ABSTRACT
This study investigates the growth and leaf composition of 2-year-old olive trees (Persian cultivars: Fishomi and Dezful and Greek cultivars: Amigdalolia and Conservolia) in response to withholding irrigation for 30 days under a hot and dry environment. Drought stress limited stem elongation, stem diameter expansion, formation of new leaves, leaf area, and specific leaf weight of the plants. However, the effect of drought on inhibition of growth of ‘Fishomi’ plants was higher than the other cultivars. ‘Dezful’, ‘Amigdalolia’ and ‘Conservolia’ were able to preserve higher levels of leaf relative water content and membrane stability index under drought stress. Higher drought tolerance of these plants was related to higher concentration of soluble carbohydrates, proline, potassium, and calcium in their leaves.
Introduction
Olive (Olea europaea L.) is traditionally known as a drought-tolerant species (Vitagliano and Sebastiani, Citation2002). Olive leaves can tolerate very low water potentials (as low as −8 MPa) and maintain their full rehydration capacity even after losing almost 40% of their water content (Rhizopoulou et al., Citation1991). Olive can sustain its normal rate of net photosynthesis until the leaf water potential drops as low as −6 MP (Zhu, Citation2001). Establishment of a gradient water potential in the leaves and roots of olive by osmoregulation facilitates water uptake even when the soil solution water potential falls to the extremities of drought, as low as −2.5 MPa (Vitagliano and Sebastiani, Citation2002). These capabilities enable olive trees to tolerate severe soil dehydration conditions (Celan et al., Citation1999).
Although olive can tolerate drought better than many of other fruit tree species, significant genotypic variations in olive drought tolerance have been reported (Bosabalidis and Kofidis, Citation2002). Some of these variations have reported to result from the differences in restricting leaf gas exchange (Angelopoulos et al., Citation1996), leaf anatomy characteristics (Bosabalidis and Kofidis, Citation2002), efficiency of antioxidant defense system (Sofo et al., Citation2005), stomatal control over water loss (Guerfel et al., Citation2009), and establishment of a low water potential in the plant (Dichio et al., Citation2006). The last mechanism is known as osmoregulation which helps olive to absorb water from drying soil for preserving protoplasm hydration under drought stress. This mechanism is crucial for preserving cell turgidity, which is essential for biological functions of leaves and the manner of root contact with soil (Zhu, Citation2001). Soluble carbohydrates (e.g., glucose and mannitol), as well as organic acids (e.g., malic acid and citric acid) and amino acids such as proline, are the major solutes which involve in osmoregulation in plants. In addition to the organic compounds, inorganic solutes may also involve in osmoregulation; however, there is limited information about the role of inorganic solutes in osmoregulation in olive trees. Accordingly, the current study was aimed at evaluating drought tolerance of four olive cultivars by investigating their growth, water content, and accumulation of organic and inorganic osmoprotectants in their leaves in response to withholding irrigation for 30 days.
Material and methods
The experiment was carried out in the Department of Horticultural Science at Shiraz University. Plant materials were obtained from four commercial olives cultivars including two Persian olives—‘Fishomi’ and ‘Dezful’—and two Greek cultivars—‘Amigdalolia’ and ‘Conservolia’. Monitoring the growth, flowering, and yield of these cultivars for 5 years had indicated that they are promising cultivars for development of olive in warm regions. However, since such areas are prone to drought, the drought tolerance of these plants was yet to be determined prior to extend their cultivation in warm climates.
Two-year-old rooted cuttings of these cultivars were transplanted into large pots, containing 12 kg of a mixture of soil, sand, and leaf mold (1:1:1). The physicochemical properties of the soil are shown in . The plants were established in a greenhouse with a day/night cycle of 30/22°C and 15% relative humidity for 60 days. Plants were pruned consistently throughout the establishment stage in order to obtain uniform shoots. Drought stress was imposed on the plants in the same environmental conditions as above, by withholding the irrigation for 30 days.
Table 1. The physicochemical characteristics of the soil used in the experiment.
Shoot length, stem diameter, and the number of fully expanded leaves of the plants were recorded twice during the experiment: at the beginning and at the termination of the drought stress period. Mean leaf area of the leaves from top half of the shoot was measured using a leaf area meter at the end of the drought period. Based on the data collected from the instances of initiation and termination of the stress period, the variations of these indices were calculated in relation to the start of the stress period. Specific leaf weight (SLW) was calculated as leaf dry weight/leaf area.
Relative water content (RWC) was determined at the end of the drought period. Fifteen uniform disks of fully developed young leaves were performed and the RWC was calculated by the following equation:
where FW is the fresh weight, DW is the dry weight of the leaf discs after drying in the oven at 75°C for 72 h, and TW is the turgid weight when re-hydration of the leaf discs occurred by soaking for 24 h in the dark.
Membrane stability index was determined according to the method described by Blum and Ebercon (Citation1981) with slight modifications. Fifteen leaf discs were thoroughly washed in deionized water and kept in 40ºC water for 30 min. After measuring the electrical conductivity (C1), the same samples were placed in boiling water bath for 10 min and their electrical conductivity was recorded as above (C2). MSI was calculated with the following equation:
Sulfosalicylic acid 3% was applied to 0.5 g of fully expanded young leaves in order to extract free proline which was later estimated via the ninhydrin reagent, according to the procedure described by Bates et al. (Citation1973). The absorbance of the fraction with toluene was determined at 520 nm, using a spectrophotometer.
To determine the concentration of soluble carbohydrates, 150 mg of dried leaf samples was extracted twice with ethanol. The samples were centrifuged at 3500 rpm for 10 min, and the volume of the supernatants was adjusted to 25 ml. Concentration of soluble carbohydrate was measured according to the method described by Buysee and Merckx (Citation1993). In summary, 1 mL of the supernatant was transferred to a test tube and 1 mL of phenol 18% was added along with 5 mL of sulfuric acid. The mixture was shaken immediately and its absorption was recorded at 490 nm using a spectrophotometer. Starch content of the residual material was determined with Anthron reagent (McCready et al., Citation1950). In this regard, 5 mL cold water and 6.5 mL perchloric acid 52% were added to the samples and the mixtures were shacked for 15 min. After adding 20 mL of water, the samples were centrifuged. The supernatants were separated and the procedure was repeated with the precipitate. 2.5 mL of cold Anthron solution 2% was added, and the samples was heated at 100°C for 7.5 min. The absorbance of the samples was measured at 630 nm by spectrophotometry.
To determine concentration of potassium (K+) and calcium (Ca2+) ions in the leaves, oven-dried plant materials were ground to a fine powder. A mass of 0.5 g dry samples was ashed at 500°C for 8 h and extracted with 5 mL 2N hydrochloric acid (HCl). After digestion, volume of the samples was made up to 100 mL with distilled deionized water (Tandon, Citation1998). Concentration of K+ was estimated by flame photometry (Jenway PFP7, ELE Instrument Co. Ltd.) and Ca2+ was measured by atomic absorption spectroscopy (Varian 220, Australia).
The experiment was conducted as a complete randomized design with factorial arrangements and four replications. Analysis of variance was performed using the SPSS software and means were compared by Duncan’s multiple range test at P ≤ 0.05.
Results
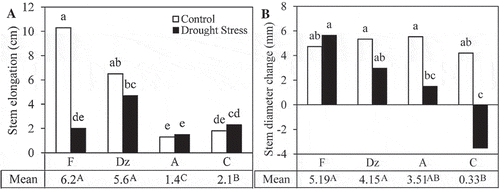
Shoot length growth and stem diameter expansion were significantly influenced by drought stress, the type of cultivars, and their interactive effect. Generally, stem elongation of drought-stressed plants was 42% lower than the irrigated plants. Under control treatment, the stem elongation of ‘Fishomi’ and ‘Dezful’ was significantly higher than that in ‘Amigdalolia’ and ‘Conservolia’. Drought stress significantly reduced stem elongation of ‘Fishomi’ (). Under drought stress, the highest increase in the shoot length was observed in ‘Dezful’ (4.7 m), and the lowest was found in ‘Amigdalolia’ (1.5 cm). Drought stress significantly reduced stem diameter expansion by 76.5%. Stem diameter growth of the stressed plants was significantly higher in ‘Fishomi’ and ‘Dezful’, and the lowest values were found in ‘Amigdalolia’ and ‘Conservolia’ (). Stem diameter of drought-stressed ‘Conservolia’ was 3.5 mm lower than that in the beginning of drought-stress period.
Figure 1. The effect of drought stress on (A) shoot length and (B) leaf number changes of olive cultivars: Fishomi (F), Dezful (Dz), Amigdalolia (A), Conservolia (C). Means (n = 4) with different letters are significantly different at 5% level of the Duncan’s multiple range test. Upper case letters indicate significant differences between the cultivars.
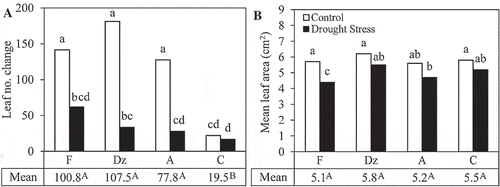
Drought stress significantly reduced the formation of new leaves in ‘Fishomi’, ‘Dezful’, and ‘Amigdalolia’ cultivars (). Leaf number changes of the cultivars were similar under drought stress. Furthermore, drought stress caused a significant reduction in the mean leaf area of ‘Fishomi’ (). Mean leaf area of the other cultivars remained unchanged under drought stress.
Figure 2. The effect of drought stress on (A) leaf number changes and (B) area of fully expanded leaves of olive cultivars: Fishomi (F), Dezful (Dz), Amigdalolia (A), Conservolia (C). Means (n = 4) with different letters are significantly different at 5% level of the Duncan’s multiple range test. Upper case letters indicate significant differences between the cultivars.
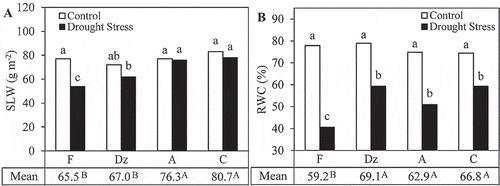
‘Conservolia’ and ‘Amigdalolia’ had the highest SLW among the olive cultivars (). SLW of ‘Fishomi’ plants significantly reduced under drought stress. However, SLW of the other cultivars was not affected by drought stress. Leaf RWC of the olive cultivars significantly reduced under drought stress. At the end of the drought period, RWC of ‘Fishomi’ was significantly lower than the RWC of other cultivars ().
Figure 3. The effect of drought stress on (A) specific leaf weight (SLW) and (B) relative water content (RWC) in the leaves of olive cultivars: Fishomi (F), Dezful (Dz), Amigdalolia (A), Conservolia (C). Means (n = 4) with different letters are significantly different at 5% level of the Duncan’s multiple range test. Upper case letters indicate significant differences between the cultivars.
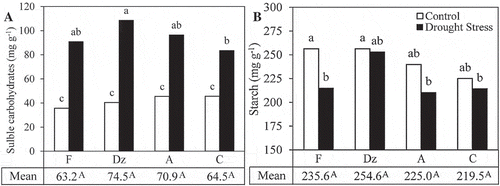
MSI significantly reduced under drought stress. Among the drought-stressed plants, ‘Fishomi’ had the lowest MSI (). No significant difference was observed in MSI of the other genotypes. Drought stress significantly increased leaf proline concentration in the leaves of ‘Amigdalolia’ and ‘Conservolia’ (). Under drought condition, the highest proline concentration was observed in the leaves of ‘Conservolia’ (157.8 µmol g−1) and the lowest value was found in the leaves of ‘Fishomi’ (116.8 µmol g−1). Concentration of soluble carbohydrates and starch content in the leaves were affected by the drought-stress treatment. Drought stress significantly increased the concentration of soluble carbohydrates in the leaves of the plants (). Among the drought-stressed plants, ‘Dezful’ had the highest and ‘Conservolia’ had the lowest concentration of soluble carbohydrates in their leaves. Drought stress significantly affected starch content in the leaves of the plants. Starch content significantly decreased in the leaves of ‘Fishomi’, ‘Amigdalolia’, and ‘Conservolia’ under drought stress. However, it remained unchanged in the leaves of drought-stressed ‘Dezful’ plants ().
Figure 4. The effect of drought stress on (A) membrane stability index (MSI) and (B) concentration of proline in leaves of olive cultivars: Fishomi (F), Dezful (Dz), Amigdalolia (A), Conservolia (C). Means (n = 4) with different letters are significantly different at 5% level of the Duncan’s multiple range test. Upper case letters indicate significant differences between the cultivars.
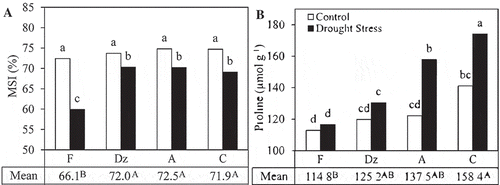
Figure 5. The effect of drought stress on (A) concentration of soluble carbohydrates and (B) starch content in the leaves of olive cultivars: Fishomi (F), Dezful (Dz), Amigdalolia (A), Conservolia (C). Means (n = 4) with different letters are significantly different at 5% level of the Duncan’s multiple range test. Upper case letters indicate significant differences between the cultivars.
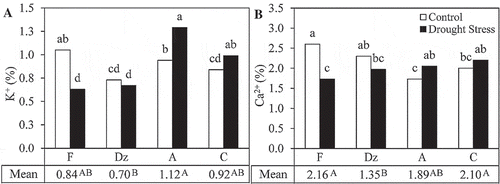
Drought stress significantly increased K+ and Ca2+ concentrations in the leaves of ‘Amigdalolia’ and ‘Conservolia’ and decreased their concentration in the leaves of ‘Fishomi’ (). In sum, the lowest concentrations of K+ and Ca2+ were detected in the leaves of drought-stressed ‘Fishomi’ plants. ‘Amigdalolia’ and ‘Conservolia’ had the highest leaf K+ and Ca2+ concentrations under drought stress.
Figure 6. The effect of drought stress on concentrations of (A) K+ and (B) Ca2+ in the leaves of olive cultivars: Fishomi (F), Dezful (Dz), Amigdalolia (A), Conservolia (C). Means (n = 4) with different letters are significantly different at 5% level of the Duncan’s multiple range test. Upper case letters indicate significant differences between the cultivars.
Discussion
The plants’ growth inhibition (limitation of stem elongation, stem diameter expansion, formation of new leaves, leaf area, and SLW) under drought stress was primarily due to decline in plant water content (Burton et al., Citation1998). According to decline of leaf RWC under drought stress, loss of turgor and limitation of cell elongation in the apex and in the developing leaves of the olive cultivars were expected (Ings et al., Citation2013). Compared to its group under normal irrigation condition, the shoot growth of drought-stressed ‘Dezful’, ‘Conservolia’, and ‘Amigdalolia’ was less affected by drought stress. Since SLW is known to be positively correlated with plant growth rate, the higher SLW of ‘Conservolia’ and ‘Amigdalolia’ confirms their higher vigor under drought stress (Osone et al., Citation2008; Scheepens et al., Citation2010). These growth responses indicated that olive ‘Fishomi’ is more drought prone than the other cultivars.
Under drought stress, stem diameter of ‘Conservolia’ and ‘Amigdalolia’ was lower than those in the other cultivars. Monitoring the stem diameter dynamics is used for evaluation of water status of plants. Ohashi et al. (Citation2006) stated that stem diameter is dependent on water status in plants and may be reduced under water stress when transpiration from leaves exceed the supply of water by roots. However, in the current study, lower stem diameter was associated with higher leaf RWC of ‘Conservolia’ and ‘Amigdalolia’. This observation suggest that these cultivars have higher ability to conduct water to their leaves under reduced water supply from the growing medium and preserve leaf RWC under this situation.
Water conservation in the plant leaves is mainly regulated by limitation of stomatal conductance, reducing plant leaf number, formation of small leaves on plant, and accumulation of osmoprotectants in the leaves (Chakhchar et al., Citation2015; Karimi et al., Citation2015). Drought stress reduced the total leaf area of the plants by reducing the leaf number and average leaf area. Among the olive cultivars, drought stress mainly restricted leaf expansion of ‘Fishomi’. Leaf expansion depends on leaf turgor, temperature, and the assimilating supply for growth (Anjum et al., Citation2011b). According to Hsiao (Citation1973), limitation of leaf area expansion under drought stress is an adaptive response for reducing water loss; however, as a side effect, decrease in green surface of plant limits plant photosynthetic capacity. Therefore, the significant decrease in leaf area and photoassimilation of ‘Fishomi’ could be the secondary cause of its growth inhibition under drought stress.
Drought-tolerant plants are able to preserve higher levels of RWC under drought stress (Karimi et al., Citation2015). Therefore, leaf RWC is widely used as a reliable index for screening drought-tolerant genotypes (Silva et al., Citation2007). In agreement with our results, a decrease in the RWC in response to drought stress has been noted in a broad spectrum of plants (Nayyar and Gupta, Citation2006). Stomatal closure is an early response to water-limited conditions and an efficient way to preserve water under this situation; on the other hand, it results in photosynthesis inhibition due to heat accumulation and limitation of CO2 diffusion in the leaves (Karimi et al., Citation2015). In this study, the decrease in SLW in parallel with the decline of leaf RWC confirmed a lower assimilation efficiency under water limitation stress. Similar responses have been reported in other species under drought and osmotic stress (Karimi et al., Citation2012a; Liu and Stützel, Citation2004). Higher SLW under drought stress is preferable, since it indicates higher leaf productivity under reduced water availability. Accordingly, the significant decline of SLW in ‘Fishomi’ confirmed that this cultivar is more drought prone than the other cultivars. Malfunction of photosynthesis due to limitation of CO2 concentration in the leaves results in overproduction of reactive oxygen species (ROS) in the leaves (Sircelj et al., Citation2007). If the plant defense system fails to detoxify the ROS, the oxidative damage symptoms (e.g., chlorophyll degradation, lipid peroxidation, and protein oxidation) will be inevitable (Tausz et al., Citation2001). Plasma membrane is of the primary targets of ROS. Peroxidation of plasma membrane lipids disrupts the functional integrity of cell membrane and results in cellular leakage (Tavallali et al., Citation2017). In this study, MSI reduced in parallel with leaf dehydration, as the lowest MSI was observed in the leaves of ‘Fishomi’ which had the lowest RWC. In addition to peroxidation of plasma membrane lipids, the reduced MSI under drought stress could partially be attributed to the significant reduction in Ca2+ concentration in the leaves of drought-sensitive genotypes (Marschner, Citation2011). In this regard, Ma et al. (Citation2009) stated that Ca2+ ions by improving the hydrophobicity of the plasma membrane and reducing its permeability play an essential role in plant drought tolerance.
Plants managed to produce and accumulate the compatible solutes to improve water absorption and preserving hydration of protoplast under drought stress (Zhu, Citation2001). This response, that is known as osmoregulation, can retard leaf senescence and improve plant performance under drought stress (Demiral and Türkan, Citation2006; Karimi et al., Citation2012b). In this experiment, accumulation of organic solutes such as soluble carbohydrates and proline was observed in the leaves of drought-stressed olive cultivars. Proline is the major osmoprotectant which accumulates in plants under different environmental stresses (Chakhchar et al., Citation2015; Rahemi et al., Citation2017; Zhu, Citation2001). Proline accumulation is the first response of dehydrating tissues which is aimed to reduce injury to the cells (Sofo et al., Citation2004). Proline can act as a signaling molecule to modulate mitochondrial functions, influence cell proliferation or cell death, and trigger specific gene expression, which can be essential for plant recovery from stress (Anjum et al., Citation2011a). In many plant species, proline accumulation has been correlated with stress tolerance (Karimi et al., Citation2012a, Citation2009; Sivritepe et al., Citation2008). In this study, the increase in proline was in parallel with higher levels of RWC and MSI in the leaves of drought-stressed ‘Amigdalolia’ and ‘Conservolia’ plants. These observations suggest that proline accumulation is crucial for olive drought tolerance. Proline maintains membrane integrity under dehydration stress and reduces the oxidation of lipid membranes, besides preventing photo-inhibition (Ashraf and Foolad, Citation2007; Bacelar et al., Citation2006; Demiral and Turkan, Citation2004; Solomon et al., Citation2006; Verslues et al., Citation2006). However, some studies have suggested that proline accumulation in plant can be considered as a general response to abiotic stresses and indicates stress pressure on plant (Karimi et al., Citation2017).
Soluble carbohydrates also involve in osmoregulation under abiotic stress situations (Chakhchar et al., Citation2015; Karimi et al., Citation2009). Accumulation of soluble carbohydrates under water stress can be related to their lower exchange rate from the leaves, lower consumption due to growth limitation, and other changes such as hydrolyzing reserved carbohydrates (Sanchez et al., Citation1998; Kameli and Losel, Citation1996). Soluble carbohydrates as osmoprotectants may substitute with water by means of hydrogen linkage and by establishing bonds with proteins and the membranes and provide protection against dehydration or peroxidation (Bacelar et al., Citation2004). Our results indicated that accumulation of soluble carbohydrate in drought-stressed olives is related to the starch hydrolysis and reduced the growth rate of the plants. The highest concentration of soluble carbohydrates was found in the leaves of drought-stressed ‘Dezful’, which had relatively low concentration of proline. However, RWC and MSI of the leaves of ‘Dezful’ were similar to those in the leaves of ‘Amigdalolia’ and ‘Conservolia’ under drought stress. These results suggested that in plants with intermediate proline concentration, accumulation of high concentration of soluble carbohydrates in the leaves improves the plants drought tolerance.
Inorganic salutes also may be involved in osmoregulation under environmental stresses (Chakhchar et al., Citation2017). K+ cations involve in osmoregulation in cell, water transport in plant, and regulation of stomatal movement (Raza et al., Citation2014). Therefore, the role of K+ in improving plant drought tolerance is not surprising (Wang et al., Citation2014). K+ absorption from soil and transportation in plant mainly depend on water availability in soil (Oliveira et al., Citation2010). K+ concentration reduced in the leaves of drought-stressed ‘Fishomi’ plants, which was considered to be related to their higher drought susceptibility. These responses suggest that during water stress, K+ is required to involve in osmotic adjustment in shoot of olive. These results are in accordance with previous investigations (Chakhchar et al., Citation2017; Huang, Citation2001). Eakes et al. (Citation1991) also emphasized on the role of K+ accumulation in active osmotic adjustment in plants subjected to drought stress. Similar to K+, concentration of Ca2+ reduced in the leaves of drought-stressed ‘Fishomi’ plants. Accumulation of Ca2+ in the leaves of drought-tolerant genotypes is required for osmoregulation (Chakhchar et al., Citation2017; Huang, Citation2001). Since Ca2+ is mainly transported in the soil and plant via mass flow, the significant decrease of Ca2+ in the leaves of ‘Fishomi’ probably indicates a lower efficiency of its roots in water and mineral acquisition from the drying soil. These results suggest that high concentration of K+ and Ca2+ in the leaves probably is a drought-tolerance mechanism of olive.
In conclusion, accumulation of proline and inorganic osmolytes was found to be crucial for preserving RWC and MSI in the leaves of olive under drought stress. Soluble carbohydrates also were found that can enhance the plant drought tolerance in the presence of intermediate-to-high concentrations of proline in the leaves. Plants with the ability of preserving water showed better growth and performance under drought stress. In sum, the olives ‘Dezful’, ‘Amigdalolia’, and ‘Conservolia’ were clarified to be more drought tolerant than ‘Fishomi’ and can be suggested for the development of olive cultivation in drought-prone areas with mild and short winters.
Disclosure statement
The authors declare that there is no conflict of interest.
Additional information
Funding
References
- Angelopoulos, K., B. Dichio, and C. Xiloyannis. 1996. Inhibition of photosynthesis in olive trees (Olea europaea L.) during water stress and rewatering. J. Exp. Bot 47(8):1093–1100. doi: 10.1093/jxb/47.8.1093.
- Anjum, S.A., L.C. Wang, M. Farooq, I. Khan, and L.L. Xue. 2011a. Methyl jasmonate-induced alteration in lipid peroxidation, antioxidative defense system and yield in soybean under drought. J. Agron. Crop. Sci 197:296–301. doi: 10.1111/j.1439-037X.2011.00468.x.
- Anjum, S.A., X. Xie, L.C. Wang, M.F. Saleem, C. Man, and W. Lei. 2011b. Morphological, physiological and biochemical responses of plants to drought stress. Afric. J. Agric. Res 6(9):2026–2032. doi: 10.5897/AJAR10.027.
- Ashraf, M., and M.R. Foolad. 2007. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot 59:206–216. doi: 10.1016/j.envexpbot.2005.12.006.
- Bacelar, E., C. Correia, D. Santos, J. Moutinho-Pereira, B. Goncalves, T.E. Ferreira, and J.M. Torres-Pereira. 2004. Leaf gas exchange, chlorophyll fluorescence and oxidative damage in olive trees under two irrigation regimes. Acta. Physiol. Plant 26(3):172.
- Bacelar, E.A., D.L. Santos, J.M. Moutinho-Pereir, C.G. Berta, H. Alves, F. Ferreira, and C.M. Correia. 2006. Immediate responses and adaptative strategies of three olive cultivars under contrasting water availability regimes: changes on structure and chemical composition of foliage and oxidative damage. Plant Sci 170:596–605. doi: 10.1016/j.plantsci.2005.10.014.
- Bates, L.S., R.P. Waldren, and I.D. Teare. 1973. Rapid determination of free proline for water-stress. Plant Soil 39:205–207. doi: 10.1007/BF00018060.
- Blum, A., and E. Ebercon. 1981. Cell membrane stability as a measure of drought and heat tolerance in wheat. Crop Sci 21:43–47. doi: 10.2135/cropsci1981.0011183X002100010013x.
- Bosabalidis, A.M., and G. Kofidis. 2002. Comparative effects of drought stress on leaf anatomy of two olive cultivars. Plant Sci 163(2):375–379. doi: 10.1016/S0168-9452(02)00135-8.
- Burton, A.J., K.S. Pregitzer, G.P. Zogg, and D.R. Zak. 1998. Drought reduces root respiration in sugar maple forests. J. App. Ecol 8:771–778. doi: 10.1890/1051-0761(1998)008[0771:DRRRIS]2.0.
- Buysee, J., and R. Merckx. 1993. An improved colorimetric method to quantify sugar content of plant tissue. J. Exp. Bot 44:1627–1629. doi: 10.1093/jxb/44.10.1627.
- Celan, G., B. Dichio, G. Montanaro, V. Nuzzo, A.M. Palese, and C. Xiloyannis. 1999. Distribution of dry matter and amount of mineral elements in irrigated and non-irrigated olive trees. Acta Hortic 474:381–384. doi: 10.17660/ActaHortic.1999.474.79.
- Chakhchar, A., M. Lamaoui, S. Aissam, A. Ferradous, S. Wahbi, A.E. Mousadik, S. Ibnsouda-Koraichi, A. Filali-Maltouf, and C. El Modafar. 2017. Electrolyte ions and glutathione enzymes as stress markers in Argania spinosa subjected to drought stress and recovery. Afric. J. Biotechnol 16(1):10–21. doi: 10.5897/AJB2016.15234.
- Chakhchar, A., M. Lamaoui, S. Wahbi, A. Ferradous, A. El Mousadik, S. Ibnsouda- Koraichi, A. Filali-Maltouf, and C.E. Modafar. 2015. Leaf water status, osmoregulation and secondary metabolism as a model for depicting drought tolerance in Argania spinosa. Acta Physiol. Planta 37(4):80–96. doi: 10.1007/s11738-015-1833-8.
- Demiral, T., and I. Turkan. 2004. Does exogenous glycinebetaine affect antioxidative system of rice seedlings under NaCl treatment? J. Plant Physiol. 161:1089–1110. doi: 10.1016/j.jplph.2004.03.009.
- Demiral, T., and I. Türkan. 2006. Exogenous glycinebetaine affects growth and proline accumulation and retards senescence in two rice cultivars under NaCl stress. Environ. Exp. Bot 56(1):72–79.2. doi: 10.1016/j.envexpbot.2005.01.005.
- Dichio, B., C. Xiloyannis, A. Sofo, and G. Montanaro. 2006. Osmotic regulation in leaves and roots of olive trees during a water deficit and rewatering. Tree Physiol 26(2):179–185. doi: 10.1093/treephys/26.2.179.
- Eakes, D.J., R.D. Wright, and R. Seiler. 1991. Potassium nutrition and moisture stress tolerance of salvia. Hortic. Sci 26:422.
- Guerfel, M., O. Baccouri, D. Boujnah, W. Chaïbi, and M. Zarrouk. 2009. Impacts of water stress on gas exchange, water relations, chlorophyll content and leaf structure in the two main Tunisian olive (Olea europaea L.) cultivars. Sci. Hortic 119(3):257–263. doi: 10.1016/j.scienta.2008.08.006.
- Hsiao, T.C. 1973. Plant responses to water stress. Ann. Rev. Plant Physiol 24:519–570. doi: 10.1146/annurev.pp.24.060173.002511.
- Huang, B. 2001. Nutrient accumulation and associated root characteristics in response to drought stress in tall fescue cultivars. HortScience 36(1):148–152.
- Ings, J., L.A. Mur, P.R. Robson, and M. Bosch. 2013. Physiological and growth responses to water deficit in the bioenergy crop Miscanthus × giganteus. Front. Plant Sci 4:468–475. doi: 10.3389/fpls.2013.00468.
- Kameli, A., and D.M. Losel. 1996. Growth and sugar accumulation in durum wheat plants under water stress. New. Phytol 132:57–62. doi: 10.1111/j.1469-8137.1996.tb04508.x.
- Karimi, S., S. Eshghi, S. Karimi, and S. Hasan-Nezhadian. 2017. Inducing salt tolerance in sweet corn by magnetic priming. Acta Agric. Slov 109(1):89–102. doi: 10.14720/aas.2017.109.1.09.
- Karimi, S., S. Hojati, S. Eshghi, R.N. Moghaddam, and S. Jandoust. 2012a. Magnetic exposure improves tolerance of fig ‘Sabz’ explants to drought stress induced in vitro. Sci. Hortic 137:95–99. doi: 10.1016/j.scienta.2012.01.018.
- Karimi, S., M. Rahemi, S. Eshghi, M. Maftoun, and V. Tavallali. 2009. Effects of long-term salinity on growth and performance of two pistachio (Pistacia vera L.) rootstocks. Aust. J. Basic. App. Sci 3(3):1630–1639.
- Karimi, S., A. Yadollahi, K. Arzani, and A. Imani. 2015. Gas exchange response of almond genotypes to water stress. Photosynthetica 53(1):29–34. doi: 10.1007/s11099-015-0070-0.
- Karimi, S., A. Yadollahi, R.A. Nazari-Moghadam, A. Imani, and K. Arzani. 2012b. In vitro screening of almond (Prunus dulcis (Mill.)) genotypes for drought tolerance. J. Biol. Environ. Sci 6(18):263–270.
- Liu, F., and H. Stützel. 2004. Biomass partitioning, specific leaf area, and water use efficiency of vegetable amaranth (Amaranthus spp.) in response to drought stress. Sci. Hortic 102:15–27. doi: 10.1016/j.scienta.2003.11.014.
- Ma, Y.Y., W.Y. Song, Z.H. Liu, H.M. Zhang, X.L. Guo, H.B. Shao, and F.T. Ni. 2009. The dynamic changing of Ca2+ cellular localization in maize leaflets under drought stress. Comp. Rend. Boil 332(4):351–362. doi: 10.1016/j.crvi.2008.12.003.
- Marschner, H. 2011. Marschner’s mineral nutrition of higher plants. Academic Press, USA. isbn 9780123849052.
- McCready, R.M., J. Guggolz, V. Silviera, and H.S. Owens. 1950. Determination of starch and amylase in vegetables. Analyt. Chem 22:1156–1158. doi: 10.1021/ac60045a016.
- Nayyar, H., and D. Gupta. 2006. Differential sensitivity of C3 and C4 plants to water deficit stress: association with oxidative stress and antioxidants. J. Biol. Environ. Exp. Bot 58:106–113. doi: 10.1016/j.envexpbot.2005.06.021.
- Ohashi, Y., N. Nakayama, H. Saneoka, and K. Fujita. 2006. Effects of drought stress on photosynthetic gas exchange, chlorophyll fluorescence and stem diameter of soybean plants. Biol. Plant 50(1):138–141. doi: 10.1016/j.envexpbot.2005.06.021.
- Oliveira, E.M.M., H.A. Ruiz, V.H. Alvarez, P.A. Ferreira, F.O. Costa, and I.C.C. Almeida. 2010. Nutrient supply by mass flow and diffusion to maize plants in response to soil aggregate size and water potential. Rev. Brasil. Ciên. Solo 34:317–327. doi: 10.1590/S0100-06832010000200005.
- Osone, Y., A. Ishida, and M. Tateno. 2008. Correlation between relative growth rate and specific leaf area requires associations of specific leaf area with nitrogen absorption rate of roots. New. Phytol 179(2):417–427. doi: 10.1111/j.1469-8137.2008.02476.x.
- Rahemi, M., S. Karimi, S. Sedaghat, and A.A. Rostami. 2017. Physiological responses of olive cultivars to salinity stress. Adv. Hortic. Sci 31(1):53–59. doi: 10.13128/ahs-20726.
- Raza, M.A.S., M.F. Saleem, G.M. Shah, I.H. Khan, and A. Raza. 2014. Exogenous application of glycinebetaine and potassium for improving water relations and grain yield of wheat under drought. J. Soil. Sci. Plant Nutr 14:348–364.
- Rhizopoulou, S., D. Meletiou-Christou, and S. Diamantoglou. 1991. Water relation for sun and shade leaves of four Mediterranean evergreen sclerophylls. J. Exp. Bot 42:627–635. doi: 10.4067/S0718-95162014005000028.
- Sanchez, F.J., M. Manzanares, E.F. De Andres, J.L. Tenorio, and L. Ayerbe. 1998. Turgor maintenance, osmotic adjustment and soluble sugar and proline accumulation in 49 pea cultivars in response to water stress. Field. Crop. Res 59:225–235. doi: 10.1016/S0378-4290(98)00125-7.
- Scheepens, J.F., E.S. Frei, and J. Stöcklin. 2010. Genotypic and environmental variation in specific leaf area in a widespread Alpine plant after transplantation to different altitudes. Oecologia 164(1):141–150. doi: 10.1007/s00442-010-1650-0.
- Silva, M.D.A., J.L. Jifon, J.A. Da Silva, and V. Sharma. 2007. Use of physiological parameters as fast tools to screen for drought tolerance in sugarcane. Braz. J. Plant Physiol. 19(3):193–201. doi: 10.1590/S1677-04202007000300003.
- Sircelj, H., M. Tausz, D. Grill, and F. Batic. 2007. Detecting different level of drought stress in apple trees (Malus domestica Borkh) with selected biochemical and physiological Parameters. Sci. Hortic 113:362–369. doi: 10.1016/j.scienta.2007.04.012.
- Sivritepe, N., U. Erturk, C. Yerlikaya, I. Turkan, M. Bor, and F. Ozdemir. 2008. Response of the cherry rootstock to water stress induced in vitro. Biol. Plant 52:573–576. doi: 10.1007/s10535-008-0114-4.
- Sofo, A., B. Dichio, C. Xiloyannis, and A. Masia. 2004. Effects of different irradiance levels on some antioxidant enzymes and on malondialdehyde content during rewatering in olive tree. Plant Sci 166:293–302. doi: 10.1016/j.plantsci.2003.09.018.
- Sofo, A., B. Dichio, C. Xiloyannis, and A. Masia. 2005. Antioxidant defenses in olive trees during drought stress: changes in activity of some antioxidant enzymes. Funct. Plant Biol 32(1):45–53. doi: 10.1071/FP04003.
- Solomon, A., S. Golubowicz, S. Yablowicz, S. Grossman, M. Bergman, and H.F. Gottlieb. 2006. Antioxidant activities and anthocyanin content of fresh fruits of common fig (Ficus carica L.). J. Agri. Food Chem 54:7717–7723. doi: 10.1021/jf060497.
- Tandon, H.L.S. 1998. Methods of analysis of soils, plants, waters and fertilizers. Fertilizer Development and Consultation Organization, New Delhi, India. doi: 10.1007/s10661-005-9133-1.
- Tausz, M., A. Wonisch, J. Peters, M.S. Jimenez, D. Morales, and D. Grill. 2001. Short-term changes in free radical scavengers and chloroplast pigments in Pinus canariensis needles as affected by mild drought stress. J. Plant Physiol 158:213–219. doi: 10.1078/0176-1617-00178.
- Tavallali, V., S. Karimi, and O. Espargham. 2017. Boron enhances antioxidative defense in the leaves of salt-affected Pistacia vera seedlings Hortic. J. doi: 10.2503/hortj.OKD-062.
- Verslues, P.E., M. Agaraal, S. Katiyar-Agaraal, and J. Zhu. 2006. Methods and concepts in quantifying resistance to drought salt and freezing, abiotic stressed that affect plant status. Plant J 45:523–539. doi: 10.1111/j.1365-313X.2005.02593.x.
- Vitagliano, C., and L. Sebastiani. 2002. Physiological and biochemical remarks on environmental stress in olive (Olea europaea L.). Acta Hortic 586:435–440. doi: 10.17660/ActaHortic.2002.586.89.
- Wang, X., I. Mohamed, Y. Xia, and F. Chen. 2014. Effects of water and potassium stresses on potassium utilization efficiency of two cotton genotypes. J. Soi. Sci. Plant Nutr 14(4):833–844.
- Zhu, J.K. 2001. Plant salt tolerance. Trend. Plant Sci 6:66–71. doi: 10.1016/S1360-1385(00)01838-0.
