillance complex cascade provides efficient off-target site rejection. Cell Rep. 2015;10:1534–1543.
- Wilkinson ME, Nakatani Y, Staals RH, et al. Structural plasticity and in vivo activity of Cas1 from the type I-F CRISPR-Cas system. Biochem J. 2016;473:1063–1072.
- Wang X, Yao D, Xu JG, et al. Structural basis of Cas3 inhibition by the bacteriophage protein AcrF3. Nat Struct Mol Biol. 2016;23:868–870.
- Wang J, Ma J, Cheng Z, et al. A CRISPR evolutionary arms race: structural insights into viral anti-CRISPR/Cas responses. Cell Res. 2016;26:1165–1168.
- Rollins MF, Chowdhury S, Carter J, et al. Cas1 and the Csy complex are opposing regulators of Cas2/3 nuclease activity. Proc Natl Acad Sci U S A. 2017;114:E5113–E21.
- Chowdhury S, Carter J, Rollins MF, et al. Structure reveals mechanisms of viral suppressors that intercept a CRISPR RNA-guided surveillance complex. Cell. 2017;169:47–57.e11.
- Guo TW, Bartesaghi A, Yang H, et al. Cryo-EM structures reveal mechanism and inhibition of DNA targeting by a CRISPR-Cas surveillance complex. Cell. 2017;171:414–26 e12.
- Pausch P, Muller-Esparza H, Gleditzsch D, et al. Structural variation of Type I-F CRISPR RNA guided DNA surveillance. Mol Cell. 2017;67:622–32 e4.
- Wiedenheft B, Zhou K, Jinek M, et al. Structural basis for DNase activity of a conserved protein implicated in CRISPR-mediated genome defense. Structure. 2009;17:904–912.
- Rollins MF, Chowdhury S, Carter J, et al. Structure reveals a mechanism of CRISPR-RNA-guided nuclease recruitment and Anti-CRISPR viral mimicry. Mol Cell. 2019;74:132–42.e5.
- Pawluk A, Staals RH, Taylor C, et al. Inactivation of CRISPR-Cas systems by anti-CRISPR proteins in diverse bacterial species. Nat Microbiol. 2016;1:16085.
- Marino NA-O, Zhang JA-O, Borges AA-O, et al. Discovery of widespread type I and type V CRISPR-Cas inhibitors. Science. 2018;362:240–242.
- Pinilla-Redondo R, Shehreen S, Marino ND, et al. Discovery of multiple anti-CRISPRs highlights anti-defense gene clustering in mobile genetic elements. Nat Commun. 2020;11:5652.
- Bondy-Denomy J, Garcia B, Strum S, et al. Multiple mechanisms for CRISPR-Cas inhibition by anti-CRISPR proteins. Nature. 2015;526:136–139.
- Maxwell KL, Garcia B, Bondy-Denomy J, et al. The solution structure of an anti-CRISPR protein. Nat Commun. 2016;7:13134.
- Peng R, Xu Y, Zhu T, et al. Alternate binding modes of anti-CRISPR viral suppressors AcrF1/2 to Csy surveillance complex revealed by cryo-EM structures. Cell Res. 2017;27:853–864.
- Kim GE, Lee SY, Park HH. A high-resolution (1.2 A) crystal structure of the anti-CRISPR protein AcrIF9. FEBS Open Bio. 2020;10:2532–2540.
- Hirschi M, Lu WT, Santiago-Frangos A, et al. AcrIF9 tethers non-sequence specific dsDNA to the CRISPR RNA-guided surveillance complex. Nat Commun. 2020;11:2730.
- Zhang K, Wang S, Li S, et al. Inhibition mechanisms of AcrF9, AcrF8, and AcrF6 against type I-F CRISPR-Cas complex revealed by cryo-EM. Proc Natl Acad Sci U S A. 2020;117:7176–7182.
- Lu WT, Trost CN, Muller-Esparza H, et al. Anti-CRISPR AcrIF9 functions by inducing the CRISPR-Cas complex to bind DNA non-specifically. Nucleic Acids Res. 2021;49:3381–3393.
- Gabel C, Li Z, Zhang H, et al. Structural basis for inhibition of the type I-F CRISPR-Cas surveillance complex by AcrIF4, AcrIF7 and AcrIF14. Nucleic Acids Res. 2021;49:584–594.
- Hong S, Ka D, Yoon SJ, et al. CRISPR RNA and anti-CRISPR protein binding to the Xanthomonas albilineans Csy1-Csy2 heterodimer in the type I-F CRISPR-Cas system. J Biol Chem. 2018;293:2744–2754.
- Kim I, Koo J, An SY, et al. Structural and mechanistic insights into the CRISPR inhibition of AcrIF7. Nucleic Acids Res. 2020;48:9959–9968.
- Niu Y, Yang L, Gao T, et al. A type I-F Anti-CRISPR protein inhibits the CRISPR-Cas surveillance complex by ADP-Ribosylation. Mol Cell. 2020;80:512–24 e5.
- Mejdani M, Pawluk A, Maxwell KL, et al. Anti-CRISPR AcrIE2 binds the Type I-E CRISPR-Cas complex but does not block DNA binding. J Mol Biol. 2021;433:166759.
- Dong L, Guan X, Li N, et al. An anti-CRISPR protein disables type V Cas12a by acetylation. Nat Struct Mol Biol. 2019;26:308–314.
- Jumper J, Evans R, Pritzel A, et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596(7873):583–589.
- Tunyasuvunakool K, Adler J, Wu Z, et al. Highly accurate protein structure prediction for the human proteome. Nature. 2021;596(7873):590–596.
- Peters JE, Makarova KS, Shmakov S, et al. Recruitment of CRISPR-Cas systems by Tn7-like transposons. Proc Natl Acad Sci U S A. 2017;114:E7358–E66.
- Klompe SE, Vo PLH, Halpin-Healy TS, et al. Transposon-encoded CRISPR-Cas systems direct RNA-guided DNA integration. Nature. 2019;571:219–225.
- Shin J, Jiang F, Liu -J-J, et al. Disabling Cas9 by an anti-CRISPR DNA mimic. Sci Adv. 2017;3:e1701620.
- Li C, Psatha N, Gil S, et al. HDAd5/35(++) adenovirus vector expressing Anti-CRISPR peptides decreases CRISPR/Cas9 toxicity in human hematopoietic stem cells. Mol Ther Methods Clin Dev. 2018;9:390–401.
- Lee JA-O, Mou HA-O, Ibraheim RA-O, et al. Tissue-restricted genome editing in vivo specified by microRNA-repressible anti-CRISPR proteins. RNA. 2019;25:1421–1431.