ABSTRACT
Biomolecular condensates, forming membrane-less organelles, orchestrate the sub-cellular compartment to execute designated biological processes. An increasing body of evidence demonstrates the involvement of these biomolecular condensates in translational regulation. This review summarizes recent discoveries concerning biomolecular condensates associated with translational regulation, including their composition, assembly, and functions. Furthermore, we discussed the common features among these biomolecular condensates and the critical questions in the translational regulation areas. These emerging discoveries shed light on the enigmatic translational machinery, refine our understanding of translational regulation, and put forth potential therapeutic targets for diseases born out of translation dysregulation.
1. Introduction
Cells effectively organize distinct compartments for various physiological processes within minute environments. Complementing the presence of the membrane-bound organelles surrounded by lipid bilayers, such as mitochondria, Golgi apparatus, endoplasmic reticulum (ER), and the nucleus, a unique class of membrane-less organelles plays instrumental roles in regulating physiological processes via orchestrating local cellular compartments. Membrane-less organelles are biomolecular condensates characterized by their transient, reversible, and highly concentrated properties. Biomolecular condensates possess the ability to assemble as needed for specific physiological functions and disassemble promptly upon task completion [Citation1]. Formed by liquid-liquid phase separation (LLPS), biomolecular condensates counteract entropy-driven solute-solution mixing and reduce system energy via demixing influenced by solute/solution intermolecular interactions [Citation1–4]. Biomolecular condensates display a tristate existence, appearing as liquid, solid, or gel [Citation3], with the potential for mutual transformation ().
Figure 1. Mechanisms of biomolecular condensation. monomers assemble into different material status condensates, including liquid-like drops, hydrogel, or solid-fibril, of which the fluidity gradually decreases (up panel). Molecules involved in biomolecular condensation include (a) proteins with multiple modular domains, (b) proteins with intrinsically disordered regions (IDRs), (c) nucleic acids, nucleic acid-binding proteins, (d) proteins capable of head-to-tail polymerization. The condensation was driven by intermolecular forces, including hydrophobic, π-π, electrostatic, and cation-π interactions, which are listed in the yellow box.
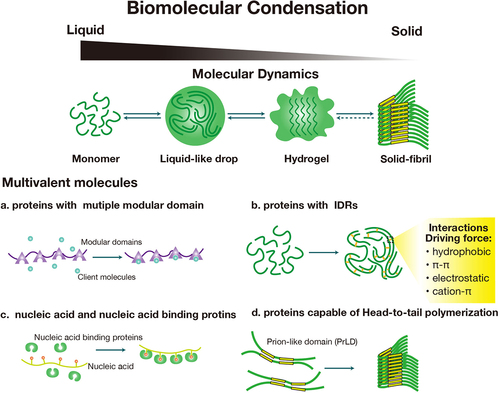
Biomolecular condensate assembly is commonly elucidated as the ‘scaffolds and clients’ model. In this model, scaffold molecules typically possess multivalent modular domains and drive condensation, serving as the primary structural elements of the condensates. Low-valency client molecules, which are dispensable for condensation, accumulate within the condensate through direct binding to scaffolds to perform specific functions [Citation1,Citation5,Citation6]. Scaffold molecules encompass multivalent biomolecules, including proteins with multiple modular domains [Citation1], proteins with intrinsically disordered regions (IDRs) [Citation1,Citation7–10], nucleic acids, nucleic acid-binding proteins [Citation5,Citation11], and proteins capable of polymerization [Citation5,Citation12]. Condensation is driven by intermolecular forces such as hydrophobic, π-π, electrostatic, and cation-π interactions [Citation13]. These interactions compel molecules to form large complexes, thereby reducing solubility to assemble into condensates and facilitating phase transitions (). Biomolecular condensates, as the membrane-less organelles, play versatile roles in cells, such as nucleolus, heterochromatin, Cajal bodies, and transport channels of nuclear pore complexes [Citation1]. In differentiated cells, biomolecular condensates enact unique functions as cell-type-related ribonucleoprotein (RNP) granules, such as neuronal granules in neurons and germinal granules in germ cells [Citation5,Citation14,Citation15].
Translational regulation is a fundamental strategy for cellular survival and adaption to environmental stress [Citation16]. The translation processes in eukaryotic cells are divided into three steps: initiation, elongation, and termination, in which initiation is the rate-limiting step [Citation17]. Remarkably, accumulating evidence implies emerging roles of biomolecular condensates in translational regulation, among which stress granules (SGs) and P-body are extensively studied [Citation18,Citation19]. Furthermore, biomolecular condensation-induced translational dysregulation has been associated with the pathogenesis of multiple diseases, including neurodegenerative diseases, cancers, and viral infections [Citation2,Citation20].
However, key questions remain unsolved. What is the common feature of the biomolecular condensate mechanisms involved in translational regulation? Is the presence of biomolecular condensate a necessity or a concomitant factor during translational regulation? Addressing these questions will aid in elucidating the indispensable roles that biomolecular condensates play during translational regulation.
A plethora of studies have provided overviews on the properties, functions, and dysregulation-related diseases of biomolecular condensates [Citation1,Citation2,Citation9,Citation11,Citation15,Citation20–22]. A recent review further summarized the relationship between biomolecular condensates and translational control from the translational repression and activation aspects [Citation23]. In this review, our focus lies on the following points: (1) we summarized the mechanisms of typical condensates involved in the translational regulation, including FXR1, FMRP, Ddx3xb condensates, and MARDO, which are not introduced in the reference [Citation23]. (2) In each section, we introduced the translation-related condensates by assembling and functioning, to emphasize the functional contributions of condensation on translational regulation. (3) We summarized the SGs-related diseases as examples to illustrate the emerging roles of condensation in treating translation-dysregulation-related diseases. All in all, we have summarized recent advances in translational regulation-related condensates, including FXR1, FMRP, Ddx3xb condensates, SGs, P-body, MARDO, and TIS granules, presenting an overview of their composition, assembly, and function. Subsequently, we discussed the common feature among condensates involved in translational regulation: the scaffold proteins assemble into condensates and effectively enrich the functionally related client molecules to perform specific biological processes, despite these condensates leading to different translational regulation outcomes. Finally, we highlight critical questions warranting future investigation and speculate on the potentiality of biomolecular condensates as novel targets for treating translation-dysregulation-related diseases.
2. FXR1 condensates
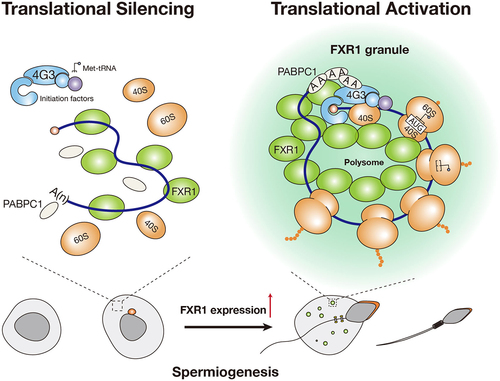
Due to the nuclear compaction causing transcription to halt in the later stages of spermatids in mammals, all the genes required for spermatid development and sperm formation must be transcribed in advance in earlier stages of male germ cells and stored as translationally repressed messenger ribonucleoproteins (mRNPs) until needed for translation [Citation24]. These inert mRNPs are typically organized into germinal granules in developing spermatids. Recent studies have revealed that both the chromatoid body (CB) and FXR1 mRNP condensates play a role in translation activation in mouse spermatids [Citation25,Citation26]. Specifically, Kang and colleagues recently demonstrated that LLPS of FXR1 is essential for translation activation of stored mRNAs in late spermatids [Citation25] ().
Figure 2. FXR1 condensates involved in mRNA translational activation in mouse late spermatozoa. during mouse spermiogenesis, FXR1 expression upregulates, and FXR1 condensation form in a concentration-dependent manner. FXR1 works as a scaffold protein that binds to mRNA, and recruits translation factors, including eIF4G3 and PABPC1, to FXR1 condensates and activates mRNA translation.
2.1. FXR1 is an RNA-binding protein related to translational regulation
The fragile X-related (FXR) family, encompassing members such as FMRP, FXR1, and FXR2, are typical RNA-binding proteins that possess a domain architecture consisting of two Agenet domains, two KH domains, and an RGG domain [Citation27]. Unlike the well-documented roles of other FXR family members in translational repression, FXR1 accelerates translation in mouse late spermatids (LS) [Citation25]. In addition to its association with multiple neurological defects, FXR1 is also involved in various functions, which include but are not limited to, oogenesis [Citation28,Citation29], eye and neural crest development [Citation30], myogenic cell differentiation [Citation31,Citation32], neural stem cell proliferation during adult neurogenesis [Citation33].
2.2. Assembly of FXR1 condensates
Intriguingly, FXR1 protein is potently upregulated in LS in mice, where FXR1 undergoes LLPS to form mRNP granules and recruits the translational machinery via direct interaction with EIF4G3, an eukaryotic transcription initiation factor [Citation25]. Other translation factors, including PABPC1, EIF4A3, and ribosomal components, are also recruited to the FXR1 mRNP granules, leading to the assembly of a unique RNP platform to regulate the translation of spermatogenic mRNAs independent of the chromatoid body (CB), the best-characterized germinal granule in mouse spermatids [Citation34,Citation35] (). Key evidence for the vital function of FXR1 condensates in translation activation and spermatid development should find a mutation that disrupts FXR1’s propensity for condensation without affecting its other functions, such as RNA binding capacity [Citation36]. To this end, Kang and colleagues screened the key residue(s) in FXR1 responsible for LLPS by testing a series of FXR1 mutants. They identified a leucine residue at position 351 in the KH2 domain of FXR1a as an essential residue for phase separation. In contrast, a fusion of the FUS IDR domain can recover the phase separation ability of the L351P mutant. Interestingly, while the L351P mutation nullifies the phase separation ability of FXR1, it leaves its RNA-binding ability largely unscathed. L351 is situated in the fourth helix (α4) in the KH2 domain of FXR1, and the L351P mutation likely disrupts the orientation of α4, thereby changing the conformation of the two adjacent aromatic residues, Y353 and Y357. These two residues are shown to be equally crucial for the phase separation of FXR1, as their mutations could alter the hydrophobic interface, thus leading to the loss of the phase separation ability of FXR1.
2.3. FXR1 condensates involving in mRNA translational activation in mouse late spermatozoa
Both wild-type FXR1 and condensation-restored mutant, but not the condensation-deficient mutant, activate the translation of FXR1 target mRNAs in co-transfected cells and significantly rescue target translation in Fxr1-depleted mouse spermatids, strongly supporting that LLPS is required for FXR1 to activate translation in late spermatids in mice. Moreover, researchers constructed the Fxr1L351P knock-in mice. They showed the knock-in mutant mice fully phenocopied the FXR1 knockout mice, directly supporting the requirement of FXR1 condensation for spermatid development and male fertility [Citation25]. Similar to the previous report about the roles of FXR1 in Ago2-mediated translation activation in quiescent conditions [Citation37], this work provides strong evidence that FXR1 condensation is functionally essential for activating target mRNA translation in mouse spermatids.
Of note, Greenblatt et al. show that FMR1 (homolog of FMRP in Drosophila) acts to activate the translation of stored mRNAs in early Drosophila embryos, echoing the function of FXR1 in mouse spermatids [Citation38]. During the tenth day of embryonic development after fertilization of Fmr1-null oocytes, the ventral nerve cord develops defectively. Ribosome-profiling analysis revealed that a group of neurodevelopmental syndrome-related genes was down-regulated in Fmr1-null embryos, such as the neurofibromatosis gene Nf1 and E3 ubiquitin ligase genes ctrip/Trip12, poe/Ubr4, and Huwe1, the human homologs of which are associated with intellectual disability, autism, early-onset dementia, and schizophrenia [Citation38]. Furthermore, the protein products of FMR1 target mRNAs appear to be large. Due to the inefficient translation of large proteins, FMR1 functions to activate the translation of such large mRNAs by isolating them from other non-translationally RNP particles in the cytoplasm [Citation38]. However, whether the condensation propensity of FMR1 is required for translation activation of its target mRNAs in Drosophila embryos remains to be characterized.
3. FMRP condensates
Fragile X mental retardation protein (FMRP) is a well-documented factor involved in regulating mRNA translation by forming condensates [Citation39,Citation40]. In neurons, the regulation of mRNA translation relies on membrane-less organelles called neural granules, which facilitate mRNA transportation from the cytoplasm to synaptic terminals [Citation41]. Translational repression is required during mRNA transportation, whereas translational activation occurs upon reaching the synapse [Citation42]. The dynamic assembly and disassembly of neural granules are associated with translationally regulated processes [Citation43]. The FMRP protein is a member of the FXR family and is ubiquitously expressed in mammalian tissues, with exceptionally high abundance in the brain and testis [Citation44]. FMRP is localized in ribonucleoprotein (RNP) granules [Citation45] and is involved in the processing of precursor mRNAs [Citation46], granule transport [Citation47,Citation48], translational regulation [Citation49], as well as the regulation of ion channel expression and activity [Citation50]. Lack of FMRP expression results in translational dysregulation and defects in synaptic maturation, leading to Fragile X syndrome (FXS), a common form of inherited intellectual disability and one of the major known causes of autism [Citation44].
3.1. Post-translational modifications (PTMs) regulate FMRP repression of synaptic mRNA translation
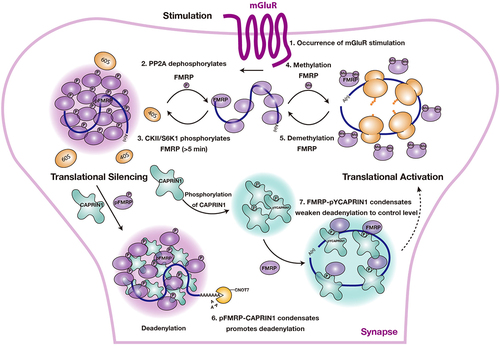
FMRP localizes to postsynaptic spaces of dendritic spines and binds a group of translationally repressed dendritic mRNAs [Citation44]. When the synapse receives stimulus signals, FMRP derepresses translation, allowing essential proteins associated with synaptic plasticity to be translated at the proper location and time [Citation44,Citation51]. This process is regulated by the PTMs state of FMRP [Citation39,Citation40]. A previous study found that phosphorylated state FMRP was associated with stalled polysomes, while non-phosphorylated FMRP was associated with actively translated polysomes [Citation52]. In neurons, FMRP proteins undergo phosphorylation in the quiescent state, leading to condensation and the subsequent sequestration of target mRNA in FMRP granules [Citation39]. However, activation of metabotropic glutamate receptor (mGluRs) by neuronal stimulation triggers the activation of phosphatase 2A (PP2A), resulting in the dephosphorylation of FMRP and subsequent mRNA translation [Citation53,Citation54]. After mGluRs stimulation (>5 min), the inhibition of PP2A activity and the activation of S6 kinase 1(S6K1) phosphorylates FMRP, restoring mRNA translation repression [Citation55]. In addition, Casein kinase II (CKII) is also involved in regulating FMRP phosphorylation [Citation56] (, steps 1–3).
Figure 3. PTMs of FMRP control the assembly and disassembly of FMRP condensate and regulate the mRNA translational state in neurons. PTMs, including phosphorylations and methylations, control the assembly of FMRP condensates and regulate the mRNA translation as the following step: (1) when a synapse receives stimulations, mGlur activates phosphatase 2A (PP2A). (2) PP2A dephosphorylates FMRP, triggering mRNA translation. (3) after mGlur stimulation (>5 min), PP2A activity is inhibited; instead, CKII/S6K1 is activated, which phosphorylates FMRP and restores mRNA translation to the inhibited status. (4) methylation weakens the binding capacity of FMRP to polyribosomes and mRNAs, impairs condensation propensity of FMRP leading towards translation activation. (5) Demethylation regulates translation in the reverse direction of step 4. (6) phosphorylation of FMRP (pFMRP) co-phase separates with CAPRIN1, promoting mRNA degradation by recruiting the CCR4-NOT complex to pFMRP-CAPRIN1 condensates. (7) FMRP co-phase separates with pYCAPRIN1 and tunes the deadenylation rates of the CCR4-NOT complex, promoting mRNA translation.
3.2. PTMs of FMRP regulate the assembly and disassembly of FMRP condensates
In vitro, RNAs harbouring G4 structures interact with the RGG motif of FMRP, present within its IDR (FMRPIDR), to mediate the formation of FMRP condensates [Citation39]. Crucial for the assembly of these condensates, phosphorylation of FMRPIDR increases the negative charge densities of the glutamic/aspartic acid-rich cluster. It enhances the condensation propensity of FMPR and the efficiency of translational repression. Meanwhile, FMRP condensates recruited translation repressors, including 4E-BP2 and microRNA-125b, to achieve inhibition of mRNA translation [Citation39].
In addition to phosphorylation, methylation of FMRP has also been reported to play a role in regulating mRNA translation [Citation39]. Methylation of the FMRP RGG motif eliminates electrostatic interactions, disassembles FMRP condensates, and relieves the inhibition of mRNA translation [Citation39]. Thus, phosphorylation and methylation of FMRP play a bi-directional role in the regulation of mRNA translation. In summary, PTMs regulate mRNA translation by modulating the assembly and disassembly of FMRP condensates (, steps 4–5).
3.3. FMRP and CAPRIN1 collaboratively regulate CCR4-NOT activity through condensation
FMRP and CAPRIN1 control synaptic plasticity in neurons, making their regulation a crucial area of investigation [Citation48,Citation57]. The C-terminal IDRs of FMRP and CAPRIN1 determine their condensation propensity [Citation39,Citation58], and these regions contain multiple phosphorylation sites [Citation59,Citation60]. The interaction between the C-terminus of CAPRIN1 and phosphorylated FMRP (pFMRP) is facilitated by Arginine-π interactions, resulting in their co-condensation [Citation40]. Additionally, tyrosine phosphorylation of CAPRIN1 (pYCAPRIN1) promotes its interaction and co-condensation with FMRP but not with pFMRP [Citation40]. Interestingly, the addition of sc1, a well-known FMRP-binding RNA containing a G4 structure, results in the formation of multiphase droplets in pFMRP-CAPRIN1 condensates but not in FMRP-pYCAPRIN1 condensates [Citation40]. Further, pFMRP-CAPRIN1 liquid highly promotes the deadenylase activity of CNOT7, the catalytic subunit of the CCR4-NOT complex, and RNA degradation. In contrast, the enzymatic activity of CNOT7 in FMRP-pYCAPRIN1 droplets is similar to that in the control [Citation40]. Collectively, these findings demonstrate that PTMs act as a switch to dynamically regulate the condensation status of the different components in the condensates, thereby conferring a subtle tuning function to these granules for the precise control of translation dynamics (, steps 6–7).
4. Stress granules
SGs are membrane-less RNP granules ranging in size from 0.1–0.2 μm and play crucial roles in regulating translation, signalling, and innate immunity [Citation5,Citation18,Citation61]. SGs form in response to cellular stressors, such as heat shock, oxidative stress, or starvation [Citation62]. The core component of the SGs consists of translationally stalled pre-initiation complexes, including pre-initiation factors (e.g. eIF4E, eIF3, eIF4A), 40S ribosomal subunits, and mRNA from polysome [Citation63]. Additionally, the composition of SGs varies depending on cell type and the type of stress experienced [Citation63]. SGs serve as hubs for mRNA triage: the recruitment of molecules from other signalling pathways to SGs determines the fate of each mRNA, i.e. whether it is stored or degraded [Citation64,Citation65]. SGs can be formed through either a stress eIF2α phosphorylation-dependent pathway [Citation66] or a PI3K-mTOR-controlled cap-binding eIF4F complex-dependent pathway [Citation67]. Drugs that inhibit translation initiation can stimulate SGs assembly; in contrast, drugs that inhibit translation elongation, such as cyclohexanone, prevent their formation. SGs formation is relevant to stress-induced resistance to cancer chemotherapy, suppressing cancer cell apoptosis by inhibiting the MAPK pathway during environmental stress [Citation68]. However, cisplatin disrupts SGs formation and causes cancer cell apoptosis by inducing ribosomal subunit aggregation [Citation69].
4.1. The formation of stress granules
The formation of SGs is mediated by the condensation of critical proteins, such as the Ras-GAP SH3 domain-binding protein (G3BP1) and T-cell intracellular antigen 1 (TIA1). G3BP1 undergoes LLPS and assembly to form SGs [Citation70–72]. However, it requires the presence of single-stranded RNA or mRNA of lengths greater than 250 nucleotides to facilitate the condensation [Citation70]. Furthermore, the N-terminal NTF2 domain and the C-terminal KH domain of G3BP1 are essential for condensation, while the IDR regions of G3BP1 regulate the mobility of G3BP1 droplets [Citation70–72]. Ubiquitin-associated protein 2-like (UBAP2L), another component of SGs, interacts with G3BP1 and determines the formation of SGs. Interfering with the UBAP2L/G3BP1 interaction disrupts the formation of SGs [Citation71,Citation73]. Additionally, the RNA multivalent binding sites of G3BP1 play a crucial role in SGs assembly [Citation70,Citation71]. USP10, another binding partner of G3BP1 [Citation58], negatively regulates SGs assembly by masking the RNA multivalent binding site of G3BP1 [Citation71]. Overexpression of either UBAP2L or FXR1 in G3BP1 knockdown cells restores SGs assembly, indicating that other key components of SGs could form their assembly nodes independently of the G3BP1 [Citation71].
TIA1, another critical component in SGs, also undergoes condensation through the IDR domain to assist in SGs formation [Citation74]. Under oxidative stress, reactive oxygen species (ROS) oxidizes Cys36 of TIA1, preventing its interaction with target RNA and inhibiting SGs formation, which leads to SGs disassembly [Citation74]. In neurodegenerative diseases, misfolded proteins trigger endoplasmic reticulum stress (ER stress) and oxidative stress [Citation74]. However, oxidative stress disrupts ER stress-induced SGs, releasing apoptotic factors sequestered in SGs and leading to neuronal apoptosis [Citation74].
Ded1p, a component of yeast SGs, is an ATP-dependent DEAD-box helicase that plays a crucial role in translation initiation [Citation75]. Ded1p unwinds the secondary structure of mRNA 5’-UTR to facilitate ribosome scanning and recognition of the ATG codon on mRNA [Citation76,Citation77]. However, condensation inactivates Ded1p. Under heat shock and pH changes, Ded1p undergoes condensation to form a gel-like condensate [Citation78]. After heat shock in yeast cells (>39 centigrade), Ded1p condensates sequester housekeeping mRNAs with complex 5’-UTR structures and prevent their translation, while stress mRNAs with simple 5’-UTR structures, such as those encoding heat shock proteins, escape translational repression of Ded1p and are expressed to enhance cell survival under severe heat stress [Citation78].
4.2. Advanced techniques reveal details of stress granule structures and functions
Cutting-edge imaging techniques such as single-molecule imaging, proteomic analysis, and condensation investigations have provided insight into functions in translational regulation and fine structure of SGs. Recent single-molecule imaging studies have revealed that not all mRNAs in SGs are translationally repressed [Citation79]. Specific mRNAs, such as activating transcription factor 4 (ATF4), which are required for initiating the stress response, localize to SGs and undergo translation within them. Additionally, the entire cycle of ATF4 protein synthesis, including translational initiation, elongation, and termination, occurs within SGs. Moreover, the translating mRNA can be transported directly between SGs and the cytoplasm without altering its translational state [Citation79].
In another study using ultra-high-resolution microscopy imaging, the interior of the SGs was found to be heterogeneous. G3BP1, PABP1, and poly(A) RNA form the concentrated and stable core of the SGs, with the surrounding less concentrated material forming the phase-separated shell through weaker interactions [Citation80]. Proteomic analysis has shown that numerous ATPases are in the core of SGs. ATP is required to maintain the liquid-like property of SGs, which affects the assembly and dynamics of SGs. ATP-dependent RNA helicases are conserved components of SGs. Nucleic acid remodelling complexes, including protein chaperones (such as Hsp70/Hsp40 and the CCT complex), disrupt SGs assembly, while various RNA and DNA helicases (such as DEAD-box proteins, MCM, and RuvB-like helicases) facilitate the persistence of SGs [Citation80]. These findings demonstrate that multiple ATP-driven machines are necessary for maintaining the dynamic assembly and disassembly of SGs.
4.3. Stress granule-related diseases
4.3.1. Stress granule-related neurodegenerative diseases
SGs form through LLPS and have garnered attention for their role in regulating translation by storing translation-stalled mRNA in a central location [Citation5,Citation18,Citation61]. However, SGs assembly or clearance goes awry may lead to the formation of inclusion bodies composed of RNP granules in the cytoplasm, disrupting cellular translation and ribostasis, which are common pathogenic mechanisms contributing to neurodegenerative diseases [Citation81,Citation82]. During transcription, mRNA assembles with nuclear RNA-binding proteins (nRBPs), including TDP-43, hnRNPA1, hnRNPA2B1, FUS, and TIA-1, which are involved in pre-mRNA processing and guide the mRNA to the cytosol [Citation81]. These nRBPs usually possess prion-like domains (PrLDs) that can self-propagate into hyper-stable amyloid aggregations [Citation81]. Under normal conditions, SGs maintain a rapid rate of protein exchange with the cytosol to maintain high dynamics [Citation81]. However, nRBPs containing PrLDs enter the SGs with the non-ribosome-bound mRNAs [Citation83], stochastically forming hyper-stable amyloid-fibril aggregates, leading to the abnormal assembly of SGs and over-accumulation of amyloid aggregates [Citation81]. For example, excessive accumulation of TDP-43 [Citation84,Citation85], FUS [Citation86,Citation87] or hnRNPA1 [Citation88], hnRNPA2B1 [Citation88] have been identified in amyotrophic lateral sclerosis (ALS).
Mutations in the PrLD-containing proteins are usually identified in neurodegenerative clinical cases. For example, fused in sarcoma (FUS), a multifunctional RNA-binding protein [Citation86], has been found to contribute to a subset of familial ALS cases [Citation86,Citation87]. ALS-related FUS mutations (ALS-FUS) may result in cytoplasmic localization of FUS, translational repression, and overactivation of the nonsense-mediated decay (NMD) pathway [Citation89]. In addition, ALS-FUS promotes FMRP condensation and sequesters FMRP in aberrant neuronal condensates, leading to translational inhibition in mouse and human FUS-ALS motor neurons [Citation90].
A pathological mutation of hnRNPA1, D262V, located in the PrLDs of hnRNPA1, has been identified in families with hereditary myopathy and Paget’s disease of the bone [Citation88]. The hnRNPA1 protein known to be recruited into SGs [Citation88] undergoes condensation upon temperature variations (25 to 4 degrees Celsius) under normal conditions, simultaneously self-assembling to form amyloid-fibril. The hnRNPA1 amyloid-fibril formation is reversible, with fibres disassembling upon temperature recovery from 4 to 25 degrees Celsius [Citation10]. Furthermore, reversible amyloid-forming cores of hnRNPA1 (hnRACs) were identified. A structural analysis of wild-type hnRAC revealed a unique ‘stacking-D’ feature with continuously stacked negatively charged Asp residues along the β-sheet, contributing to fibril instability. Interestingly, the cross-β architecture of hnRACs possesses hydrophilic interfaces, contributing to the reversible property of fibrils, in contrast to the hydrophobic interfaces of irreversible fibrils. However, the pathological mutation, D262V, disrupted the D-stacking, leading to irreversible amyloid aggregation and pathogenesis of ALS [Citation91].
Another example of a deleterious mutation associated with altered condensation is related to Marie-Tooth type 2 neuropathies (CMT2), which causes nerve damage primarily in the extremities [Citation92]. A pathological mutant of glycyl-tRNA synthetase (GlyRS), which leads to CMT2 (CMT2-GlyRS), translocates from the cytosol into SGs upon stress, while the wild-type GlyRS would not [Citation92]. The CMT2-GlyRS aberrantly binds to G3BP and integrates into SGs. In vitro studies revealed the CMT2-GlyRS mutant exhibited a strong co-condensation tendency with G3BP1 and converted a fraction of G3BP1 from spherical droplets to dense condensates. CMT2-GlyRS disturbs the normal cellular stress response, leading to a pronounced decrease in the capacity of motor neurons to resist adverse environmental stimuli, making motor neurons susceptible to axonal degeneration [Citation92].
C9ORF72 is a mutant gene associated with condensation-related pathology. It has a hexanucleotide (G4C2)n repeat expansion in the non-coding region of chromosome 9 open reading frame 72. C9ORF72 generates dipeptide repeat proteins (DRP), which link to ALS and frontotemporal dementia (FTLD) [Citation5,Citation93]. These arginine-containing DRPs undergo condensation and interact with cellular membrane-less organelles, such as nucleoli, the nuclear pore complex, and SGs [Citation94]. Toxic DRPs perturb the dynamics of SGs and impact RNA metabolism, which is the cornerstone underlying ALS/FTLD pathogenesis [Citation95].
Autism spectrum disorder (ASD) is a complex neurodevelopmental disorder characterized by abnormal social interactions, impaired communication skills, and repetitive or stereotypical behaviours [Citation96]. One of the mechanisms contributing to the pathogenesis of ASD is the misregulation of alternative splicing of variable microexons [Citation96]. Alternative Microexons are a class of neuron-specific, frame-preservation elements conserved in vertebrates, ranging from 3–27 nt in length. They mediate protein interactions by being located on the protein surface [Citation97]. Most microexon splicing is controlled by the serine/arginine repetitive matrix protein 4 (SRRM4) [Citation96,Citation98]. One-third of individuals with congenital ASD exhibit exons that undergo increased skipping, corresponding to downregulated SRRM4 levels [Citation99]. Additionally, translational dysregulation during neurodevelopment is also a contributing factor to ASD [Citation100]. Recent research has shown that microexon protein products regulate neuron translation via condensation and are associated with the neurodevelopmental and behavioural phenotypes of ASD [Citation101].
Two ASD-associated neuron-specific microexons were identified from the eIF4G homologs, eIF4G1 and eIF4G3. The alternative splicing of these two eIF4G microexons was regulated by SRRM4. The protein products of the eIF4G microexons interact with various cytoplasmic mRNPs, such as Fxr1, Ataxin-2, Larp1, and Stau2. Furthermore, it was revealed that the eIF4G microexon protein product is embedded in the PrLD of the eIF4G protein. The GGFRxxQ consensus sequence in the microexon protein enhances the condensation propensity of eIF4G and promotes the condensation of pFMRP. As a result, the FMRP condensates are ‘hijacked’, which promotes ribosomal arrest, ultimately leading to the downregulation of synaptic proteins critical for normal cognitive function [Citation101].
In summary, over-accumulating condensated proteins in SGs are a common cause of neurodegenerative diseases. Therapeutic strategies targeting the accumulation of SGs in neurons could potentially arrest the disease progression in neurodegenerative diseases [Citation102].
4.3.2. Stress granule-related and viral infection
Viral infection-induced SGs (V-SGs) negatively regulate translation and protect the host cell from viral parasites. However, viruses evolved mechanisms to confront V-SGs formation, ensuring efficient viral replication. Reviews have summarized how viruses disrupt the V-SGs formations or prevent V-SGs assembly for a robust and productive infection [Citation103]. In short, during V-SGs formations, activation of eIF2α kinases (PRK/PERK/HRI/GCN2) triggers a translation stall. To prevent V-SGs formation at the translational initiation stage, some viruses (West Nile, Influenza, vaccinia, poliovirus, rotavirus, SFV) inhibit PRK, while some inhibit PERK (reovirus). Other viruses, such as junin virus, hepatitis C virus, sindbis virus, and respiratory syncytial virus, hijack the SGs components or reconstitute novel foci through viral proteins in the host cytosol to segregate and sequester critical SGs components. However, West Nile and Dengue viruses interfere with SGs formation by sequestering TIA1 through interaction with the 3’UTR of viral minus-strand RNAs [Citation104]. Additionally, poliovirus inhibits SGs assembly by cleaving G3BP1 in a manner dependent on viral protease 3C [Citation105]. This knowledge helps us understand the mechanism of virus impeding V-SGs formation and provides us with many potential targets for anti-viral drug discovery.
5. P-body
The processing body (P-body) is a condensate containing translationally stalled mRNA that is predominantly associated with the regulation of RNA storage [Citation19], translation control [Citation19,Citation106,Citation107], and signal transduction [Citation108]. P-body is the centre for the regulation of mRNA fate. Accumulated evidence shows that P-body are biomolecular condensates formed through condensation. In this review, we highlight recent progress in P-body-related research. We discuss the newly identified P-body components and the working model of P-body as illustrated by the ultra-resolution single-molecule fluorescence imaging technology, which aids in our understanding of the molecular details of P-body-mediated translational regulation.
5.1. Components of P-body
The classical and newly identified components of P-body have been summarized in detail [Citation109–111]. Core components of P-body mainly include the decapping complex (the decapping enzyme Dcp1p/Dcp2p and Edc4), the decapping activator (Dhh1, Pat1p, Edc3p), the Lsm1-7p complex, the 5’ to 3’ nucleic acid exonuclease (Xrn1p), and the deadenylation complex (CCR4/POP2/NOT complex). Additional components include miRNA repression factors (Argonautes, GW182, and TNRC6B), as well as translation repressors (Spb1p and eIF4E) [Citation109].
Recently identified P-body components, including NoBody (NBDY), YTHDF2, and CCHCR1, are reviewed in reference [Citation111]. Additionally, meiosis arrest female 1 (MARF1), another newly identified P-body component, was discovered to mediate the CCR4-NOT-independent RNA decay and play a crucial role in development [Citation112–114]. MARF1 comprises an active nuclease domain, NYN, and eight LOTUS domains (RNA binding domains) [Citation115]. In mammals, MARF1 has two isoforms: a somatic isoform that promotes neuronal differentiation [Citation116], and an oocyte-specific isoform vital for mouse oocyte maturation and meiosis recovery [Citation115,Citation117]. Studies on MARF1 knockout mice revealed upregulation of certain mRNAs, including the protein phosphatase two catalytic subunits (PPP2CB) and transposons such as Iap and LINE1, leading to female sterility [Citation115]. The nuclease domain, NYN, of MARF1, responsible for the CCR4-NOT-independent mRNA decay, is required for mouse oocyte maturation [Citation112,Citation118]. MARF1 enters P-body via EDC4 interaction in mammalian cell lines while its nuclease activity is inhibited. Notably, the nuclease activity of MARF1 is recovered when released to the cytosol from P-body [Citation114].
A mass spectrometry-based proteomics study revealed a comprehensive landscape of the P-body interaction network. Three recent works have identified components of the interacting proteomes of human-derived DDX6 and the yeast homolog Dhh1p, largely overlapping with P-body components [Citation119–121]. Hubstenberger et al. used fluorescence-activated particle sorting (FAPS) combined with proteomic analysis to identify many translationally repressed mRNAs and mRNA regulators clustered in the P-body condensate. The comprehensive interaction network of the P-body condensate positions DDX6 as the central node, interacting with half of the P-body proteins. According to transcriptome data, mRNAs in P-body are not degraded but rather stored in a translationally repressed status [Citation120].
5.2. Ultra-resolution single-molecule fluorescence imaging revealed a detailed working model of P-body
P-body is considered the liquid-like condensate because P-body components are usually IDR-containing proteins [Citation122]. Spherical morphology of P-body, droplet fusion, and fluorescence recovery after photobleaching (FRAP) experiments support the liquid-like property of P-body [Citation65,Citation123,Citation124]. Examples of condensation-mediated P-body component assembly, such as Lsm4, the decapping enzyme Dcp1/Dcp2, and Edc3, have been summarized in the reference [Citation111]. However, using ultra-resolution single-molecule fluorescence imaging, Pitchiaya et al. revealed P-body condensates are not homogenous but hierarchically composed of the core and periphery parts. Each part is resident with different kinds of RNA and possesses different kinetics to execute the corresponding molecular function [Citation125].
By tracking the trajectory of miRNAs in the P-body in living cells, researchers found that the localization of miRNAs in the P-body depends on the function of the miRNAs, i.e. P-body stable sequester the target-less miRNAs in its core for surveillance. In contrast, functional miRNAs targeting mRNA targets transiently interact with P-body and localize to P-body periphery [Citation125]. Moreover, miRNAs targeting the 3’ UTR lead to stable interactions with P-body compared to miRNAs targeting the 5’ UTR. Nevertheless, translating mRNAs rarely localize to P-body, which means the P-body localization of mRNA increases translation repression. Furthermore, mRNA degradation is mainly catalysed by the degradation enzymes diffused in the cytosol, while only a tiny fraction of repressed mRNAs is degraded in the P-body. The P-body interacting lncRNAs transiently interact with the P-body periphery, which differs from miRNAs and translational stalled mRNAs stably captured in P-body [Citation125]. This work provides a working model of how P-body stores the repressed mRNA and unused miRNA for surveillance. Moreover, it shows us that ultra-resolution fluorescence microscopy is a powerful tool for mechanistically probing the dynamic assembly of RNP granules by condensation at single-molecule resolution [Citation125].
6. Other translational related condensates
6.1. MARDO
Recent studies have shed light on the mechanism of condensation and regulation of translation repression in mouse oocytes [Citation126]. During oocyte growth, transcription ceases and only resumes when the embryonic genome is activated after fertilization, similar to spermatogenesis [Citation127]. The oocyte and embryo utilize stored mRNAs to synthesize new proteins [Citation128]. A mitochondria-associated ribonucleoprotein domain (MARDO) has recently been identified as a membrane-less organelle involved in the translation repression of mRNA and essential for fertility in mammals [Citation126].
MARDO comprises mRNAs, RNA-binding proteins (ZAR1, YBX2, DDX6, LSM14B, and 4E-T), and clustering mitochondria. The formation of MARDO depends on increased mitochondrial membrane potential. MARDO coalescence and mitochondria clustering are promoted by Zygote Arrest 1 (ZAR1). The N-terminal IDR of ZAR1 formed hydrogel-like condensates in vivo, which was necessary and sufficient for MARDO coalescence. In vitro, the N-terminal IDR of ZAR1 fused to a small ubiquitin-related modifier tag SMT3 formed liquid-like condensates. Both MARDO and ZAR1 repress mRNA translation. ZAR1-knockout oocytes exhibit premature loss of mRNAs and severe defects in spindle assembly, chromosome alignment, and cytokinesis, contributing to meiotic and embryonic developmental defects. However, MARDO dissolution, regulated by the proteasomal degradation of ZAR1, is required for timely maternal mRNA degradation during oocyte meiotic maturation. These condensates formed through condensation coordinate maternal mRNA storage, translation repression, and degradation, contributing to maintaining mammalian fertility [Citation126].
6.2. Ddx3xb condensates
Shi et al. recently reported that LLPS of the RNA helicase Ddx3xb promotes maternal mRNA translation during the maternal-to-zygotic transition (MZT) in zebrafish. Mechanistically, condensation of Ddx3xb, mediated by the IDR at the N-terminal domain, facilitates the Ddx3xb helicase activity and enhances the unwinding of 5’ UTR structures of maternal mRNAs for their translation. Moreover, condensation of Ddx3xb is involved in degrading specific maternal mRNAs and activating the translation of a subset of zygotic mRNAs, thereby contributing to MZT [Citation129].
6.3. TIS granules
TIS granules were identified to be condensates associated with the endoplasmic reticulum (ER). The main component of TIS granules is TIS11B, an RNA-binding protein that forms gel-like condensates in vivo. TIS11B prefers to bind AU-rich elements (AREs) of an RNA. TIS11B orchestrates the assembly of TIS granules, serving as a scaffold protein through condensation driven by charge distribution but not the IDR. TIS granules and the ER form a subcellular compartment, the TIGER domain, which specifically recruits membrane-protein-coding mRNAs that contain AREs at 3’UTR. Meanwhile, the TIGER domain enriches HuR, folding chaperone HSPA8, and SET protein to promote surface expression and folding of membrane proteins on ER, such as CD47 and PD-L1 [Citation130]. Moreover, mRNAs serve as scaffold molecules to form the condensation skeleton through extensive intermolecular mRNA interactions. This RNA matrix prevents TIS11B from forming liquid-like condensates and drives it to form irregularly shaped membrane-less organelles [Citation131]. This example provides a paradigm in which biomolecular condensates constitute a subcellular compartment that enriches the functionally related molecules to fulfill a specific physiologic process that couldn’t be conducted outside the condensates.
7. Concluding and perspective
In this review, we have summarized the translational-related condensates. We provided an overview of these biomolecular condensates’ composition, assembly, and functions, summarized in and . The translation process in eukaryotic cells encompasses initiation, elongation, and termination. Condensation occurs at each translation stage related to translational activation, repression, mRNA storage, and degradation.
Figure 4. Translational regulation-related biomolecular condensates in eukaryotic cells. the translation process in eukaryotic cells includes initiation, elongation, and termination. FXR1 and Ddx3xb condensates regulate mRNA translation at the initiation stage, in which, FXR1condensates promote translation activation in mouse late spermatids and Ddx3xb condensates are involved in the translational activation of zygotic mRNAs. FMPR condensates inhibit mRNA translation in neurons at the initiation, elongation, and termination stages. stress granules predominantly repress the translation at the elongation stages. P-body hosts RNA degradation complexes and regulates translational processes at the termination stages. MARDO is a coalescence of RNP condensates (including ZAR1, DDX6, LSM14B, 4E-T, and YBX2) and mitochondria, which stores translational repressed mRNA and is crucial for mammalian oocyte growth. TIGER domain, composed of TIS granules and the ER, provides a unique location to promote the translation of the membrane-protein-coding, AREs-containing mRNA and assist protein folding on the ER surface.
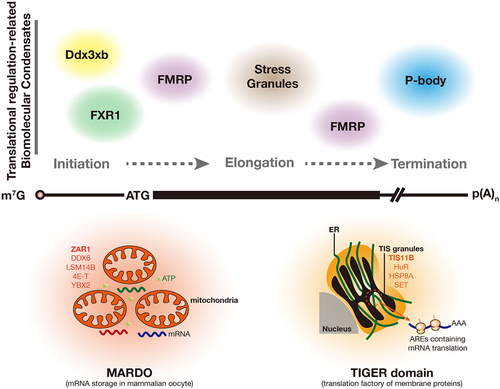
Table 1. Translational-related biomolecular condensation in eukaryotic cells.
At the translation initiation stage, the distinctive roles of FXR family members in translational regulation have been extensively documented. FXR1 assembles into condensates through condensation, facilitating translation in mouse spermatids to guarantee proper spermatogenesis. Conversely, FMRP, another FXR family member, forms condensates to regulate translation negatively in neurons. FMRP condensates regulate translation at the initiation, elongation, and termination stages [Citation39,Citation40,Citation51]. The condensation propensity of FMRP is carefully regulated by PTMs, including phosphorylation and methylation, which finely modulate the assembly and disassembly of FMRP condensates, ultimately leading to the repression or activation of translation. Moreover, Ddx3xb condensates are involved in the translational activation at the initiation stage.
SGs are repositories of pre-initiation factors and translation-stalled mRNAs, which predominantly regulate the translation at the elongation stage. The assembly and disassembly of SGs are controlled by critical components such as G3BP1, TIA1, and Ded1p, among others, through their condensation propensity. Notably, not all stress granular mRNAs are in a translationally repressed state. The entire synthesis cycle of some proteins required for stress response, including initiation, elongation, and termination, occurs in SGs. This signifies that SGs are temporary storage stations for translation-stalled mRNA but do not repress mRNA translation.
P-body is a subcellular RNP condensate that hosts RNA degradation complexes and regulates translational processes at the termination step. Recently, the discovery of novel P-body components, such as Nobody, YTHDF2, and MARF1, has shed light on the intricate regulation of mRNA clearance and translational control. Notably, RNA-degrading enzymes within the P-body activity are inhibited, and mRNA degradation predominantly occurs in the cytoplasm.
Some subcellular compartments formed by the RNP condensates and membrane-bound organelles can be interpreted as temporary warehouses or factories for mRNA storing or translation in the specific cell type, such as the MARDO and the TIGER domain. MARDO is a coalescence of RNP condensates and mitochondria, which stores translational repressed mRNA and is crucial for mammalian oocyte growth. The TIGER domain, composed of TIS granules and the ER, provides a unique location to promote the translation of the membrane-protein-coding, AREs-containing mRNA and assist protein folding on the ER surface.
All these findings provide a clearer understanding of how RNP condensates regulate translational processes. Although these condensates work at different times and spaces with various biological outcomes, one common feature they share is that the scaffold proteins assemble into condensates, recruiting and concentrating the functionally related client molecules to execute a specific biological process effectively.
Although significant progress has been made, key questions warrant further investigation, helping us better understand the mechanisms of biomolecular condensates involving translational regulation and fully utilize the knowledge for disease therapeutics in the future.
RNA modifications also play significant roles in condensation-mediated translational regulation. Accumulating evidence shows that RNA modifications, including N6-methyladenosine (m6A), 5-methylcytosine (m5C), and N7-methylguanosine (m7G), play essential roles in regulating translation through condensation. Modified RNAs as multivalent molecules recruit the modification reader proteins and form LLPS-mediated condensates. For example, the condensation of m6A-binding proteins, YTHDF1–3, is mediated by LLPS and is markedly enhanced by mRNAs containing multiple m6A residues but not by the ones with a single m6A residue [Citation132–134]. Functionally, nuclear YTHDC1-m6A condensates (nYACs) play an essential role in acute myeloid leukaemia (AML) cells [Citation135]. nYACs enable YTHDC1 to protect m6A-modification, maintain mRNA stability, control cancer cell survival, and regulate differentiation. YBX2, a reader protein of m5C, also forms condensates with m5C containing RNA both in vitro and in cells. However, the functions of these condensates warrant further investigation [Citation136].
A recent work reported that Quaking proteins (QKIs) could bind to m7G modification of mRNA and transport them to SGs by interacting with G3BP1, thereby regulating mRNA stability and translation under stress conditions. These examples highlight how RNA modifications play significant roles in condensation-mediated translational regulation and inspire us to further explore the functions of RNA modifications and the underlying mechanisms.
Moreover, evaluating whether a biomolecular condensate’s role in translational regulation is necessary or merely concomitant is critical. Phase separation is a prevalent phenomenon involved in many biological processes. A recent study suggests a more general principle that condensation is primarily based on electrostatic complementarity, which occurs widely in a heterogeneous cellular environment between nucleic acid and protein components [Citation137]. This encourages us to put more effort into considering the actual contribution of condensates to physiological functions. A more effective way to answer this question might be to induce a mutation that only affects the protein’s propensity to form condensates without affecting its other functions, following the examination of the in vivo phenotype of the mutant. The study that FXR1 condensates activate translation in late spermatozoa is a good example of this approach. Researchers identified a mutant, FXR1L351P, which shows a deficiency in condensate formation yet maintains its RNA binding ability. The germline-specific FXR1L351P knock-in mouse exhibits defects in spermatozoa development and male sterility, strongly supporting that the propensity of FXR1 to form condensates is involved in the translational activation in late spermatozoa and is indispensable for mammalian male fertility. Of note, mounting evidence suggests that structured domains, such as the KH2 domain of FXR1, contribute significantly to the condensation propensity of the entire protein, indicating that this propensity extends beyond the IDR of a protein. This observation underscores the need for more precise prediction tools for condensation, including the utilization of cutting-edge technologies such as Alpha-fold and artificial intelligence technology (AIT) to develop sophisticated condensation prediction tools, grounded in accumulated experimental knowledge.
Furthermore, it holds promise to utilize mechanisms of translational regulation mediated by condensation for drug design. Translation is a fundamental biological process crucial for cell survival and function. Growing evidence shows that the dysregulation of biomolecular condensates involved in translation is associated with diseases, including neurodegeneration, cancer, and viral infections – providing numerous potential therapeutic targets for translation-dysregulation-related diseases. Moreover, a recent study reported that antineoplastic drugs, such as cisplatin and tamoxifen, can be concentrated in specific protein condensates in vitro and in tumour cells [Citation138]. Based on these findings, researchers have proposed novel approaches for new drug design, which include uncovering new drugs via condensates assay that perturb condensate formation, augmenting drug efficacy by incorporating them into condensates for heightened concentration, tailoring drugs based on the physicochemical properties of cellular condensates, and leveraging existing knowledge of drug mechanisms for condensate-based drug design [Citation13,Citation139]. Integrating these drug design strategies with the emerging mechanisms of condensation-mediated translational regulation could facilitate the discovery of more potential therapeutic targets and new drugs.
Author contributions
YH and ML conceived and designed the manuscript. YZ and JK performed the literature search. YZ wrote the manuscript. ML critically reviewed the manuscript. YH provided overall supervision and prepared the final draft.
Declaration
This research did not involve the use of any animal or human data or tissue.
Acknowledgments
We would like to thank all the lab members for stimulating discussions for this manuscript. We sincerely apologize to the authors whose work we could not cite or discuss. We would like to express our gratitude to Adam Weiss for extending us the invitation to write this review.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.
Additional information
Funding
References
- Banani SF, Lee HO, Hyman AA, et al. Biomolecular condensates: organizers of cellular biochemistry. Nat Rev Mol Cell Biol. 2017;18(5):285–298. doi: 10.1038/nrm.2017.7
- Alberti S, Dormann D. Liquid–Liquid Phase Separation in Disease. Ann Rev Genet. 2019;53(1):171–194. doi: 10.1146/annurev-genet-112618-043527
- Boeynaems S, Alberti S, Fawzi NL, et al. Protein phase separation: a new phase in Cell Biology. Trends Cell Biol. 2018;28(6):420–435. doi: 10.1016/j.tcb.2018.02.004
- Hyman AA, Weber CA, Julicher F. Liquid-liquid phase separation in biology. Annu Rev Cell Dev Biol. 2014;30(1):39–58. doi: 10.1146/annurev-cellbio-100913-013325
- Alberti S, Hyman AA. Biomolecular condensates at the nexus of cellular stress, protein aggregation disease and ageing. Nat Rev Mol Cell Biol. 2021;22(3):196–213. doi: 10.1038/s41580-020-00326-6
- Banani SF, Rice AM, Peeples WB, et al. Compositional control of phase-separated cellular bodies. Cell. 2016;166(3):651–663. doi: 10.1016/j.cell.2016.06.010
- Patel A, Lee HO, Jawerth L, et al. A liquid-to-solid phase transition of the ALS protein FUS accelerated by disease mutation. Cell. 2015;162(5):1066–1077. doi: 10.1016/j.cell.2015.07.047
- Nott TJ, Petsalaki E, Farber P, et al. Phase transition of a disordered nuage protein generates environmentally responsive membraneless organelles. Mol Cell. 2015;57(5):936–947. doi: 10.1016/j.molcel.2015.01.013
- Martin EW, Mittag T. Relationship of sequence and phase separation in protein low-complexity regions. Biochemistry. 2018;57(17):2478–2487. doi: 10.1021/acs.biochem.8b00008
- Molliex A, Temirov J, Lee J, et al. Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell. 2015;163(1):123–133. doi: 10.1016/j.cell.2015.09.015
- Roden C, Gladfelter AS. RNA contributions to the form and function of biomolecular condensates. Nat Rev Mol Cell Biol. 2021;22(3):183–195. doi: 10.1038/s41580-020-0264-6
- Bienz M. Head-to-tail polymerization in the assembly of biomolecular condensates. Cell. 2020;182(4):799–811. doi: 10.1016/j.cell.2020.07.037
- Boija A, Klein IA, Young RA. Biomolecular Condensates and Cancer. Cancer Cell. 2021;39(2):174–192. doi: 10.1016/j.ccell.2020.12.003
- Thomas L, Putnam A, Folkmann A. Germ granules in development. Development. 2023;150(2). doi: 10.1242/dev.201037
- Formicola N, Vijayakumar J, Besse F. Neuronal ribonucleoprotein granules: Dynamic sensors of localized signals. Traffic. 2019;20(9):639–649. doi: 10.1111/tra.12672
- Spriggs KA, Bushell M, Willis AE. Translational regulation of gene expression during conditions of cell stress. Mol Cell. 2010;40(2):228–237. doi: 10.1016/j.molcel.2010.09.028
- Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell. 2009;136(4):731–745. doi: 10.1016/j.cell.2009.01.042
- Protter DSW, Parker R. Principles and properties of stress granules. Trends Cell Biol. 2016;26(9):668–679. doi: 10.1016/j.tcb.2016.05.004
- Standart N, Weil D. P-Bodies: cytosolic droplets for coordinated mRNA storage. Trends Genet. 2018;34(8):612–626. doi: 10.1016/j.tig.2018.05.005
- Shin Y, Brangwynne CP. Liquid phase condensation in cell physiology and disease. Science. 2017;357(6357). doi: 10.1126/science.aaf4382
- Shorter J. Phase separation of RNA-binding proteins in physiology and disease: an introduction to the JBC reviews thematic series. J Biol Chem. 2019;294(18):7113–7114. doi: 10.1074/jbc.REV119.007944
- Snead WT, Gladfelter AS. The control centers of biomolecular phase separation: how membrane surfaces, PTMs, and active processes regulate condensation. Mol Cell. 2019;76(2):295–305. doi: 10.1016/j.molcel.2019.09.016
- Parker DM, Winkenbach LP, Osborne Nishimura E. It’s just a phase: exploring the relationship between mRNA, biomolecular condensates, and translational control. Front Genet. 2022;13:931220. doi: 10.3389/fgene.2022.931220
- Sassone-Corsi P. Unique chromatin remodeling and transcriptional regulation in spermatogenesis. Science. 2002;296(5576):2176–2178. doi: 10.1126/science.1070963
- Kang JY, Wen Z, Pan D, et al. LLPS of FXR1 drives spermiogenesis by activating translation of stored mRnas. Science. 2022;377(6607):eabj6647. doi: 10.1126/science.abj6647
- Dai P, Wang X, Gou LT, et al. A translation-activating function of MIWI/piRNA during mouse spermiogenesis. Cell. 2019;179(7):1566–1581 e16. doi: 10.1016/j.cell.2019.11.022
- Majumder M, Johnson RH, Palanisamy V. Fragile X-related protein family: a double-edged sword in neurodevelopmental disorders and cancer. Crit Rev Biochem Mol Biol. 2020;55(5):409–424. doi: 10.1080/10409238.2020.1810621
- Truesdell SS, Mortensen RD, Seo M, et al. MicroRNA-mediated mRNA translation activation in quiescent cells and oocytes involves recruitment of a nuclear microRNP. Sci Rep. 2012;2(1):842. doi: 10.1038/srep00842
- Mortensen RD, Serra M, Steitz JA, et al. Posttranscriptional activation of gene expression in xenopus laevis oocytes by microRNA-protein complexes (microRnps). Proc Natl Acad Sci U S A. 2011;108(20):8281–8286. doi: 10.1073/pnas.1105401108
- Gessert S, Bugner V, Tecza A, et al. FMR1/FXR1 and the miRNA pathway are required for eye and neural crest development. Dev Biol. 2010;341(1):222–235. doi: 10.1016/j.ydbio.2010.02.031
- Khandjian EW, Bardoni B, Corbin F, et al. Novel isoforms of the fragile X related protein FXR1P are expressed during myogenesis. Hum Mol Genet. 1998;7(13):2121–2128. doi: 10.1093/hmg/7.13.2121
- Davidovic L, Durand N, Khalfallah O, et al. A novel role for the RNA-binding protein FXR1P in myoblasts cell-cycle progression by modulating p21/Cdkn1a/Cip1/Waf1 mRNA stability. PLoS Genet. 2013;9(3):e1003367. doi: 10.1371/journal.pgen.1003367
- Patzlaff NE, Nemec KM, Malone SG, et al. Fragile X related protein 1 (FXR1P) regulates proliferation of adult neural stem cells. Hum Mol Genet. 2017;26(7):1340–1352. doi: 10.1093/hmg/ddx034
- Kotaja N, Sassone-Corsi P. The chromatoid body: a germ-cell-specific RNA-processing centre. Nat Rev Mol Cell Biol. 2007;8(1):85–90. doi: 10.1038/nrm2081
- Dodson AE, Kennedy S. Phase separation in germ cells and development. Dev Cell. 2020;55(1):4–17. doi: 10.1016/j.devcel.2020.09.004
- Alberti S, Gladfelter A, Mittag T. Considerations and Challenges in Studying Liquid-Liquid Phase Separation and Biomolecular Condensates. Cell. 2019;176(3):419–434. doi: 10.1016/j.cell.2018.12.035
- Vasudevan S, Steitz JA. AU-rich-element-mediated upregulation of translation by FXR1 and Argonaute 2. Cell. 2007;128(6):1105–1118. doi: 10.1016/j.cell.2007.01.038
- Greenblatt EJ, Spradling AC. Fragile X mental retardation 1 gene enhances the translation of large autism-related proteins. Science. 2018;361(6403):709–712. doi: 10.1126/science.aas9963
- Tsang B, Arsenault J, Vernon RM, et al. Phosphoregulated FMRP phase separation models activity-dependent translation through bidirectional control of mRNA granule formation. Proc Natl Acad Sci U S A. 2019;116(10):4218–4227. doi: 10.1073/pnas.1814385116
- Kim TH, Tsang B, Vernon RM, et al. Phospho-dependent phase separation of FMRP and CAPRIN1 recapitulates regulation of translation and deadenylation. Science. 2019;365(6455):825–829. doi: 10.1126/science.aax4240
- Krichevsky AM, Kosik KS. Neuronal RNA granules: a link between RNA localization and stimulation-dependent translation. Neuron. 2001;32(4):683–696. doi: 10.1016/S0896-6273(01)00508-6
- Jung H, Gkogkas CG, Sonenberg N, et al. Remote control of gene function by local translation. Cell. 2014;157(1):26–40. doi: 10.1016/j.cell.2014.03.005
- Buxbaum AR, Wu B, Singer RH. Single beta-actin mRNA detection in neurons reveals a mechanism for regulating its translatability. Science. 2014;343(6169):419–422. doi: 10.1126/science.1242939
- Santoro MR, Bray SM, Warren ST. Molecular mechanisms of fragile X syndrome: a twenty-year perspective. Annu Rev Pathol. 2012;7(1):219–245. doi: 10.1146/annurev-pathol-011811-132457
- El Fatimy R, Davidovic L, Tremblay S, et al. Tracking the fragile X mental retardation protein in a highly ordered neuronal RiboNucleoParticles population: a link between stalled polyribosomes and RNA granules. PLoS Genet. 2016;12(7):e1006192. doi: 10.1371/journal.pgen.1006192
- Zhou LT, Ye SH, Yang HX, et al. A novel role of fragile X mental retardation protein in pre-mRNA alternative splicing through RNA-binding protein 14. Neuroscience. 2017;349:64–75. doi: 10.1016/j.neuroscience.2017.02.044
- Kao DI, Aldridge GM, Weiler IJ, et al. Altered mRNA transport, docking, and protein translation in neurons lacking fragile X mental retardation protein. Proc Natl Acad Sci U S A. 2010;107(35):15601–15606. doi: 10.1073/pnas.1010564107
- Kiebler MA, Bassell GJ. Neuronal RNA granules: movers and makers. Neuron. 2006;51(6):685–690. doi: 10.1016/j.neuron.2006.08.021
- Costa-Mattioli M, Sossin WS, Klann E, et al. Translational control of long-lasting synaptic plasticity and memory. Neuron. 2009;61(1):10–26. doi: 10.1016/j.neuron.2008.10.055
- De Rubeis S, Bagni C. Fragile X mental retardation protein control of neuronal mRNA metabolism: Insights into mRNA stability. Mol Cell Neurosci. 2010;43(1):43–50. doi: 10.1016/j.mcn.2009.09.013
- Darnell JC, Van Driesche SJ, Zhang C, et al. FMRP stalls ribosomal translocation on mRnas linked to synaptic function and autism. Cell. 2011;146(2):247–261. doi: 10.1016/j.cell.2011.06.013
- Ceman S, O’Donnell WT, Reed M, et al. Phosphorylation influences the translation state of FMRP-associated polyribosomes. Hum Mol Genet. 2003;12(24):3295–3305. doi: 10.1093/hmg/ddg350
- Narayanan U, Nalavadi V, Nakamoto M, et al. FMRP phosphorylation reveals an immediate-early signaling pathway triggered by group I mGlur and mediated by PP2A. J Neurosci. 2007;27(52):14349–14357. doi: 10.1523/JNEUROSCI.2969-07.2007
- Richter JD, Bassell GJ, Klann E. Dysregulation and restoration of translational homeostasis in fragile X syndrome. Nat Rev Neurosci. 2015;16(10):595–605. doi: 10.1038/nrn4001
- Narayanan U, Nalavadi V, Nakamoto M, et al. S6K1 phosphorylates and regulates fragile X mental retardation protein (FMRP) with the neuronal protein synthesis-dependent mammalian target of rapamycin (mTOR) signaling cascade. J Biol Chem. 2008;283(27):18478–18482. doi: 10.1074/jbc.C800055200
- Bartley CM, O’Keefe RA, Blice-Baum A, et al. Mammalian FMRP S499 is phosphorylated by CK2 and promotes secondary phosphorylation of FMRP. eNeuro. 2016;3(6):ENEURO.0092–16.2016. doi: 10.1523/ENEURO.0092-16.2016
- Nakayama K, Ohashi R, Shinoda Y, et al. RNG105/caprin1, an RNA granule protein for dendritic mRNA localization, is essential for long-term memory formation. Elife. 2017;6:6. doi: 10.7554/eLife.29677
- Solomon S, Xu Y, Wang B, et al. Distinct structural features of caprin-1 mediate its interaction with G3BP-1 and its induction of phosphorylation of eukaryotic translation initiation factor 2alpha, entry to cytoplasmic stress granules, and selective interaction with a subset of mRnas. Mol Cell Biol. 2007;27(6):2324–2342. doi: 10.1128/MCB.02300-06
- Hornbeck PV, Zhang B, Murray B, et al. PhosphoSitePlus, 2014: mutations, PTMs and recalibrations. Nucleic Acids Res. 2015;43(Database issue):D512–20. doi: 10.1093/nar/gku1267
- Siomi MC, Higashijima K, Ishizuka A, et al. Casein kinase II phosphorylates the fragile X mental retardation protein and modulates its biological properties. Mol Cell Biol. 2002;22(24):8438–8447. doi: 10.1128/MCB.22.24.8438-8447.2002
- Panas MD, Ivanov P, Anderson P. Mechanistic insights into mammalian stress granule dynamics. J Cell Bio. 2016;215(3):313–323. doi: 10.1083/jcb.201609081
- Anderson P, Kedersha N. Stress granules. Curr Biol. 2009;19(10):R397–8. doi: 10.1016/j.cub.2009.03.013
- Buchan JR, Parker R. Eukaryotic stress granules: the ins and outs of translation. Mol Cell. 2009;36(6):932–941. doi: 10.1016/j.molcel.2009.11.020
- Kedersha N, Ivanov P, Anderson P. Stress granules and cell signaling: more than just a passing phase? Trends Biochem Sci. 2013;38(10):494–506. doi: 10.1016/j.tibs.2013.07.004
- Kedersha N, Stoecklin G, Ayodele M, et al. Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J Cell Bio. 2005;169(6):871–884. doi: 10.1083/jcb.200502088
- Hilliker A, Gao Z, Jankowsky E, et al. The DEAD-box protein Ded1 modulates translation by the formation and resolution of an eIF4F-mRNA complex. Mol Cell. 2011;43(6):962–972. doi: 10.1016/j.molcel.2011.08.008
- Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–293. doi: 10.1016/j.cell.2012.03.017
- Arimoto K, Fukuda H, Imajoh-Ohmi S, et al. Formation of stress granules inhibits apoptosis by suppressing stress-responsive MAPK pathways. Nat Cell Biol. 2008;10(11):1324–1332. doi: 10.1038/ncb1791
- Pietras P, Aulas A, Fay MM, et al. Translation inhibition and suppression of stress granules formation by cisplatin. Biomed Pharmacother. 2022;145:112382. doi: 10.1016/j.biopha.2021.112382
- Yang P, Mathieu C, Kolaitis RM, et al. G3BP1 is a Tunable switch that triggers phase separation to assemble stress granules. Cell. 2020;181(2):325–345 e28. doi: 10.1016/j.cell.2020.03.046
- Sanders DW, Kedersha N, Lee DSW, et al. Competing protein-RNA interaction networks control multiphase intracellular organization. Cell. 2020;181(2):306–324 e28. doi: 10.1016/j.cell.2020.03.050
- Guillen-Boixet J, Kopach A, Holehouse AS, et al. RNA-Induced conformational switching and clustering of G3BP drive stress granule assembly by condensation. Cell. 2020;181(2):346–361 e17. doi: 10.1016/j.cell.2020.03.049
- Cirillo L, Cieren A, Barbieri S, et al. UBAP2L forms distinct cores that act in nucleating stress granules upstream of G3BP1. Curr Biol. 2020;30(4):698–707 e6. doi: 10.1016/j.cub.2019.12.020
- Arimoto-Matsuzaki K, Saito H, Takekawa M. TIA1 oxidation inhibits stress granule assembly and sensitizes cells to stress-induced apoptosis. Nat Commun. 2016;7(1):10252. doi: 10.1038/ncomms10252
- Kedersha N, Chen S, Gilks N, et al. Evidence that ternary complex (eIF2-GTP-tRNA(i)(Met))-deficient preinitiation complexes are core constituents of mammalian stress granules. Mol Biol Cell. 2002;13(1):195–210. doi: 10.1091/mbc.01-05-0221
- Guenther UP, Weinberg DE, Zubradt MM, et al. The helicase Ded1p controls use of near-cognate translation initiation codons in 5’ UTRs. Nature. 2018;559(7712):130–134. doi: 10.1038/s41586-018-0258-0
- Sen ND, Zhou F, Harris MS, et al. eIF4B stimulates translation of long mRnas with structured 5’ UTRs and low closed-loop potential but weak dependence on eIF4G. Proc Natl Acad Sci U S A. 2016;113(38):10464–10472. doi: 10.1073/pnas.1612398113
- Iserman C, Desroches Altamirano C, Jegers C, et al. Condensation of Ded1p promotes a translational switch from housekeeping to stress protein production. Cell. 2020;181(4):818–831 e19. doi: 10.1016/j.cell.2020.04.009
- Mateju D, Eichenberger B, Voigt F, et al. Single-molecule imaging reveals translation of mRnas localized to stress granules. Cell. 2020;183(7):1801–1812 e13. doi: 10.1016/j.cell.2020.11.010
- Jain S, Wheeler JR, Walters RW, et al. ATPase-Modulated Stress Granules Contain a Diverse Proteome and Substructure. Cell. 2016;164(3):487–498. doi: 10.1016/j.cell.2015.12.038
- Ramaswami M, Taylor JP, Parker R. Altered ribostasis: RNA-protein granules in degenerative disorders. Cell. 2013;154(4):727–736. doi: 10.1016/j.cell.2013.07.038
- Wolozin B, Ivanov P. Stress granules and neurodegeneration. Nat Rev Neurosci. 2019;20(11):649–666. doi: 10.1038/s41583-019-0222-5
- Moore MJ. From birth to death: the complex lives of eukaryotic mRnas. Science. 2005;309(5740):1514–1518. doi: 10.1126/science.1111443
- Dormann D, Haass C. TDP-43 and FUS: a nuclear affair. Trends Neurosci. 2011;34(7):339–348. doi: 10.1016/j.tins.2011.05.002
- Mann JR, Gleixner AM, Mauna JC, et al. RNA binding antagonizes neurotoxic phase transitions of TDP-43. Neuron. 2019;102(2):321–338 e8. doi: 10.1016/j.neuron.2019.01.048
- Vance C, Rogelj B, Hortobagyi T, et al. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science. 2009;323(5918):1208–1211. doi: 10.1126/science.1165942
- Kwiatkowski TJ Jr., Bosco DA, Leclerc AL, et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009;323(5918):1205–1208. doi: 10.1126/science.1166066
- Kim HJ, Kim NC, Wang YD, et al. Mutations in prion-like domains in hnRNPA2B1 and hnRNPA1 cause multisystem proteinopathy and ALS. Nature. 2013;495(7442):467–473. doi: 10.1038/nature11922
- Kamelgarn M, Chen J, Kuang L, et al. ALS mutations of FUS suppress protein translation and disrupt the regulation of nonsense-mediated decay. Proc Natl Acad Sci U S A. 2018;115(51):E11904–E11913. doi: 10.1073/pnas.1810413115
- Birsa N, Ule AM, Garone MG, et al. FUS-ALS mutants alter FMRP phase separation equilibrium and impair protein translation. Sci Adv. 2021;7(30). doi: 10.1126/sciadv.abf8660
- Gui X, Luo F, Li Y, et al. Structural basis for reversible amyloids of hnRNPA1 elucidates their role in stress granule assembly. Nat Commun. 2019;10(1):2006. doi: 10.1038/s41467-019-09902-7
- Cui Q, Bi H, Lv Z, et al. Diverse CMT2 neuropathies are linked to aberrant G3BP interactions in stress granules. Cell. 2023;186(4):803–820 e25. doi: 10.1016/j.cell.2022.12.046
- Smeyers J, Banchi EG, Latouche M. C9ORF72: what it is, what it does, and why it matters. Front Cell Neurosci. 2021;15:661447. doi: 10.3389/fncel.2021.661447
- Lee KH, Zhang P, Kim HJ, et al. C9orf72 dipeptide repeats impair the assembly, dynamics, and function of membrane-less organelles. Cell. 2016;167(3):774–788 e17. doi: 10.1016/j.cell.2016.10.002
- Boeynaems S, Bogaert E, Kovacs D, et al. Phase separation of C9orf72 dipeptide repeats perturbs stress granule dynamics. Mol Cell. 2017;65(6):1044–1055 e5. doi: 10.1016/j.molcel.2017.02.013
- Quesnel-Vallieres M, Weatheritt RJ, Cordes SP, et al. Autism spectrum disorder: insights into convergent mechanisms from transcriptomics. Nat Rev Genet. 2019;20(1):51–63. doi: 10.1038/s41576-018-0066-2
- Dergai M, Tsyba L, Dergai O, et al. Microexon-based regulation of ITSN1 and src SH3 domains specificity relies on introduction of charged amino acids into the interaction interface. Biochem Biophys Res Commun. 2010;399(2):307–312. doi: 10.1016/j.bbrc.2010.07.080
- Quesnel-Vallieres M, Dargaei Z, Irimia M, et al. Misregulation of an activity-dependent splicing network as a common mechanism underlying autism spectrum disorders. Mol Cell. 2016;64(6):1023–1034. doi: 10.1016/j.molcel.2016.11.033
- Irimia M, Weatheritt RJ, Ellis JD, et al. A highly conserved program of neuronal microexons is misregulated in autistic brains. Cell. 2014;159(7):1511–1523. doi: 10.1016/j.cell.2014.11.035
- Borrie SC, Brems H, Legius E, et al. Cognitive dysfunctions in intellectual disabilities: the contributions of the ras-MAPK and PI3K-AKT-mTOR pathways. Annu Rev Genomics Hum Genet. 2017;18(1):115–142. doi: 10.1146/annurev-genom-091416-035332
- Gonatopoulos-Pournatzis T, Niibori R, Salter EW, et al. Autism-misregulated eIF4G microexons control synaptic translation and higher order cognitive functions. Mol Cell. 2020;77(6):1176–1192 e16. doi: 10.1016/j.molcel.2020.01.006
- Kim HJ, Raphael AR, LaDow ES, et al. Therapeutic modulation of eIf2alpha phosphorylation rescues TDP-43 toxicity in amyotrophic lateral sclerosis disease models. Nat Genet. 2014;46(2):152–160. doi: 10.1038/ng.2853
- Reineke LC, Lloyd RE. Diversion of stress granules and P-bodies during viral infection. Virology. 2013;436(2):255–267. doi: 10.1016/j.virol.2012.11.017
- Emara MM, Brinton MA. Interaction of TIA-1/TIAR with West Nile and dengue virus products in infected cells interferes with stress granule formation and processing body assembly. Proc Natl Acad Sci U S A. 2007;104(21):9041–9046. doi: 10.1073/pnas.0703348104
- White JP, Cardenas AM, Marissen WE, et al. Inhibition of cytoplasmic mRNA stress granule formation by a viral proteinase. Cell Host Microbe. 2007;2(5):295–305. doi: 10.1016/j.chom.2007.08.006
- Corbet GA, Parker R. RNP Granule Formation: Lessons from P-Bodies and Stress Granules. Cold Spring Harb Symp Quant Biol. 2019;84:203–215. doi: 10.1101/sqb.2019.84.040329
- Ivanov P, Kedersha N, Anderson P. Stress granules and processing bodies in translational control. Cold Spring Harb Perspect Biol. 2019;11(5):a032813. doi: 10.1101/cshperspect.a032813
- Zhang B, Herman PK. It is all about the process(ing): P-body granules and the regulation of signal transduction. Curr Genet. 2020;66(1):73–77. doi: 10.1007/s00294-019-01016-3
- Parker R, Sheth U. P bodies and the control of mRNA translation and degradation. Mol Cell. 2007;25(5):635–646. doi: 10.1016/j.molcel.2007.02.011
- Decker CJ, Parker R. P-bodies and stress granules: possible roles in the control of translation and mRNA degradation. Cold Spring Harb Perspect Biol. 2012;4(9):a012286. doi: 10.1101/cshperspect.a012286
- Luo Y, Na Z, Slavoff SA. P-Bodies: composition, properties, and functions. Biochemistry. 2018;57(17):2424–2431. doi: 10.1021/acs.biochem.7b01162
- Nishimura T, Fakim H, Brandmann T, et al. Human MARF1 is an endoribonuclease that interacts with the DCP1: 2 decapping complex and degrades target mRnas. Nucleic Acids Res. 2018;46(22):12008–12021. doi: 10.1093/nar/gky1011
- Brothers WR, Fakim H, Kajjo S, et al. P-bodies directly regulate MARF1-mediated mRNA decay in human cells. Nucleic Acids Res. 2022;50(13):7623–7636. doi: 10.1093/nar/gkac557
- Zhu L, Kandasamy SK, Liao SE, et al. LOTUS domain protein MARF1 binds CCR4-NOT deadenylase complex to post-transcriptionally regulate gene expression in oocytes. Nat Commun. 2018;9(1):4031. doi: 10.1038/s41467-018-06404-w
- Su YQ, Sugiura K, Sun F, et al. MARF1 regulates essential oogenic processes in mice. Science. 2012;335(6075):1496–1499. doi: 10.1126/science.1214680
- Kanemitsu Y, Fujitani M, Fujita Y, et al. The RNA-binding protein MARF1 promotes cortical neurogenesis through its RNase activity domain. Sci Rep. 2017;7(1):1155. doi: 10.1038/s41598-017-01317-y
- Su YQ, Sun F, Handel MA, et al. Meiosis arrest female 1 (MARF1) has nuage-like function in mammalian oocytes. Proc Natl Acad Sci U S A. 2012;109(46):18653–18660. doi: 10.1073/pnas.1216904109
- Yao Q, Cao G, Li M, et al. Ribonuclease activity of MARF1 controls oocyte RNA homeostasis and genome integrity in mice. Proc Natl Acad Sci U S A. 2018;115(44):11250–11255. doi: 10.1073/pnas.1809744115
- Ayache J, Benard M, Ernoult-Lange M, et al. P-body assembly requires DDX6 repression complexes rather than decay or Ataxin2/2L complexes. Mol Biol Cell. 2015;26(14):2579–2595. doi: 10.1091/mbc.E15-03-0136
- Hubstenberger A, Courel M, Benard M, et al. P-Body purification reveals the condensation of repressed mRNA regulons. Mol Cell. 2017;68(1):144–157 e5. doi: 10.1016/j.molcel.2017.09.003
- Cary GA, Vinh DB, May P, et al. Proteomic analysis of Dhh1 complexes reveals a role for Hsp40 chaperone Ydj1 in yeast P-Body assembly. G3 (Bethesda). 2015;5(11):2497–2511. doi: 10.1534/g3.115.021444
- Jonas S, Izaurralde E. The role of disordered protein regions in the assembly of decapping complexes and RNP granules. Genes Dev. 2013;27(24):2628–2641. doi: 10.1101/gad.227843.113
- Andrei MA, Ingelfinger D, Heintzmann R, et al. A role for eIF4E and eIF4E-transporter in targeting mRnps to mammalian processing bodies. RNA. 2005;11(5):717–727. doi: 10.1261/rna.2340405
- Kroschwald S, Maharana S, Mateju D, et al. Promiscuous interactions and protein disaggregases determine the material state of stress-inducible RNP granules. Elife. 2015;4:e06807. doi: 10.7554/eLife.06807
- Pitchiaya S, Mourao MDA, Jalihal AP, et al. Dynamic recruitment of single RNAs to processing bodies depends on RNA functionality. Mol Cell. 2019;74(3):521–533 e6. doi: 10.1016/j.molcel.2019.03.001
- Cheng S, Altmeppen G, So C, et al. Mammalian oocytes store mRNAs in a mitochondria-associated membraneless compartment. Science. 2022;378(6617):eabq4835. doi: 10.1126/science.abq4835
- Jukam D, Shariati SAM, Skotheim JM. Zygotic genome activation in vertebrates. Dev Cell. 2017;42(4):316–332. doi: 10.1016/j.devcel.2017.07.026
- Li L, Zheng P, Dean J. Maternal control of early mouse development. Development. 2010;137(6):859–870. doi: 10.1242/dev.039487
- Shi B, Heng J, Zhou JY, et al. Phase separation of Ddx3xb helicase regulates maternal-to-zygotic transition in zebrafish. Cell Res. 2022;32(8):715–728. doi: 10.1038/s41422-022-00655-5
- Ma W, Mayr C. A Membraneless Organelle Associated with the Endoplasmic Reticulum Enables 3‘UTR-Mediated Protein-Protein Interactions. Cell. 2018;175(6):1492–1506 e19. doi: 10.1016/j.cell.2018.10.007
- Ma W, Zhen G, Xie W, et al. In vivo reconstitution finds multivalent RNA–RNA interactions as drivers of mesh-like condensates. Elife. 2021;10:10. doi: 10.7554/eLife.64252
- Ries RJ, Zaccara S, Klein P, et al. m(6)A enhances the phase separation potential of mRNA. Nature. 2019;571(7765):424–428. doi: 10.1038/s41586-019-1374-1
- Gao Y, Pei G, Li D, et al. Multivalent m(6)A motifs promote phase separation of YTHDF proteins. Cell Res. 2019;29(9):767–769. doi: 10.1038/s41422-019-0210-3
- Fu Y, Zhuang X. m(6)A-binding YTHDF proteins promote stress granule formation. Nat Chem Biol. 2020;16(9):955–963. doi: 10.1038/s41589-020-0524-y
- Cheng Y, Xie W, Pickering BF, et al. N(6)-methyladenosine on mRNA facilitates a phase-separated nuclear body that suppresses myeloid leukemic differentiation. Cancer Cell. 2021;39(7):958–972 e8. doi: 10.1016/j.ccell.2021.04.017
- Zhao Z, Qing Y, Dong L, et al. QKI shuttles internal m(7)G-modified transcripts into stress granules and modulates mRNA metabolism. Cell. 2023;186(15):3208–3226 e27. doi: 10.1016/j.cell.2023.05.047
- Dutagaci B, Nawrocki G, Goodluck J, et al. Charge-driven condensation of RNA and proteins suggests broad role of phase separation in cytoplasmic environments. Elife. 2021;10:10. doi: 10.7554/eLife.64004
- Klein IA, Boija A, Afeyan LK, et al. Partitioning of cancer therapeutics in nuclear condensates. Science. 2020;368(6497):1386–1392. doi: 10.1126/science.aaz4427
- Mitrea DM, Mittasch M, Gomes BF, et al. Modulating biomolecular condensates: a novel approach to drug discovery. Nat Rev Drug Discov. 2022;21(11):841–862. doi: 10.1038/s41573-022-00505-4
