Abstract
The present paper represents a comprehensive up-to-date review of β -glucans, their chemical and biological properties, and their role in immunological reactions. β -D-Glucans belong to a group of physiologically active compounds called biological response modifiers and represent highly conserved structural components of cell walls in yeast, fungi, or seaweed. Despite almost 150 years of research, the exact mechanisms of their action remain unclear. The present review starts with the history of glucans. Next, attention is focused on sources and structure, comparing the effects of physicochemical properties, and sources on biological effects. As glucans belong to natural products useful in preventing various diseases, they have been highly sought after throughout human history. Based on extensive recent research, this paper explains the various mechanisms of effects and the ways glucans mediate their effects on defense reactions against infections. Despite the fact that predominately pharmacological effects of glucans are positive, their unfavorable and potentially toxic side effects were not overlooked. In addition, attention was focused on the future research, possible alternatives such as synthetic oligosaccharides, and on clinical applications.
1. INTRODUCTION
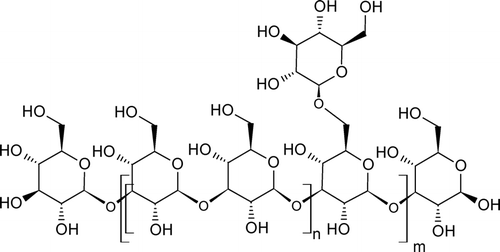
Generally, β -glucan is a chemical name of a polymer of β -glucose. There are more of such polymers, differing in glycosidic bond position. Cellulose, (1 → 4)-β -d-glucan, is an example. The following data refer to homopolymers of glucose having a linear molecule with (1 → 3)-β -d-glycosidic linkages or a branched one, with side chains bound by (1 → 6)-β -d-glycosidic linkages. Although chemically heterogeneous, these polysaccharides are usually termed by common name “β -glucans.” It is necessary to take into account that these compounds, which otherwise caused similar or nearly identical immune reactions in macroorganisms, can differ in origin as well as in their primary, secondary, or tertiary structures or their solubility in water or alkalies. Due to these factors, numerous discrepancies can be found in the literature. Some sources of β -glucans are listed in .
TABLE 1 Some β -glucans with immunomodulatory effects
β − Glucans show notable physiological effects; this is their most important quality and the reason why so much attention has been devoted to them. They belong to a group of physiologically active compounds, collectively termed biological response modifiers (BRM). According to their effects, BRM can be classified into two groups – cytokines, responsible for communication between immune system cells and regulation of the system, and immuno-modulators. The immunomodulators are able to positively (i.e., immunopotentiation) or negatively (i.e., immunosuppression) manipulate the immune system. Different known immunomodulators can be classified into three groups (Werner, Citation1987): 1) Intact microbes (e.g., Bacille Calmette-Guérin) and components of microbial cells (e.g., endotoxin of Gram-negative microorganisms (LPS), muramyldipeptide (MDP), fungal polysaccharides (zymosan, β -glucans), polynucleotides, bestatine); 2) Natural components of a normal immune system (e.g., thymic hormones, lymphokines, monokines); and, 3) Synthetic compounds (e.g., levamisole, isoprinosine, diethyldithiocarbamate).
Thus far, among many known and tested immunomodulators of the first group, polysaccharides isolated from different microorganisms and plants are the most important. A large number of such polysaccharides, which act only as immunpotentiators, is known (Whistler et al., Citation1976). As mentioned earlier, β -glucans belong to this group.
2. HISTORY
At the beginning of the 18th Century, it was already known that certain infectious diseases showed a therapeutic effect on malignant processes. Purposeful use of such therapy dates approximately from the middle of the 19th Century, when Bush (Bush, 1850) performed experiments in curing sarcoma by infecting patients with erysipelas, i.e., with β -hemolytic streptococci of the Group A. These therapeutical procedures were repeated toward the end of the 19th Century by Coley (Coley, Citation1894), who applied less dangerous extracts of microbial cultures, in this case from cultures of Bacillus prodigiosus (now Serratia marcescens). This extract was at these times known as “Coley's toxin,” and was lately determined as interleukin IL-12 (Tsung and Norton, Citation2006).
However, Coley does not have priority in this regard. At the time, it was already known that certain components of microorganisms caused in macroorganisms, especially mammalian ones, ferocious reactions comparable to pathophysiological conditions during infection by intact microbes. It is likely that the first investigated substance with these properties was LPS of Gram-negative bacteria (e.g., Escherichia, Salmonella, Shigella, Pseudomonas, Neisseria, and Haemophilus spp.); a paper describing the endotoxin was published in 1865 (Billroth, Citation1865). In both humans and experimental animals, LPS induces increased phagocytosis with a potential protective effect for host. However, its toxic effects (such as fever, diarrhea, hypotensive shock, intravascular coagulation, multiple organ dysfunctions) completely predominate. A toxic principle of LPS is its lipid moiety, while the saccharidic moiety, with prevailing glucose, galactose and mannose content (Nowotny, Citation1969), is non-toxic and bears immunomodulating activity as well. It is apparent that even polysaccharides themselves can act as immunomodulators, while their toxicity is negligible.
Proper history of polysaccharides as immunomodulators goes back to the 1940s when Shear and coworkers (Shear et al., Citation1943) described a substance, again from Serratia marcescens cultures, that caused necrosis of tumors. Subsequently, this substance (so-called Shear's polysaccharide) was identified as a mixture of three polysaccharides with the main chain consisting of d-glucose and d-mannose units connected by (1 → 3) glycosidic linkages (CitationSrivastava and Adams, 1994).
In time, other polysaccharide immunomodulators were sought and among them were β -glucans. Their investigation began in the 1960s and 1970s. Two lines can be traced in β -glucan history, each based on different starting points but gradually converging. The first took place chiefly in the United States and Europe, the second in Asia, primarily in Japan. Research on β -glucans in the Euro-American milieu was based on knowledge of immunomodulatory effects of zymosan, a mixture of polysaccharides isolated from the cell walls of Saccharomyces cerevisiae. Zymosan was for the first time prepared and investigated by Pillemer and Ecker (Citation1941) in the 1940s and since that time, it has been used in many physiological and immunological studies. Zymosan is potent stimulator especially of alveolar macrophages and, among others, it induces the release of a series of cytokines, mainly interleukin (IL-8), from human neutrophils.
Although zymosan was able to stimulate non-specific immune response, at the onset it was not clear what component of this rather crude composition is responsible for that activity. When zymosan was examined in detail, β -glucan was identified as the component of primary effect. It was subsequently isolated and its immunological effects were investigated. This research was pioneered by Nicholas R. DiLuzio (i.e., see DiLuzio and Riggi, Citation1970, Williams et al., Citation1980) at Tulane University in New Orleans.
The arrival of β -glucan in Japan was different. In Asian medicine, consumption of different medicinal mushrooms (e.g., shiitake, maitake, reishi, etc.) has a long tradition. In detailed studies of the biological effects of these mushrooms, especially the anticancer actions, β -glucans were again found to be a main cause of non-specific immunomodulation. The source of this investigation is associated with Goro Chihara from Teikyo University in Kawasaki, who isolated β -glucan, named by him lentinan, from mushroom shiitake (Lentinus edodes, now Lentinula edodes) (Chihara et al., Citation1969).
All sufficiently-purified polysaccharidic immunomodulators distinguish themselves by very low toxicity (e.g., lentinan has an LD50 (mouse) > 1600 mg/kg [Chihara et al., Citation1982]). Time incorporation of β -glucans in the history of immunomodulation is shown in .
TABLE 2 History of immunomodulators
The history of the investigation of the chemical composition of β -glucans is rather long and not straightforward. Composition of the cell walls of different microbial producers of β -glucan, in particular yeasts, was previously investigated in the 19th Century. Van Wisselingh (Citation1898) published an opinion that in the fungal cell wall, either chitin or cellulose could prevail.
β-Glucan from the cell wall of Saccharomyces cerevisiae can serve as an example of how difficult an exact determination of chemical structure was in the first half of the last century. In the 1950s and 1960s, β-glucan isolated from the cell walls of S. cerevisiae was subjected to investigation by the technique of sugar analysis, i.e., partial hydrolysis, methylation analysis, periodate oxidation, Smith degradation (periodate oxidation, reduction by NaBH4, and subsequent partial hydrolysis), etc. However, the results obtained were rather discrepant (Bell and Northcote, Citation1950; Manners and Patterson, Citation1966). Only after finding that in the yeast cell wall several types of β -glucans exist (Bacon and Farmer, Citation1968), detailed fractionation of cell wall components and their characterization was made (Manners et al., Citation1973). It is believed now that the main component of β -glucan from the yeast cell wall is a slightly branched, high-molecular (1 → 3)-β -d-glucan (DP about 1500, molecular weight ca. 240 kDa), with about 3% of β (1 → 6) branching.
Considerable heterogeneity of all natural β -glucans, not only from saccharomycetes but also from other sources, obviously was and continues to be the cause of a series of mutually contradicting conclusions. Recently, an attempt was made to solve this problem using semisynthetic and synthetic probes, suitable for accurate immunological research (Jamois et al., Citation2005).
3. SOURCES AND STRUCTURE OF β -GLUCAN
There are various natural sources of β -glucans; however, they are most frequently prepared from fungal cell walls. In addition to the fungal cell walls, β -glucan is also isolated from seaweed (laminaran from Laminaria sp., linear β (1 → 3)-d-glucan [Black et al., Citation1951], commercially sold as Phycarine [Vetvicka and Yvin, 2005]). It is also produced by bacteria (curdlan from Alcaligenes faecalis) (Harada et al., Citation1968) and included in cereals. The composition of the cereal β -glucan is somewhat different; it contains, in addition, β (1 → 4) bound glucose.
Taxonomic conception of kingdom Fungi went through a certain progression; many microorganisms earlier classified as fungi are now categorized into other kingdoms. In the following text, the name fungi will be written in quotation marks to distinguish between taxonomic and practical definition. Examples of composition of cell wall polysaccharides are presented in . The table makes evident that the most important producers of β -glucans are ascomycetes (where yeasts and certain filamentous moulds pertain) and basidiomycetes. To basidiomycetes belong edible or non-edible mushrooms, either found in nature or artificially cultivated.
TABLE 3 Composition of cell wall polysaccharides of some “fungi”
The cell wall composes an appreciable part of fungal cell mass; in yeasts it represents between 15% and 25% of total cell mass. The cell wall is not an inert structure but a vital organelle that performs a range of functions – mechanical protection, osmotic stabilization, compounds binding, mutual cellular adhesion, etc. The cell wall also serves as enzyme support and a selectively permeable barrier.
Research on the cell wall of different “fungal” species does not lead to a straightforward model of its structure, and concepts of its organization underwent certain development. According to Stratford (Stratford, Citation1994), the yeast cell wall resembles reinforced concrete. An armature, representing about 35% of wall mass and formed by fibrils of alkali insoluble β (1 → 3)-glucan, is dipped into mannoproteins (about 25–35% of wall mass) and bound to the armature through amorphous β -glucan and chitin. The cell wall of other “fungi” is constructed in a similar way. Essentially, the same model of the fungal cell wall was published by Selitrennikoff (Citation2001).
Recently, Grün (2001) published another model of the cell wall of ascomycetes and basidiomycetes. According to this model, the cell wall of most “fungi” contains five main components: (1 → 3)-β -d-glucan, (1 → 6)-β -d-glucan, (1 → 3)-α -d-glucan, chitin, and glycoproteins. On the other hand, (1 → 3)-α -d-Glucan is not present in yeasts (e.g., in S. cerevisiae and Candida albicans). However, in many other species of the Ascomycetes and Basidiomycetes, it forms 9–46% of cell wall mass. Chitin at saccharomycetes is found only in bud scars.
Though different models of the fungal cell wall differ somewhat, they agree that β -glucan is not located on the surface of the wall but is more or less immersed in the wall material. With regard to both immunological research and pharmaceutical utilization of β -glucans, an important conclusion can be reached. In macroorganisms, β -glucans act first of all as markers of fungal invasion so that activity of β -glucan preparations will increase with the degree of denudation of glucan fibrils. A general definition of glucans of any origin is given in .
TABLE 4 General division of β -glucans of any origin
Until recently, biologically efficient β -glucans were supposed to have similar structure – the main chain of β (1 → 3) bound d-glucopyranose moieties to which some d-glucopyranoses are randomly connected by β (1 → 6) linkages (). The degree of branching (DB) of some β -glucans is presented in . However, the detailed structure of β -glucans from dissimilar sources differs as well as their biological activity (Rees and Scott, Citation1971; Wagner et al., Citation1988; Adachi et al., Citation1989; Jamas et al., Citation1991; Kraus and Franz, Citation1992). In native β -glucans, their fibrils are composed from organized parts in which the main chain is coiled to triple helix. These regions are combined with single or double filaments of β (1 → 3)-d-gluco-pyranoses (CitationSaito et al., 1987, Ohno et al., Citation1988). The triple helix, formed by three H-bonds in C-2 position and stabilized by side chains, is probably present only in high-molecular β -glucans with molecular weight over 90 kDa (Chuah et al., Citation1983; Ohno et al., Citation1988). The H-bonds of triple helices can be interrupted by increased temperature, high pH, or certain solvents.
TABLE 5 Degree of branching (DB) of different β -glucans
Diverse data on the comparison of structure, molecular size, and biological effect can be found in the literature. For example, anti-tumor activity of schizophyllan is supposedly conditioned by triple helix presence and a molecular weight higher than 100 kDa (Kojima et al., Citation1986). However, the triple helix structure most likely should not be a solely effective form of β -glucan because alkaline treatment, used in most of isolation procedures, destroys this structure (Young and Jacobs, Citation1998; Saito et al., 1991; Miura et al., Citation1995). In addition, the most recent opinions do not confirm established ideas of the necessity of high molecular mass and branching of biologically active β -glucans. Thirty years ago, Kabat (Citation1976) found that for antigen polysaccharidic determinants, the size of the binding site on an antibody corresponds to six or seven monosaccharide units. Size of the binding site for β -glucan – in this case on a receptor of an immunocompetent cell, e.g., the macrophage – seems also to correspond to this number of glucose residues.
4. GLUCAN AND IMMUNOLOGICAL REACTIONS
Natural products useful in preventing or treating disease have been highly sought after throughout human history. A major problem in characterizing many natural products is that they represent a complex mixture of ingredients, each one of which may contribute to their bioactivity. β -Glucans from fungi, yeast and seaweed are well-known biologic response modifiers that function as immunostimulants against infectious diseases and cancer (Borchers et al., Citation1999; Brown and Gordon, Citation2003). Unlike most other natural products, purified β -glucans retain their bioactivity, which permits the characterization of how β -glucans work on a cellular and molecular level.
β-Glucan has been used as an immunoadjuvant therapy for cancer since 1980, primarily in Japan (Takeshita et al., Citation1991; Kimura et al., Citation1994; Matsuoka et al., Citation1997; Yan et al., Citation1999). Another activity demonstrated with β -glucan in the mid-1980s was its ability to stimulate hematopoiesis in an analogous manner as granulocyte monocyte-colony stimulating factor (Patchen et al., Citation1983). Both particulate and soluble β -glucans, all of which were administered intravenously, caused significantly enhanced recovery of blood cell counts after gamma radiation (Patchen et al., Citation1985). Others showed that β -glucan could reverse the myelosuppression produced with chemotherapeutic drugs (Wagnerova et al., Citation1993).
In addition to the effect in treatment of cancer, β -glucans have been demonstrated to protect against infection with both bacteria and protozoa in several experimental models and were shown to enhance antibiotic efficacy in infections with antibiotic-resistant bacteria. The protective effect of β -glucans was shown in experimental infection with Leishmania major (Al Tuwaiji et al., Citation1987) and L. donovani (Cook and Holbrook, Citation1984), C. albicans (Bacon and Farmer, Citation1968), Toxoplasma gondii (Bousquet et al., Citation1988), Streptococcus suis (Dritz et al., Citation1995), Plasmodium berghei (Kumar and Ahmad, Citation1985), Staphylococcus aureus (Liang et al., Citation1998), Escherichia coli (Rasmussen and Seljelid, Citation1990), Mesocestoides corti (White et al., Citation1988), Trypanosoma cruzi (Williams et al., Citation1989), Eimeria vermiformis (Yun et al., Citation1998), and anthrax infection (Vetvicka et al., 2002). Most published studies described effects of injected β -glucans (either intraperitoneal [IP], intravenous [IV], or subcutaneous [SC]). It is however necessary, for possible clinical practice, to evaluate the possibility of oral delivery. These experiments are summarized in .
TABLE 6 Oral effects of glucan
Yeast β -glucan is able to absorb mycotoxins (such as zearalenon, aflatoxin B1, deoxynivalenol, ochratoxin A, and patulin), probably through hydrogen bonds and van der Waals forces; this β -glucan effect is important particularly for livestock.
The influence of certain cereals (barley, oats) and edible mushrooms (e.g., Grifola frondosa, L. edodes and Flammulina velutipes [Fukushima et al., Citation2001], Pleurotus ostreatus [Bobek et al., 1996], or Agaricus bisporus [CitationFukushima et al., 2000]) on decreasing levels of serum cholesterol and liver low-density lipoproteins, leading to lowering of arteriosclerosis and heart disease hazards, is also mediated by β -glucan. It is known that cereals, mushrooms and yeast facilitate bowel motility and can be used in amelioration of intestinal problems, particularly obstipation (Battilana et al., Citation2001; Dongowski et al., Citation2002). Non-digestible β -glucans, forming a remarkable portion of these materials, are also able to modulate mucosal immunity of the intestinal tract (Tsukada et al., Citation2003). In the central nervous system, β -glucans activate microglial cells (Muller et al., Citation1994). These cells act as scavengers of the brain cell debris and play a positive role in Alzheimer's disease, AIDS, and multiple sclerosis (Haga et al., Citation1989).
Possible effects of β -glucans on macroorganisms are thus very diverse and impinge upon not only the immune system, but probably in most of the described activities, are in some form more or less connected with that system.
5. MECHANISMS OF ACTION
The most pronounced effect of β -glucans consists of augmentation of phagocytosis and proliferative activities of professional phagocytes–granulocytes, monocytes, macrophages, and dendritic cells. In this regard, macrophages (Chihara et al., Citation1982; Quinn, Citation1990; Vetvicka et al., Citation1996), considered the basic effector cells in host defense against bacteria, viruses, multicellular parasites, tumor cells. and erroneous clones of own somatic cells, play the most important role.
Macrophages are constituents of the non-specific (innate, non-adaptive), evolutionary older, immune system, which beyond phagocytes is comprised of a complicated family of serum proteins called complement and a number of other soluble recognizing and effector molecules. This innate immunity is based on non-clonal receptors (pattern recognition receptors, PRRs) that recognize certain molecules on the surface of invading microorganisms and are collectively termed as pathogen-associated molecular patterns (PAMPs). Regardless of their name, PAMPs are not unique for pathogens only, but are fundamental for the survival and pathogenicity of a given microorganism. The PAMPs differ from host molecules, are not subjected to variability, and are evolutionary highly conserved. Different biopolymers, including β -glucans, belong to the PAMPs.
Macrophages detect PAMPs through a number of different receptors. For β -glucan recognition the macrophages keep several receptors at their disposal: TLR-2 (toll-like receptor 2), dectin-1, CR3 (complement receptor 3), lactosylceramide and probably even other. Generally, the receptors are not too specific and usually detect several different PAMPs.
Toll-like receptors (TLRs) were not discovered until quite recently, although they possibly represent the most important receptor molecules of the non-adaptive component of the immune system. They are typical PRRs, which when bound together with PAMPs facilitate activation of the adaptive immune system in vertebrates. The name of these receptors is derived from sequential homology with a protein coded by the Toll gene. This gene occurs in Drosophilla flies where it plays a role in embryogenesis and, in mature flies, helps in defense against fungal infection (Lemaitre et al., Citation1996; Bilak et al., Citation2003). TLRs are transmembrane proteins with extracellular repetitive sequences rich in leucine. Thus far, approximately eleven TLRs are known. β -Glucan (and also zymosan, intact yeast cells, and/or LPS) is initially bound to TLR-2 (Underhill et al., Citation1999).
Dectin-1 is a lectin located on the macrophage surface and has special involvement in the detection and phagocytosis of fungal pathogens. In certain cases, it cooperates with the TLR-2. It is also a transmembrane protein with many particular functions, e.g., binding of a fungal PAMP, uptake and killing of invading cells, and induction of the production of cytokines and chemokines.
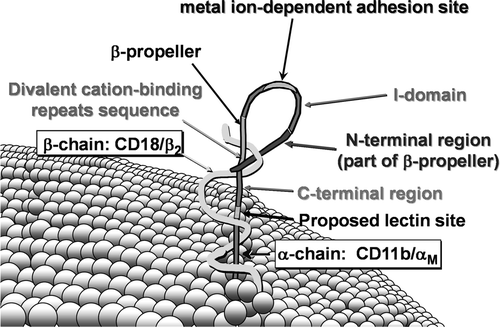
The complement receptor 3 (CR3, also denoted as integrin CD11b/CD18, 〈 M,2 and MAC1) is one of the most promiscuous pattern-recognition receptors. In addition to comple-ment components, it recognizes a large number of other ligands, among them β -glucan. The CR3 receptor is created from several domains; for saccharide recognition, it is a lectin domain (Ross et al., Citation1999) (). When a fragment iC3b of complement is simultaneously bound, binding of β -glucan triggers phagocytosis and degranulation.
FIG. 2 Schematic representation of CR3 showing its intertwined two-chain structure and the major domains of CD11b (From Ross, G.D. et al., Immunopharmacology, 42, 61, 1999. With permission).
Lactosylceramide (also CDw17 or Galβ 4Glcβ 1Cer) is a glycosphingolipid PRR located in the plasmatic membrane. β -Glucan recognition mediated by lactosylceramide causes different cellular responses, including production of cytokines and respiratory burst (Brown, Citation2006). Examples of macrophage receptors and the corresponding PAMPs are given in .
TABLE 7 Examples of macrophage receptors and corresponding PAMPs
Binding of β -glucan to a receptor activates macrophages. The activation consists of several interconnected processes including increased chemokinesis, chemotaxis, migration of macrophages to particles to be phagocyted, degranulation leading to increased expression of adhesive molecules on the macrophage surface, adhesion to the endothelium, and migration of macrophages to tissues. In addition, β -glucan binding also triggers intracellular processes, characterized by the respiratory burst after phagocytosis of invading cells (formation of reactive oxygen species and free radicals - hydrogen peroxide, superoxide radical, NO, HOCl [hypochlorous acid], HOI [hypoiodous acid] etc.), increasing of content and activity of hydrolytic and metabolic enzymes, and signaling processes leading to activation of other phagocytes and secretion of cytokines and other substances initiating inflammation reactions (e.g., IL-1, IL-9, tumor necrosis factor–α [TNFα]). For an excellent review regarding the interaction of glucans with macrophages, see Schepetkin and Quinn (Citation2006).
It is important for the pharmacological effect of β -glucan that activated macrophages do not act only against the activator but also against any present antigen, microorganism or tumor cell. Due to the fact that mammals lack β -glucanases in their enzyme equipment, macrophages represent what is probably the only tool for liquidation of β -glucan in the body. Within the macrophages, phagocytized β -glucan is degraded by an oxidative pathway (Nono et al., Citation1991).
6. SIDE EFFECTS
While the predominant pharmacological effects of β -glucans may be positive, their unfavorable side effects cannot be overlooked. At this time, few adverse effects have been described. It can be presumed, however, that with improved knowledge of the effects of β -glucans, this area will broaden.
Intramuscularly administered β -glucan induces an inflammatory reaction and granuloma formation at the puncture site. This is a painful method of application. The fact that β -glucan can be the cause of the inflammatory reaction itself represents a certain risk. Physiological inflammation occurs at the extent and rate corresponding with an inducing noxa, and also to the β -glucan present. If the noxious impact persists, a pathological inflammation can take place, manifested by excessive tissue damage subsequently ending in immune disorders and development of immunopathological (e.g., autoimmune) processes. In most serious cases, generalization of inflammatory processes can occur with shock development and subsequent fatal multiple organ dysfunction syndrome (MODS) (Tanriverdi et al., Citation2005).
The function of nitric oxide is double-edged. Nitric oxide, the “Molecule of Year, 1992” for which the discoverers of its physiological effects earned the Nobel Prize in Physiology and Medicine in 1998, is produced in macrophages by inducible nitric oxide synthase (iNOS). Synthesis of this enzyme is triggered by binding of β -glucan to a PRR on the macrophage surface. Formed nitric oxide induces a cytotoxic effect upon tumor cells (Hibbs et al., Citation1984; Stuehr and Nathan, Citation1989) and shows distinct impact on many pathogens (Grangeer et al., 1988; James and Claven, Citation1989).On the other hand, it can also damage tissues and DNA (Billar, Citation1995) and high concentrations can cause septic shock. The sustained action of the activator induces expression of iNOS, and increased formation of NO results in vasodilatation of veins. The latter, in turn, brings about an intense drop of venous resistance and blood pressure (Vincent et al., Citation2000). As of now, such an effect of β -glucan has not been described, yet it is quite conceivable.
Inhalation of intact cells or cellular detritus of fungi or yeasts - ingredients of home dust (Beijer et al., Citation2002) or different agricultural and industrial dusts (Rylander et al., Citation1999) - induces so-called syndrome of toxic organic dust (STOD) which is characterized by lung reactions that include pneumonia, cough, chronic bronchitis (Sato and Sano, Citation2003), rhinitis, headache, and irritation of the eyes and throat (Alwis et al., Citation1999). The cause of these complaints is β -glucan which, through activation of macrophages, monocytes, and leukocytes, causes increased secretion of inflammatory components (i.e., TNFα and IL-8).
Due to the fact that β -glucan administration brings about inflammatory processes in the human body, it is possible to hypothesize competitive interaction with anti-inflammatory drugs. Induced lethal toxicity elicited by a combination of β -glucan and a nonsteroidal anti-inflammatory drug (indomethacin), was described in mice (Yoshioka et al., Citation1998). Results strongly suggest that such a combination induces lethality by compromising the cytokine network that leads to systemic inflammation response syndrome (SIRS) and death.
7. POSSIBLE ALTERNATIVES OF FURTHER RESEARCH
Different natural sources, polymer character, methods of isolation, insolubility or limited solubility of preparations, (and thus unrealistic fractionation), all resulted in the fact that each preparation of β -glucan, especially the particulate one, is necessarily heterogeneous. Even if in some cases the heterogeneity of β -glucan (from the point of view of molecular size, branching, and crystalline or amorphous structure) does not principally change the in vivo activities, for trustworthy pharmacological research as well as for regulatory authorities (such as the USFDA) it represents substantial complications. It follows then that most preparations containing β -glucan – with the aim of avoiding complications – are classified as healthy food or nutritional supplements. As a result, the market is saturated with many products containing greater or lesser amounts of β -glucan that are often of questionable quality, with uncertain effects, and recommended by delusory advertisement.
Reliable research techniques allow the problems of heterogeneity and nonexistent standards of various natural β -glucans to be solved. A first possibility is to improve isolation techniques to obtain products with closely defined chemical composition and to use up-to-date physicochemical methods for infallible identification and analyses of these products. Chemically modified β -glucans, which due to their solubility can be easily fractionated, represents a second possibility. Unfortunately, biological activity of some of these products is decreased. Recently, an attempt has been made to solve this problem by construction of semi-synthetic or synthetic probes that are suitable for exact immunological research. A general solution is the binding of short oligomers of glucose containing β (1 → 3) and β (1 → 6) linkages to a polymer carrier of defined size and structure. A reasonable assumption is that such “synthetic” β -glucans will interact with receptors of immunocompetent cells and elicit analogous reactions as natural β -glucan (Descroix et al., Citation2006). From the immunopharmacological point of view, such probes would eventually replace the natural β -glucan.
8. CONCLUSION
Among the many thus far known and tested immunomodulators, polysaccharides isolated from various natural sources occupy a prominent position. An important group of these polysaccharides is represented by homopolymers of β -glucose, β -glucans. These contain either linear chains with (1 → 3)-β -d-glycosidic linkages or branched ones containing additional (1 → 6)-β -d-glycosidic linkages.
Due to their very low-to-negligible toxicity, there was a time when β -glucans were considered merely a matter of fashion. This cannot be said about many other immuno-modulators. Effects of β -glucans on a variety of diseases, such as infections, irradiation diseases, and foremost on neoplastic growth, were investigated. A flood of various food additives and “alternative remedies,” usually offered by faintly enlightened non-specialists, is a holdover from these pioneer years. After some time, naturally, wave of enthusiasm declined. β -Glucans were criticized by many regulatory authorities, the main reasons being insufficiently defined preparations and non-specific and/or complex effects.
Fortunately, in the last ten years, research in reputable laboratories has reached a phase where the basic mechanisms of glucan effects are known and the relationship between structure and activity is clearly outlined. It seems now that β -glucans will finally take the position which was ascribed to them fifty years ago.
REFERENCES
- Adachi Y., Ohno N., Ohsawa M., Sato K., Oikawa S., Yadomae T. Physiochemical properties and antitumor activities of chemically modified derivatives of antitumor glucan “grifolan LE” from Grifola frondosa. Chem. Pharm. Bull. (Tokyo) 1989; 37: 1837–1843
- Al Tuwaiji A. S., Mahmoud A. A., Al Mofleh I. A., Al Khuwaitir S. A. Effect of glucan on Leishmania major infection in BALB/c mice. J. Med. Microbiol. 1987; 23: 363–365
- Alwis K. U., Mandryk J., Hocking A. D. Exposure to biohazards in wood dust: Bacteria, fungi, endotoxins, and (1 → 3)-β -D-glucans. Appl. Occup. Environ. Hyg. 1999; 14: 598–608
- Aoyagi K., Itoh N., Abe F., Uchida K., Ishizuka M., Takeuchi T., Yamaguchi H. Enhancement by ubenimex (Betastatin) of host resistance to Candida albicans infections. J. Antibiot. 1992; 45: 1778–1784
- Bacon J. S. D., Farmer V. S. The presence of predominantly β -(1-6)-component in preparations of yeast glucan. Biochem. J. 1968; 110: 34–35P
- Battilana P., Ornstein K., Minehira K., Schwarz J. M., Acheson K., Schneiter P., Burri J., Jequier E., Tappy L. Mechanisms of action of beta-glucan in postprandial glucose metabolism in healthy men. Eur. J. Clin. Nutr. 2001; 55: 327–333
- Bell D. J., Northcote D. H. The structure of a cell-wall polysaccharide of baker's yeast. Part II. J. Chem. Soc. 1950; 47: 1944–1947
- Beijer L., Thorn J., Rylander R. Effects after inhalation of (1 → 3)-β -D-glucan and relation to mould exposure in the home. Mediators Inflam. 2002; 11: 149–153
- Bilak H., Tauszig-Delamasure S., Imler J. L. Toll and Toll-like receptors in Drosophila. Biochem. Soc. Trans. 2003; 31: 648–651
- Billar T. R. Nitric oxide, novel biology and clinical relevance. Ann. Surg. 1995; 221: 339–349
- Billroth T. Beobachtung-Studien uber Wundfieber und accidentelle Wundkrankheiter. Arch. Klin. Chir. 1865; 6: 372–399
- Black W. A. P., Cornhill W. J., Dewar E. T., Woodward F. N. Manufacture of algal chemicals. III. Laboratory scale isolation of laminarin from brown marine algae. J. Appl. Chem. 1951; 1: 505–507
- Bobek P., Ozdin L., Kuniak L. Effect of oyster mushroom (Pleurotus ostreatus) and its ethanolic extract in diet on absorption and turnover of cholesterol in hypercholesterolemic rat. Nahrung 1996; 40: 222–224
- Borchers A. T., Stern J. S., Hackman R. M., Keen C. L., Gershwin M. E. Mushrooms, tumors, and immunity. Proc. Soc. Exp. Biol. Med. 1999; 221: 281–293
- Bousquet M., Escoula L., Pipy B., Bessieres M. H., Chavant L., Seguela J. P. Two β -1-3, β -1-6 polysaccharides (PSAT and Scleroglucan) enhance the resistance of mice to Toxoplasma gondii. Ann. Parasitol. Hum. Comp. 1988; 63: 398–409
- Brown J. L., Kossaczka Z., Jiang B., Bussey H. A mutational analysis of killer toxin resistance in Saccharomyces cerevisiae identifies new genes involved in cell wall (1 → 6)-β -glucan synthesis. Genetics 1993; 133: 837–849
- Brown G. D., Gordon S. Fungal β -glucans and mammalian immunity. Immunity 2003; 19: 311–315
- Brown G. D. Dectin-1: A signaling non-TLR pattern-recognition receptor. Nat. Rev. Immunol. 2006; 6: 33–44
- Busch W. VII. Verhandlungen artzlicher gesellschaften. Berl. Klin. Wochenschr. 1850; 5: 137–138
- Chihara G., Maeda Y. Y., Hamuro J., Sasaki T., Fukuoka F. Inhibition of mouse sarcoma 180 by polysaccharides from Lentinus edodes (Berk.) sing. Nature 1969; 222: 687–688
- Chihara G., Maeda Y. Y., Hamuro J. Current status and perspectives of immunomodulators of microbial origin. Int. J. Tissue React. 1982; IV: 207–225
- Coley W. B. Treatment of inoperable malignant tumors with the toxins of erysipelas and the Bacillus prodigiosus. Am. J. Med. Sci. 1894; 108: 50–66
- Cook J. A., Holbrook T. W. Immunogenicity of soluble and particulate antigens from Leishmania donovani: Effect of glucan as an adjuvant. Infect. Immun. 1984; 40: 1038–1043
- Chuah C. T., Sarko Y., Deslandes Y., Marchessault R. H. Triple helical crystalline structure of curdlan and paramylon hydrates. Macromolecules 1983; 16: 1375–1382
- Descroix K., Ferrieres V., Jamois F., Yvin J. C., Plusquellec D. Recent progress in the field of β -(1,3)-glucans and new applications. Mini-Rev. Med. Chem. 2006; 6: 1341–1349
- DiLuzio N. R., Riggi S. J. The effects of laminarin, sulfated glucan and oligosaccharides of glucan on reticuloendothelial activity. J. Reticuloendothel. Soc. 1970; 8: 465–473
- Dongowski G., Huth M., Gebhardt E., Flamme W. Dietary fiber-rich barley products beneficially affect the intestinal tract of rats. J. Nutr. 2002; 132: 3704–3714
- Dritz S. S., Shi J., Klelian T. L., Goodband R. D., Nelssen J. L., Tokach M. D., Chengappa M. M., Smith J. E., Blecha F. Influence of dietary β -glucan on growth performance, non-specific immunity, and resistance to Streptococcus suis infection in weanling pigs. J. Anim. Sci. 1995; 73: 3341–3350
- Ebina T., Fujimiya Y. Antitumor effect of a peptide-glucan preparation extracted from Agaricus blazei in a double-grafted tumor system in mice. Biotherapy 1998; 11: 259–265
- Fukushima M., Ohashi T., Fujiwara Y., Sonoyama K., Nakano M. Cholesterol-lowering effects of maitake (Grifola frondosa) fiber, shiitake (Lentinus edodes) fiber, and enokitake (Flammulina velutipes) fiber in rats. Exp. Biol. Med. 2001; 226: 758–765
- Fukushima M., Nakano M., Morii Y. T., Ohashi T., Fujiwara Y., Sonoyama K. Cholesterol-lowering effects of maitake (Grifola frondosa) fiber, shiitake (Lentinus edodes) fiber, and enokitake (Flammulina velutipes) fiber in rats. J. Nutr. 2000; 130: 2151–2156
- Granger D. L., Hibbs J. B., Perfect J. A. Specific amino acid (L-arginine) requirement for the microbiostatic activity of murine macrophages. J. Clin. Invest. 1988; 81: 1096–1102
- Structure and Biosynthesis of Fungal α -Glucans, C. H. Grün. Proef. Univer., Utrechtthe Netherlands 2001
- Haga S., Akai K., Ishii T. Demonstration of microglial cells in and around senile (neuritic) plaques in the Alzheimer brain. An immunohistochemical study using a novel monoclonal antibody. Acta Neuropathol. 1989; 77: 569–575
- Harada T., Misaki A., Saitô H. Curdlan: A bacterial gel-forming β -1,3-glucan. Arch. Biochem. Biophys. 1968; 124: 292–298
- Hibbs J. B., Taintor R. R., Vavrin Z. Iron depletion: Possible cause of tumor cell cytotoxicity induced by activated macrophages. Biochem. Biophys. Res. Commun. 1984; 123: 716–723
- Hong F., Yan J., Baran J. T., Allendorf D. J., Hansen R. D., Ostroff G. R., Xing P. X., Cheung N. K., Ross G. D. Mechanism by which orally administered β -1,3-glucans enhance the tumoricidal activity of anti-tumor monoclonal antibodies in murine tumor models. J. Immunol. 2004; 173: 797–806
- Hotta H., Hagiwara K., Tabata K., Ito W., Homma M. Augmentation of protective immune responses against Sendai virus infection by fungal polysaccharide schizophyllan. Int. J. Immunopharmacol. 1993; 5: 55–60
- Jamas S., Easson D. D., Ostroff G. R., Onderdonk A. B. A novel class of macrophage activity immunomodulators. ACS Symp. Ser. 1991; 1469: 44–51
- James S., Claven J. Macrophage cytotoxicity against schistosomula of Schistosoma mansoni involves arginine-dependent production of reactive nitrogen intermediates. J. Immunol. 1989; 143: 4208–4212
- Jamois F., Ferriéres V., Guégan J. P., Yvin J. C., Plusquellec D., Vìtvièka V. Glucan-like synthetic oligosaccharides. Iterative synthesis of linear oligo-β -(1,3)-glucans and immunostimulatory effects. Glycobiology 2005; 15: 393–407
- Structural Concepts in Immunology and Immunochemistry, E. A. Kabat. Holt, Rinehart and Winston, New York 1976
- Kaibara N., Soejima K., Nakamura T., Inokuchi K. Postoperative long-term chemotherapy for advanced gastric cancer. Jpn. J. Surg. 1976; 6: 54–59
- Kimura Y., Tojima H., Fukase S., Takeda K. Clinical evaluation of sizofilan as assistant immunotherapy in treatment of head and neck cancer. Acta Otolaryngol. (Stockh.) Suppl. 1994; 511: 192–195
- Kojima T., Tabata K., Itoh W., Yanaki T. Molecular weight dependence of the antitumor activity of Schizophyllan. Agr. Biol. Chem. 1986; 50: 231–232
- Kraus J., Franz G. Immunomodulating effects of polysaccharides from medicinal plants. Adv. Exp. Med. Biol. 1992; 319: 299–308
- Kubo K., Nanba H. The effect of maitake mushrooms on liver and serum lipids. Altern. Ther. Health Med. 1996; 2: 62–66
- Kumar P., Ahmad S. Glucan-induced immunity in mice against Plasmodium bergei. Ann. Trop. Med. Parasitol. 1985; 79: 211–213
- Kurashige S., Akuzawa Y., Endo F. Effects of Lentinus edodes Grifola frondosa Pleurotus ostreatus administration on cancer outbreak, and activities of macrophages and lymphocytes in mice treated with a carcinogen, N-butyl-N-butanolnitrosoamine. Immunopharmacol. Immunotoxicol. 1997; 19: 175–183
- Lemaitre B., Nicolas E., Michaut L., Reichhart J. M., Hoffmann J. A. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 1996; 86: 973–983
- Liang J., Melican D., Cafro L., Palace G., Fisette L., Armstrong R., Patchen M. L. Enhanced clearance of a multiple antibiotic resistant Staphylococcus aureus in rats treated with PGG-glucan is associated with increased leukocyte counts and increased neutrophils oxidative burst activity. Int. J. Immunopharmacol. 1998; 20: 595–614
- Manners D. J., Masson A. J., Patterson J. C., Bjorndal H., Lindberg B. The structure of a β -(1-6)-D-glucan from yeast cell walls. Biochem. J. 1973; 135: 19–36
- Manners D. J., Patterson J. C. A re-examination of the molecular structure of yeast glucan. Biochem. J. 1966; 105: 19C–20C
- Matsuoka H., Seo Y., Wakasugi H., Saito T., Tomoda H. Lentinan potentiates immunity and prolongs survival time of some patients. Anticancer Res. 1997; 17: 2751–2755
- Miura T., Miura N. N., Ohno N., Adachi Y., Shimada S., Yadomae T. Failure in anti-tumor activity by overdose of an immunomodulating β -glucan preparation, sonifilan. Biol. Pharm. Bull. 2000; 23: 249–253
- Miura N. N., Ohno N., Adachi Y., Aketagawa J., Tamura H., Tanaka S., Yadomae T. Comparison of the blood clearance of triple- and single-helical schizophyllan in mice. Biol. Pharmacol. Bull. 1995; 18: 185–189
- Muller C. D., Bocchini V., Giaimis J., Guerrieri P., Lombard Y., Poindron P. Functional β -glucan receptor expression by a microglial cell line. Res. Immunol. 1994; 145: 267–275
- Nanba H. Results of non-controlled clinical study for various cancer patients using maitake D-fraction. Explore 1995; 6: 19–21
- Nio Y., Tshuchitani T., Imai S., Shiraishi T., Kan N., Ohgaki K., Tobe T. Immunomodulating effects of oral administration of PSK. II. Its effects on mice with cecal tumors. Nippon Gan. Chiryo Gakkai Shi. 1988; 23: 1068–1076
- Nono I., Ohno N., Masuda A., Oikawa S., Yadomae T. Oxidative degradation of an antitumor (1-3)-β -D-glucan, grifolan. J. Pharmacobiol. Dyn. 1991; 14: 9–19
- Nowotny A. Molecular aspects of endotoxic reactions. Bact. Rev. 1969; 33: 72–98
- Ohno N., Kurachi Y., Yadomae T. Physicochemical properties and antitumor activities of carboxymethylated derivatives of glucan from Sclerotinia sclerotiorum. Chem. Pharmacol. Bull. 1988; 36: 1198–1125
- Ohno N., Miura N. N., Nakajima M., Yadomae T. Antitumor 1,3-beta-glucan from cultured fruit body of Sparassis crisp. Biol. Pharm. Bull. 2000; 23: 866–872
- Patchen M. L., MacVitti T. J. Dose-dependent responses of murine pluripotent stem cells and myeloid and erythroid progenitor cells following administration of the immunomodulating agent glucan. Immunopharmacology 1983; 5: 303–313
- Patchen M. L., MacVittie T. J. Stimulated hemopoiesis and enhanced survival following glucan treatment in sublethally- and lethally-irradiated mice. Int. J. Immunopharmacol. 1985; 7: 923–932
- Pillemer L., Ecker E. E. Anti-complementary factor in fresh yeast. J. Biol. Chem. 1941; 137: 139–142
- Quinn P. J. Mechanisms of action of some immunomodulators used in veterinary medicine. Adv. Vet. Sci. Comp. Med. 1990; 35: 43–99
- Rasmussen T. L., Seljelid R. Dynamics of blood compontents and peritoneal fluid during treatment of murine E. coli sepsis with β -1,3-D-polyglucose derivates. I. Cells. Scand. J. Immunol. 1990; 31: 321–331
- Rees D. A., Scott W. E. J. Polysaccharide conformation. Part VI Chem. Soc. (B) 1971; 6: 469–479
- Ross G. D., Vetvicka V., Yan J., Xia Y., Vetvicková J. Therapeutic intervention with complement and β -glucan in cancer. J. Immunopharmacol. 1999; 42: 61–74
- Rylander R., Thorn J., Attefors R. Airways inflammation among workers in a paper industry. Eur. Respir. J. 1999; 13: 1151–1158
- Schepetkin I. A., Quinn M. T. Botanical polysaccharides: Macrophage immunomodulation and therapeutic potential. Int. Immunopharmacol. 2006; 6: 317–333
- Saitô H., Ohki T., Takasuka N., Sasaki T. A 13C-N.M.R.-Spectral study of a gel-forming, branched (1 → 3)-β –glucan, (lentinan) from Lentinus edodes, and its acid-degraded fractions. Structure, and dependence of conformation on the molecular weight. Carbohydr. Res. 1977; 58: 293–305
- Saitô H., Tabeta R., Yoshioka Y., Hara Ch., Kiho T., Ukai S. A high-resolution solid-state 13C NMR study of the secondary structure of branched (1 → 3)-β -D-glucans from fungi: Evidence of two kinds of conformers, curdlan-type single-helix and laminaran-type triple helix forms, as manifested from the conformation-dependent 13C chemical shifts. Bull. Chem. Soc. Japan 1987; 60: 4267–4272
- Saitô H., Yoshioka Y., Uehara N., Aketagawa J., Tanaka S., Shibata Y. Relationship between conformation and biological response for (1-3)-β -D-glucans in the activation of coagulation factor G from limulus amebocyte lysate and host-mediated antitumor. Carbohydr. Res. 1991; 217: 181–190
- Sakurai T., Hashimoto K., Suzuki I., Ohno N., Oikawa S., Masuda A., Yadomae T. Enhancement of murine alveolar macrophage functions by orally administered β -glucan. Int. J. Immunopharmacol. 1992; 14: 821–830
- Sato M., Sano H. Direct binding to Toll-like receptor 2 to zymosan, and zymosan-induced NF-κ B activation and TNF-α secretion are down-regulated by lung collection surfactant protein. J. Immunol. 2003; 171: 417–425
- Selitrennikoff C. P. Antifungal proteins. Appl. Environ. Microbiol. 2001; 67: 2883–2894
- Shear M. J., Turner F. C., Perrault A., Shovelton T. Chemical treatment of tumors. V. Isolation of the hemorrhage-producing fraction from Serratia marcescens (Bacillus prodigiosus) culture filtrates. J. Natl. Cancer Inst. 1943; 4: 81–97
- Srivastava H. C., Adams G. A. Preparation and properties of polysaccharide-lipid complexes from Serratia marcescens. Can. J. Chem. 1962; 40: 905–918
- Stratford M. Another brick in the wall? Recent developments concerning the yeast cell envelope. Yeast 1994; 10: 1741–1752
- Stuehr D. J., Nathan C. F. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J. Exp. Med. 1989; 169: 1543–1555
- Suzuki I., Sakurai T., Hashimoto K., Oikawa S., Masuda A., Ohsawa M., Yadomae T. Effect of orally administered beta-glucan on macrophage function in mice. Chem. Pharm. Bull. 1991; 39: 1606–1608
- Takeshita K., Saito N., Sato Y., Maruyama M., Sanagawa M., Habu H., Endo M. Diversity of complement activation by lentinan, an antitumor polysaccharide, in gastric cancer patients. Nippon Geka Gakkai Zasshi 1991; 92: 5–11
- Tanriverdi P., Yuksel B. C., Rasa K., Guler G., Iskit A. B., Guc M. O., Korkmaz A. The effects of selective nitric oxide synthase blocker on survival, mesenteric blood flow and multiple organ failure induced by zymosan. J. Surg. Res. 2005; 124: 67–73
- Tsukada C., Yokoyama H., Miyaji C., Ishimoto Y., Kawamura H., Abo T. Immunopotentiation of intraepithelial lymphocytes in the intestine by oral administrations of beta-glucan. Cell. Imunol. 2003; 221: 1–5
- Tsung K., Norton J. A. Lessons from Coley's Toxin. Surgic. Oncol. 2006; 15: 25–28
- Ueno H. Beta-1,3-D-glucan, its immune effect and its clinical use. J. Soc. Term. Syst. Dis. 2000; 6: 151–154
- Underhill D. M., Ozinsky A., Hajjar A. M., Stevens A., Wilson C. B., Bassetti M., Aderem A. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 1999; 401: 811–815
- van Wisselingh C. Mikrochemische Untersuchungen über die Zellwande der Fungi. Jahrb. Wiss. Botan. 1898; 31: 619–687
- Vetvicka V., Dvorak M., Vetvickova J., Richter J., Krizan J., Sima P., Yvin J. C. Orally-administered marine β -1,3 glucan phycarine stimulates both humoral and cellular immunity. Int. J. Biol. Macromol. 2007; 40: 291–298
- Větvička V., Terayama K., Mandeville R., Brousseau P., Kournikakis B., Ostroff G. Pilot study: Orally administered yeast β -1,3-glucan prophylactically protects against anthrax infection and cancer in mice. J. Am. Nutraceut. Assoc. 2002; 5: 1–6
- Vetvicka V., Thornton B. P., Ross G. D. Soluble β -glucan polysaccharide binding to the lectin site of neutrophil or natural killer cell complement receptor type 3 (CD11b/CD18) generates a primed state of the receptor capable of mediating cytotoxicity of iC3b-opsonized target cells. J. Clin. Invest. 1996; 98: 50–61
- Vetvicka V., Yvin J. C. Effects of marine β -glucan on immune reaction. Int. Immunopharmacol. 2004; 4: 721–730
- Vincent J. L., Zhang H., Szabo C., Preiser J. C. Effects of nitric oxide in septic shock. Am. J. Respir. Crit. Care Med. 2000; 161: 1781–1785
- Wagner H., Stuppner H., Schafer W., Zenk M. Immunologically active polysaccharides of Echinacea purpurea cell cultures. Phytochem. 1988; 27: 119–126
- Wagnerova J., Liskova A., Navarova J., Kristofova A., Trnovec T., Ferencik M. The effect of two glucan carboxymethyl derivatives with various substitution degrees on cyclophosphamide immunosuppression in mice. Immunopharmacol. Immunotoxicol. 1993; 15: 227–242
- Werner G. H. Prospective views on unexplored pathways of immunostimulation. Immunostimulants: Now and Tomorrow, I. Azuma, G. Jolles. Japan Sci. Press: Tokyo/Springer Verlag, Berlin 1987; 207–215
- Whistler R. L., Bushway A. A., Singh P. P., Nakahara W., Tokuzen R. Non-cytotoxic anti-tumor polysaccharides. Adv. Carbohydr. Chem. Biochem. 1976; 32: 235–275
- White T. R., Thompson R. C., Penhale W. J., Chihara G. The effects of lentinan on the resistance of mice to Mesocestoides corti. Parasitol. Res. 1988; 74: 563–568
- Williams D. L., Al-Tuwaijri A., DiLuzio N. R. Influence of glucan on experimental infections in mice. Int. J. Immunopharmacol. 1980; 2: 189–198
- Williams D. L., Yaeger R. G., Pretus H. A., Browder I. W., McNamee R. B., Jones E. L. Immunization against Trypanosoma cruzi: Adjuvant effect of glucan. Int. J. Immunopharmacol. 1989; 11: 403–410
- Yan J., Vetvicka V., Xia Y., Coxon A., Carroll M. C., Mayadas T. N., Ross G. D. β -glucan, a “specific” biologic response modifier that uses antibodies to target tumors for cytotoxic recognition by leukocyte complement receptor type 3 (CD11b/CD18). J. Immunol. 1999; 163: 3045–3052
- Young S. H., Jacobs R. R. Sodium hydroxide-induced conformational change in schizophyllan detected by the fluorescence dye, aniline blue. Carbohydr. Res. 1998; 310: 91–99
- Yoshioka S., Ohno N., Miura T., Adachi Y., Yadomae T. Immunotoxicity of soluble β -glucans induced by indomethacin treatment. FEMS Immunol. Med. Microbiol. 1998; 21: 171–179
- Yun C. H., Estrada A., Van Kessel A., Gajadhar A., Redmond M., Laarveld B. Immunomodulatory effects of oat β -glucan administered intragastrically or parenterally on mice infected with Eimeria vermiformis. Microbiol. Immunol. 1998; 42: 457–465