Abstract
Degradation of autophagic vacuoles (AVs) via lysosomes is an important homeostatic process in cells. Neurons are highly polarized cells with long axons, thus facing special challenges to transport AVs generated at distal processes toward the soma where mature acidic lysosomes are relatively enriched. We recently revealed a new motor-adaptor sharing mechanism driving autophagosome transport to the soma. Late endosome (LE)-loaded dynein-SNAPIN motor-adaptor complexes mediate the retrograde transport of autophagosomes upon their fusion with LEs in distal axons. This motor-adaptor sharing mechanism enables neurons to maintain effective autophagic clearance in the soma, thus reducing autophagic stress in axons. Therefore, our study reveals a new cellular mechanism underlying the removal of distal AVs engulfing aggregated misfolded proteins and dysfunctional organelles associated with several major neurodegenerative diseases.
Autophagy is an important cellular degradative process to eliminate defective organelles and aggregated proteins over a neuron's lifetime. Autophagy undergoes stepwise maturation: bulk cytoplasmic components and organelles are engulfed within double-membrane organelles termed autophagosomes, followed by fusion with LEs into hybrid organelles called amphisomes, or fusion with lysosomes into autolysosomes for degradation. Newly formed autophagosomes in distal axons are transported to the soma for degradation. Therefore, the efficient retrograde transport of autophagosomes is critical to maintain axonal homeostasis. However, it is unknown how nascent autophagosomes acquire their retrograde motility. Although dynein motors were suggested to drive AV retrograde transport, a mechanistic question remains: How are dynein motors recruited to newly formed autophagosomes? Addressing this fundamental issue will advance our understanding of several neurodegenerative diseases associated with autophagic stress in distal axons and at synapses.
First, we determined whether autophagosomes or amphisomes are the predominant AVs moving in a retrograde direction in axons. Because mature acidic lysosomes are mainly enriched in the soma, we speculated that autophagosomes in distal axons undergo fusion with LEs to form amphisomes, and then transport toward the soma. We tested our hypothesis in live dorsal root ganglion (DRG) neurons transfected with the autophagy marker GFP-LC3 and LE marker monomeric RFP (mRFP)-RAB7. Upon starvation, almost all GFP-LC3-labeled AVs (97.62% ± 0.65) colocalize with LEs, indicating their amphisomic nature following fusion with LEs. Those amphisomes share a retrograde motility pattern similar to that of LEs, displaying predominantly retrograde transport from distal axons toward the soma. These observations prompted us to propose that autophagosomes acquire their retrograde motility by sharing LE-loaded dynein motors upon their fusion to generate amphisomes ().
Figure 1. The motor-adaptor sharing model enabling axonal autophagosomes to be transported from distal axons toward the soma through fusion with LEs. (A) Autophagosomes gain the LE-loaded dynein motor complexes they need to move away from axonal terminals by fusing with LEs to form intermediate organelles known as amphisomes. SNAPIN serves as an adaptor of the dynein motor by interacting with dynein DNAI and attaching the motor to LEs. SNAPIN-dynein complexes drive long-distance transport of amphisomes from distal processes to the soma, where mature acidic lysosomes are relatively enriched. Such a mechanism enables neurons to efficiently reduce autophagic stress in distal axons and at synapses, thus maintaining axonal homeostasis. (B) Blocking dynein recruitment to LEs by disrupting dynein-SNAPIN coupling impairs the movement of amphisomes toward the cell body. (C) Reducing the ability of autophagosomes to fuse with LEs results in aberrant accumulation of immobile autophagic compartments in axon terminals.
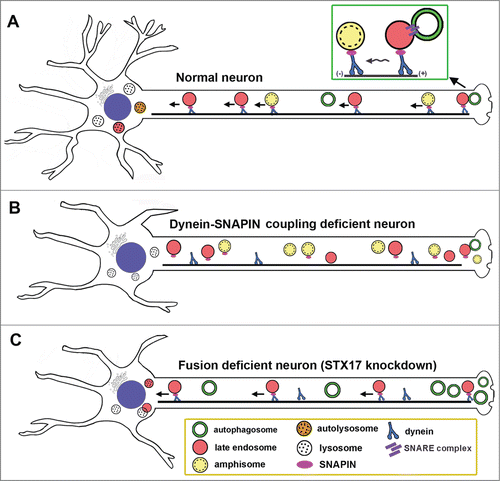
Next, we asked whether LE-loaded dynein-adaptor complexes drive amphisome trafficking. Dynein is the primary motor for retrograde transport of LEs and AVs. Our previous study reveals that SNAPIN acts as a dynein adaptor by binding DNAI/DIC (dynein, axonemal, intermediate chain) and dynein-SNAPIN coupling recruits dynein motors to LEs, thus mediating LE retrograde transport in neurons. We examined the motility of amphisomes after disrupting the dynein-SNAPIN coupling. Expressing SNAPINL99K, a dominant-negative mutant defective in DNAI binding, in DRG neurons significantly reduced retrograde transport of both amphisomes and LEs along the same axons. With transmission electron microscopy (TEM), we detected a striking accumulation of double-membrane AVs within snapin−/− neurites and presynaptic terminals; these AV-like structures were rarely observed in wild-type neurons. These results suggest that LE-loaded dynein-SNAPIN complexes drive the retrograde transport of amphisomes. Deficiency in the dynein-SNAPIN coupling thus impairs the retrograde transport of axonal amphisomes ().
We further tested our hypothesis that autophagosomes acquire retrograde motility through fusion with LEs. It is critical to choose a molecular tool that specifically retains autophagosomes by blocking their fusion with LEs. Recent studies established that STX17 (syntaxin 17) mediates the fusion of autophagosomes with endolysosomal organelles by forming a SNARE fusion complex with SNAP29 and VAMP8. Unlike SNAP29 and VAMP8, which associate with LEs and lysosomes, STX17 targets autophagosomes so that STX17 knockdown would have less impact on endolysosomal trafficking. By applying 2 alternative Stx17-targeted siRNAs combined with rescue experiments using a siRNA-resistant Stx17 silent mutant, we consistently found that depleting STX17 in DRG neurons effectively blocks formation of axonal amphisomes upon starvation: there was a robust increase in the number of autophagosomes and a decrease in amphisomes in axons relative to control neurons (P < 0.001). Surprisingly, almost all autophagosomes (96.93%) are stationary where the fusion is blocked by STX17 knockdown, while LEs moved, passing through stationary AV clusters along the same axons. Thus, our study provides compelling evidence that autophagosomes recruit dynein motors upon their fusion with LEs.
Intriguingly, we captured dynamic de novo autophagosomal biogenesis, fusion with LEs, and retrograde transport in growth cones and along distal axon shafts of live DRG neurons immediately after starvation. The time frame for such fusion and transport events is much faster (within 1 min of autophagosomal formation) than in non-neuronal cells, where similar fusion events occur much more slowly. We speculate that local calcium signaling in axons and synapses might speed up autophagosome fusion with LEs. In contrast, AVs remained stationary if they did not fuse with LEs during the recording time, further indicating that autophagosomes acquire retrograde transport upon fusion with LEs. Depleting STX17 significantly decreased the recruitment of dynein DNAI to AVs (P < 0.001) relative to control neurons. We further examined the ultrastructure of DRG neurons following STX17 knockdown and starvation. The majority of AVs in distal neurites are autophagosomes (initial AVs [AVi]) with sealed double-membrane bilayers. In control neurons, the majority of AVs are those engulfing electron-dense material, suggesting amphisomes or autolysosomes (degradative AVs [AVd]) following fusion with late endocytic organelles. These results consistently support our conclusion that amphisome formation is a prerequisite step to acquire retrograde motility; blocking the fusion impairs transport of autophagosomes from distal axons to the soma ().
In summary, our study reveals a new mechanism underlying recruitment of dynein motors to amphisomes, thus driving AV retrograde transport from distal axons toward the soma. This trafficking route is crucial for neurons to maintain effective autophagic clearance through lysosomal degradation in the soma. Thus, our study reveals, for the first time, the motor-adaptor sharing mechanism that allows 2 different organelles to take the “ride-on service” for long-distance trafficking by forming hybrid intermediate organelles ().
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the Intramural Research Program of NINDS, NIH ZIA NS002946 and ZIA NS003029 (Z-H. S.).
