ABSTRACT
Secretion of antimicrobial proteins is an important host defense mechanism against bacteria, yet how secretory cells maintain function during bacterial invasion has been unclear. We discovered that Paneth cells, specialized secretory cells in the small intestine, react to bacterial invasion by rerouting a critical secreted antibacterial protein through a macroautophagy/autophagy-based secretion system termed secretory autophagy. Mice harboring a mutation in an essential autophagy gene, a mutation which is common in Crohn disease patients, cannot reroute their antimicrobial cargo during bacterial invasion and thus have compromised innate immunity. We showed that this alternative secretion system is triggered by both a cell-intrinsic mechanism, involving the ER stress response, and a cell-extrinsic mechanism, involving subepithelial innate immune cells. Our findings uncover a new role for secretory autophagy in host defense and suggest how a mutation in an autophagy gene can predispose individuals to Crohn disease.
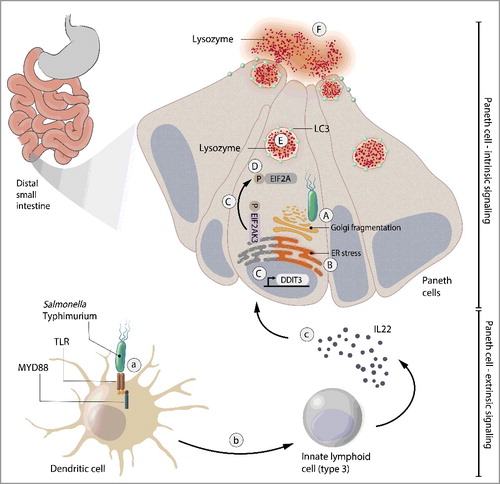
Paneth cells are specialized intestinal epithelial cells that secrete antimicrobial proteins including lysozyme into the intestinal lumen. These antimicrobial proteins disperse throughout the mucus layer that covers the intestinal epithelium, creating a chemical barrier that separates the host from the dense microbial communities inhabiting the gut. This chemical barrier enables exchange of small molecules and nutrients between host and microbiota while protecting against bacterial invasion. The location of Paneth cells on the intestinal frontline leaves them vulnerable to invasion by bacteria such as the foodborne pathogen Salmonella enterica serovar Typhimurium (S. Typhimurium). Bacterial invasion of host cells can impede their secretory function, and in the case of Paneth cells would impair their essential antimicrobial functions. Yet how Paneth cells maintain their functionality during infection was unclear.
Conventional autophagy involves the formation of phagophores (precursors to autophagosomes), double-membraned LC3-decorated transient compartments that engulf cytosolic cargo for subsequent delivery to the lysosomes for degradation. We recently discovered that Paneth cells respond to bacterial invasion by rerouting lysozyme through an autophagy-based alternative secretion system termed secretory autophagy. We found that during infection, S. Typhimurium invades Paneth cells and induces Golgi fragmentation that disrupts conventional cellular secretion. Paneth cells react to this disruption by packaging lysozyme into vesicles that are double-membraned and LC3-decorated, features of conventional autophagosomes. However, instead of fusing with lysosomes, these lysozyme-containing vesicles are released into the intestinal lumen ().
We also found that secretory autophagy of lysozyme is regulated by cell-intrinsic and cell-extrinsic mechanisms. Bacteria-induced Golgi fragmentation disrupts the normal Golgi-dependent secretory pathway and activates the endoplasmic reticulum (ER) stress response. This EIF2AK3/PERK-EIF2A/eIF2α-dependent ER stress response pathway is essential for activation of secretory autophagy (). Pharmacologically inhibiting the ER stress response blocks secretory autophagy in Paneth cells of S. Typhimurium-challenged mice and leaves them vulnerable to infection. Supplementing the mice with exogenous lysozyme rescues this vulnerability, highlighting the importance of secretory autophagy for antimicrobial defense of the intestine.
Interestingly, we found that the innate immune system is also required to “license” Paneth cells for activation of secretory autophagy. Bacteria are sensed by Toll-like receptors on dendritic cells (DCs), leading them to activate innate immune cells (ILCs) in the intestinal lamina propria. The ILCs then secrete the cytokine IL22 (interleukin 22), which primes Paneth cells for activation of secretory autophagy (). This multicellular relay system likely enables amplification of the “alarm” that DCs sound when encountering a pathogen and allows coordination of the epithelial response to invasion throughout the intestine.
Our findings reveal secretory autophagy as a potential new link between host genetics and inflammatory bowel diseases (IDB). Crohn disease, a form of IBD, is characterized by chronic inflammation that usually manifests in the distal small intestine. Intriguingly, people carrying a Thr300→Ala300 (T300A) mutation in the essential autophagy gene ATG16L1 are at increased risk for developing Crohn disease. These patients also harbor dysfunctional Paneth cells that cannot properly package and secrete lysozyme. However, the role of autophagy in these cells remained unclear. We found that Paneth cells from mice carrying this mutation (ATG16L1T300A) cannot secrete lysozyme through secretory autophagy when infected with S. Typhimurium. Imaging of Paneth cells from the infected ATG16L1T300A mice revealed that they do not properly package their lysozyme cargo, similar to the Paneth cells of Crohn disease patients. Our discovery of a role for autophagy in secretion of antimicrobial proteins from Paneth cells could thus help explain the Paneth cell abnormalities seen in Crohn disease.
IBD development likely depends on a combination of host genetics and environmental factors, such as the gut microbiota. Previous studies revealed increased secretion of pro-inflammatory cytokines by innate immune cells in response to bacterial signals in humans and mice carrying the ATG16L1 T300A mutation. Reflecting on our recent findings, we postulate that a mutation in ATG16L1 could leave the host vulnerable when encountering invasive bacteria, as Paneth cells will not be able to effectively defend the intestine during infection. These bacteria might be introduced through contaminated food or could be opportunistic commensals. A combination of dysfunctional antimicrobial defense and hyperactive secretion of pro-inflammatory cytokines in genetically susceptible individuals might then create a feedback loop that fuels chronic inflammation.
An intriguing remaining question is the mechanism by which Paneth cells reroute lysozyme for unconventional secretion. Recent work has elucidated the mechanism underlying secretory autophagy of IL1B/IL-1β (interleukin 1 beta), a leaderless protein, in macrophages. IL1B normally circumvents the conventional secretion pathway altogether and resides in the cytoplasm, where it is recruited to secretory autophagosomes upon inflammasome activation. The mechanism underlying secretory autophagy of lysozyme is likely to be different as lysozyme contains a leader peptide sequence that normally targets it for conventional secretion. Golgi fragmentation by invasive bacteria would leave newly-synthesized lysozyme stranded in the ER and the ER-to-Golgi intermediate compartment, both of which likely donate membranes to the phagophore. It is thus possible that activation of autophagy through ER stress promotes recruitment of lysozyme from these sites to newly forming autophagosomes. How these lysozyme-containing autophagosomes avoid fusion with the lysosome and subsequent degradation will need to be explored. It is possible that a SNARE-dependent membrane selection process, similar to that which facilitates secretory autophagy of IL1B, allows these autophagosomes to escape fusion with the lysosome.
Interestingly, deletion of the essential autophagy gene Atg5 in macrophages impairs lysozyme secretion when these cells are activated by bacterial molecules. Thus, it seems possible that lysozyme, which is expressed at other body sites such as the lungs and in secretions such as saliva and tears, is secreted via secretory autophagy in cells other than Paneth cells. If true, this raises the possibility that some unique characteristic of lysozyme (structure, sequence, etc.) allows its specific selection for secretory autophagy. Understanding the molecular basis for lysozyme's selection could help identify other cargos selected for secretory autophagy.
To conclude, our results provide clues about how genetic and environmental factors combine to facilitate development of IBD. Additionally, our discovery of secretory autophagy of lysozyme in Paneth cells reveals a physiological function for secretory autophagy in animals and discloses its importance for host defense.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.