Abstract
Food microencapsulation has been an efficient way of raising food shelf life during storage. Passion fruit juice was encapsulated in Capsul® and stored at different temperatures. The shelf life of vitamin C was analyzed and X-ray diffraction, scanning electronic microscopy, and laser diffraction analyses were performed. Samples stored at 7, 25, and 37°C retained 91.4, 81.1, and 36.2%, respectively, of vitamin C after storage. Capsules had an average size of 205.7 ± 0.09 μm and were presented in an amorphous form. Capsul® was shown to be an interesting material for the encapsulation of passion fruit juice.
INTRODUCTION
Passion fruit (Passiflora) is in the botanical family Passifloraceae and is found in tropical climates. The plant has its origin in North America, but is also found in Brazil, Peru, Mexico, the Mediterranean region of Europe, and North Africa (CitationRain Tree Nutrition, 2011). In Brazil, 150 varieties are known (CitationEmbrapa, 2011). Productivity in Brazil is high with around 20 to 40 tons produced per hectare (CitationIAC, 1998). Passion fruit is rich in vitamin C, vitamins B1 and B2, and the pro-vitamin A, and β-carotene, as well as minerals, such as K, P, Ca, Fe, and fiber. In the Food Composition and Nutrition Tables (CitationSouci et al., 2008) the following values are presented for 100 g of passion fruit: 75.8% water, 63 kcal, 9.5 g carbohydrates, 0.4 g lipids, 2.4 g proteins, 1.5 g dietary fiber, 3.9 g organic acids, and 0.9 g minerals. Vitamin C values have been reported as 40 mg in 100 g of natural passion fruit juice (CitationSuntornsuk et al., 2001).
The production and commercialization of passion fruit juice has faced many difficulties regarding transport and shelf life. The fruit spoils easily and needs to be processed before it loses water. Concentrations of 14° Brix for natural juice and 50° Brix for concentrated juice are considered safe for commercialization (CitationIAC, 1998). A common method of preservation is pasteurization. This method improves shelf life, but on the other hand, alters significantly the taste, color, and nutritional value. Therefore, a promising technology is microencapsulation, defined as the packaging of solid, liquid, and gaseous material in sealed capsules of sizes between nanometers and millimeters. The packaging isolates and protects the material from ambient conditions, such as light, temperature, oxygen, humidity, and from interaction with other substances. If desired, the material can be released from the capsules in a controlled way and under specific conditions (CitationDziezak, 1988; CitationRisch, 1995; CitationRé, 1998). Microencapsulation also protects materials from evaporation (CitationJackson and Lee, 1991). In food systems, microencapsulation can be utilized for acids, lipids, enzymes, microorganisms, flavors, vitamins, minerals, growth agents, and colorants (CitationBakan, 1973; CitationPothakamury and Barbosa-Cánovas, 1995). There are many methods of encapsulation with their main difference being in the combination of the encapsulating materials and the nucleus, which can be of physical, chemical, or physicochemical in nature (CitationShahidi and Han, 1993; CitationGouin, 2004).
One of the most common microencapsulation techniques is freeze-drying. The product is rapidly frozen and the taste and flavor are preserved. Subsequently, the frozen material is submitted to a vacuum, which enables dehydration with residual humidity of less than 2%. The encapsulating agent should not react with the nucleus. The most common materials are gums, carbohydrates, cellulose, lipids, and proteins (CitationGharsallaoui et al., 2007).
A common material for microencapsulation in the food industry is Capsul®, a modified amid. This modification consists in the addition of a lipophylic component, octenyl-succinate, which increases the emulsion stability (CitationArburto et al., 1998). Capsul® is used by the food and pharmaceutical industry with approval of the FDA as a food additive since the concentration of octenyl-succinate does not exceed 3% (CitationBastos et al., 2009).
The aim of this work was to create stable capsules of passion fruit juice with the aid of Capsul® using freeze-drying and to characterize the capsules with respect to their size and surface morphology. The stability of the capsules was tested by vitamin C analyses during 12 weeks of storage at different temperatures.
MATERIALS AND METHODS
Production of Passion Fruit Microcapsules with Capsul® (Starch—Octenylsuccinate)
Passion fruits were acquired at local markets in Rio de Janeiro. Fruits were washed, the juice was obtained using a commercial food processor, and filtered before being mixed with 20% Capsul®. Then the mixtures were frozen and subsequently freeze-dried in a Labconco FreeZoneR Freeze Dry System (model 740020, Labconco, Kansas City, MO, USA).
Shelf Life Analysis by Vitamin C Stability
The shelf life of the capsules was evaluated by vitamin C analysis during 12 weeks. Vitamin C is easily degraded by oxidation and is, therefore, used as a chemical indicator of sample stability. It represents a useful indicator of the efficiency of the encapsulating material as a nutrient and vitamin protector. The capsules were stored in Falcon tubes and submitted to different temperatures and storage times. Samples were divided into three parts. One part was stored at an ambient temperature of 25°C, the second part was stored in a refrigerator at 7°C, and the third part at an elevated temperature of 37°C in an oven. Storage was performed in duplicates at each temperature. All samples were maintained in the dark during 12 weeks. An additional analysis of the sample stored at 7°C was performed after 43 weeks of storage. In parallel, as a control, lyophilized samples of passion fruit juice without Capsul® were submitted to the same treatment. Samples were analyzed at 2-week intervals in triplicate following the method of Tillmans, according to the CitationAnalytic Norms of the Adolfo Lutz Institute (1985). This method is based on the strong reduction capacity of vitamin C, which reduces 2-6-diclorophenol-indophenol.
X-Ray Diffraction
Measurement of the degree of crystallinity of the samples was performed in a Mastersizer 2000 (Malvern Instruments, Worchestershire, UK) in triplicates, after 50 days of storage. Capsules were dissolved in ethanol and submitted to ultrasound treatment prior to analysis. A sample of pure Capsul® was also measured in order to compare it to the samples. The degree of crystallinity was obtained by comparison of the peak areas between sample and control sample.
Scanning Electronic Microscopy
Characterization of the microstructure of the microcapsules' surface was performed in a JEOL, JSM-5460LV scanning electron microscope (JEOL, Tokyo, Japan), following the methodology described by CitationSheu and Rosenberg (1998). Microcapsules were fixed in aluminium stubs using double-coated carbon conducting adhesive tapes. Samples were then sputter coated with gold and analyzed. The methodology allows visualizing the structure of the microcapsules and their integrity and porosity (CitationShahidi and Han, 1993).
Laser Diffraction
The particle size was analyzed in a Shimadzu laser diffraction particle size analyzer SALD-2201 (Shimadzu, São Paulo, Brazil) and the results were processed with Wing software (Shimadzu, São Paulo, Brazil). Samples were analyzed by light scattering after dilution in isopropanol and homogenization in ultra sound.
Statistical Analysis
Obtained data were compared by variance analysis at p < 0.05. Differences between the means were determined with the Tukey multiple test.
RESULTS AND DISCUSSION
Production of Passion Fruit Microcapsules with Capsul®
The mixture of fresh filtered passion fruit juice with 20% of Capsul® was freeze-dried and resulted in a white powder, which was diluted in cold water in order to reconstitute the juice for consumption. Freeze-dried control samples without Capsul® formed a yellow powder.
It is important to consider that the addition of 20% of Capsul® leads to a significant increase in the energy value of the resulting product. Since the natural passion fruit juice has a very low energy value of around 60 kcal/100 g, the addition of 20 g of Capsul® adds about 90 kcal/100 g to the capsules and approximately doubles the dry weight of the product. However, a lower amount of Capsul® did not result in a complete encapsulation of the passion fruit juice and, therefore, this calorie increment needs to be accepted for the present objective.
Shelf Life Analysis by Vitamin C Stability
Encapsulated passion fruit samples were stored at 7, 25, and 37°C, in the dark, for 12 weeks. In parallel, samples of freeze-dried passion fruit juice were submitted to the same treatment. After each 2 weeks, samples were analyzed for vitamin C concentration in triplicates. All samples, Capsul® and controls, stored at 7 and 25°C maintained their physical appearance during 12 weeks of storage, presenting a homogeneous powder. Samples stored at 37°C suffered significant modifications caused by the high storage temperature. These samples turned dark brown and sticky, ceasing to be a powder, and turning into a solid block. However, it was observed that the microencapsulated samples suffered less from this browning and hardening than the non-encapsulated control samples. Here, the Capsul® probably had offered an efficient physical protection against the effects of heat on the passion fruit juice.
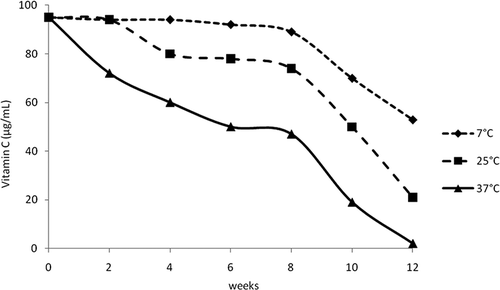
The passion fruit samples showed a substantial loss of vitamin C concentration during storage. shows that the vitamin C concentration was reduced to varying degrees depending on the storage temperature, until almost complete disappearance. Storage at 7°C, as expected, promoted a delay in vitamin C degradation, while storage at 37°C accelerated the process. At 25°C, the vitamin was degraded, but there was a delay, probably due to carotenoid activity, present in high levels in passion fruit juice. After 12 weeks, samples stored at 7, 25, and 37°C had maintained 55.8, 22.1, and 2.1% of vitamin C, respectively.
FIGURE 1 Vitamin C concentration in samples of freeze-dried passion fruit juice, stored 12 weeks in the dark at 7, 25, and 37°C.
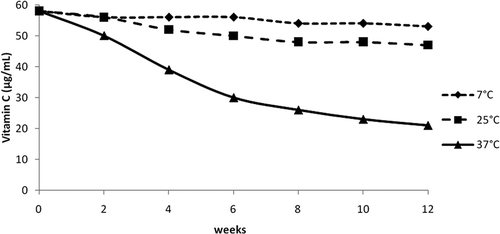
Microencapsulated samples showed a different behavior. There also was a significant loss of vitamin C concentration, but much lower than in the control samples (). Degradation was less intense and more delayed, compared to the control samples. The most impressive result was the vitamin C protection in the samples stored at 37°C, where 36.2% of the vitamin was preserved. At 7 and 25°C, 91.4 and 81.0% of the vitamin was not degraded, which is notably different from the non-encapsulated samples. Additionally, the samples that had been stored at 7°C were analyzed again after 43 weeks of storage and contained 50 μg/mL of vitamin C, which represents 86.2% of the initial vitamin C concentration.
FIGURE 2 Vitamin C concentration in samples of passion fruit juice, encapsulated in Capsul®, freeze-dried, and stored 12 weeks in the dark at 7, 25, and 37°C.
These results show the efficiency of Capsul® as a vitamin C protector during 12 weeks of storage. Together with a low storage temperature, the main part of the vitamin C can be preserved during a very long time.
X-Ray Diffraction
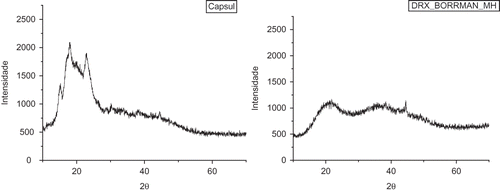
The degree of crystallinity of the samples was analyzed by X-ray diffraction. The encapsulated passion fruit juice and a sample of pure Capsul® were measured () after 50 days of storage. Images proved, as expected, that both Capsul® and the capsules of passion fruit juice were represented in an amorphous, disorganized form. Capsul® usually shows a small tendency to crystallize, and is represented by the peaks in the graph. This is reduced when mixed with the passion fruit juice, leading to a more amorphous sample, with no peaks accusing crystallization. This is particularly interesting, since amorphous samples tend to be very hygroscopic and to absorb water during the storage. Samples then tend to become sticky and form agglomerates as the amorphous sugars are transformed into crystalline sugars in order to reach an energetically more stable state. This characteristic prejudices the storage of the samples since the water absorption implicates in weight gain, microstructure collapse, and potential microbiological instability, for example. Nevertheless, the samples maintained their amorphous state during 50 days and remained as a loose and dry powder.
Scanning Electronic Microscopy
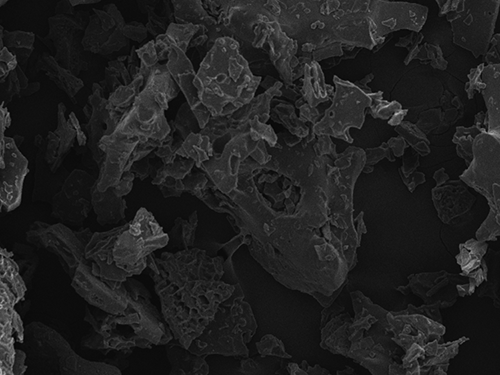
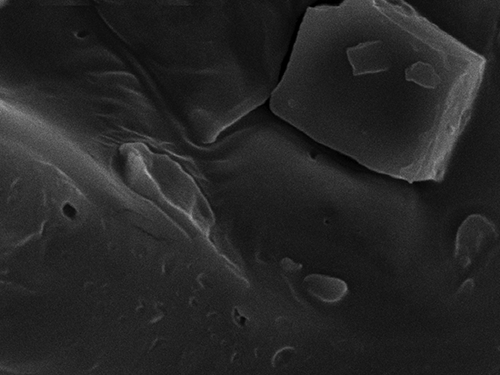
With the help of scanning electron microscopy, it was possible to physically characterize the surface of the passion fruit juice microcapsules. Measurements were performed at magnifications of 50, 100, 500, 1000, and 3000× ( and ). As the samples have been freeze-dried, they do not present a defined nucleus, neither can they be classified as typical microcapsules or microspheres, but as a matrix of microparticles. It was noticed that the microcapsules were presented in variable geometric structures and a plain surface.
Laser Diffraction
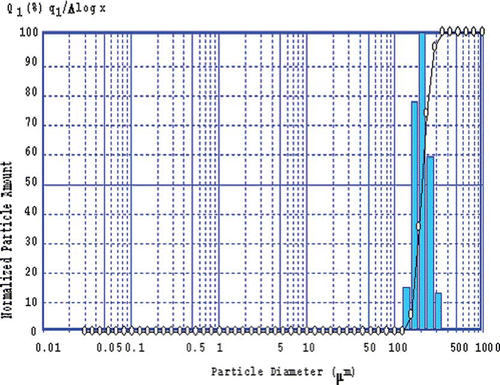
The particle size analysis by laser diffraction showed a mean size of 205.7 ± 0.09 μm (). This result proves that the present capsules can be considered microcapsules since their diameter is of micrometer size. The figure shows that the particles have very similar sizes, which means that the powder is very homogeneous.
CONCLUSIONS
Passion fruit juice was shown to be compatible with encapsulation using Capsul®, which is an inexpensive and easily obtained material having optimum properties for its use in the food industry. Capsul® doesn't alter the passion fruit taste or aroma, and is easily diluted in cold water after freeze-drying and storage. Freeze-drying of passion fruit juice encapsulated in Capsul® results in very stable microcapsules, in the form of a homogeneous white powder. Furthermore, this work has shown that Capsul® is an excellent means of preserving vitamin C and is capable of retaining almost all the vitamin C present in the samples during an extended long storage period. Once encapsulated, the passion fruit juice can be transported and stored in its powder form until reaching its destination for further industrialization without suffering any significant loss of nutritional properties. The obtained powder preserves a strong and pleasant smell of passion fruit and the juice is easy to be reconstituted. These parameters will be studied further by sensory analyses in order to test consumers' acceptance.
ACKNOWLEDGMENT
The authors wish to thank CNPq for financial support and a researcher's scholarship.
LITERATURE CITED
- Analytic Norms of the Adolfo Lutz Institute . 1985 . Normas Analíticas do Instituto Adolfo Lutz , 533 Brazil, p : São Paulo .
- Arburto , L.C. , Tavares , D.Q. and Martucci , E.T. 1998 . Microencapsulation of orange essential oil . Ciencia. Tecnol. Alime , 18 : 45 – 48 .
- Bakan , J.Á . 1973 . Microencapsulation of food and related products . Food. Technol.—Chicago , 27 ( 11 ) : 33 – 34 .
- Bastos , D.S. , Araújo , K.G.L. and Rocha-Leão , M.H.M. 2009 . Ascorbic acid retaining using a new calcium alginate—Capsul® based edible film . J. Microencapsulation , 26 : 97 – 103 .
- Dziezak , J.D. 1988 . Microencapsulation and encapsulated ingredients . Food. Technol.—Chicago , 2 : 36 – 51 .
- Embrapa (Brazilian Agricultural Research Corporation). 2011. Embrapa Cassava & Fruits. www.cnpmf.embrapa.br/index.php?p=pesquisa-culturas_pesquisadas-maracuja.phpandmenu=3 (http://www.cnpmf.embrapa.br/index.php?p=pesquisa-culturas_pesquisadas-maracuja.phpandmenu=3) (Accessed: 21 March 2011 ).
- Gharsallaoui , A.A. , Roudant , G. , Chambin , O. , Voilley , A. and Saurel , R. 2007 . Applications of spray-drying in microencapsulation of food ingredients: A review . Food. Res. Intl , 40 : 1107 – 1121 .
- Gouin , S. 2004 . Microencapsulation: Industrial appraisal of existing technologies and trends . Trends Food. Sci. Technol , 15 : 330 – 347 .
- IAC (Instituto Agronômico de Campinas). 1998. www.iac.sp.gov.br/UniPesquisa/Fruta/Frutiferas/Maracuja.asp (http://www.iac.sp.gov.br/UniPesquisa/Fruta/Frutiferas/Maracuja.asp) (Accessed: 21 March 2011 ).
- Jackson , L.S. and Lee , K. 1991 . Microencapsulation in the food industry . Lebensm. Wiss. Technol , 24 : 289 – 297 .
- Pothakamury , U.R. and Barbosa-Cánovas , G.V. 1995 . Fundamental aspects of controlled release in food . Trends. Food. Sci. Tech , 6 : 397 – 407 .
- Rain Tree Nutrition. 2011. Tropical Plant database—Passion flower. www.rain-tree.com/maracuja.htm (http://www.rain-tree.com/maracuja.htm) (Accessed: 21 March 2011 ).
- Ré , M.I. 1998 . Microencapsulation by spray-drying . Drying Technol , 16 : 1195 – 1236 .
- Risch , S.J. 1995 . “ Encapsulation: Overview of uses and techniques ” . In American Chemist's Society, Symposium Series 590 Edited by: Risch , S.J. and Reineccius , G.A. 2 – 7 . Washington, DC, p
- Shahidi , A. and Han , X.Q. 1993 . Encapsulation of food ingredients . Crit. Rev. Food. Sci , 33 : 501 – 547 .
- Sheu , T.Y. and Rosenberg , M. 1998 . Microstructure of microcapsules consisting of whey proteins and carbohydrates . J. Food. Sci , 63 : 491 – 494 .
- Souci , S.W. , Fachmann , W. and Kraut , H. 2008 . Food composition and nutrition tables , 7th , MedPharm Scientific Publishers, Stuttgart, Germany .
- Suntornsuk , L. , Gritsanapun , W. , Nilkamhank , S. and Paochom , A. 2001 . Quantification of vitamin C content in herbal juice using direct titration . J. Pharmaceut. Biomed , 1 : 849 – 855 .