ABSTRACT
The capacity of cells and organisms to sustain, and to eventually adapt to, environmental and genetic insults declines with age. Because macroautophagy/autophagy is regarded as one of the major determinants of cellular fitness in vitro and in vivo, maneuvers that aim at promoting autophagy may slow down aging and promote health span. Caloric restriction (CR), a reduction in caloric intake without malnutrition, efficiently counteracts aging-associated features, yet is difficult to be applied to humans. Caloric-restriction mimetics (CRMs) are pharmacological agents that recapitulate the main biochemical properties of CR, namely a global reduction of protein acetylation and the induction of autophagy. We found that the ancient drug aspirin and its active metabolite salicylate stimulate autophagic flux by virtue of their inhibitory action on acetyltransferase EP300. The inhibition of EP300 results from a direct competition between salicylate and acetyl coenzyme A for binding to the catalytic domain of the enzyme. This mode of action appears to be conserved across evolution as it accounts for the induction of autophagy by aspirin in various mouse models and in the nematode Caenorhabditis elegans. In sum, aspirin acts as a CRM.
Autophagy is required for the maintenance of cellular and organismal fitness due to its role in eliminating damaged organelles and potentially harmful protein aggregates as well as its unique capacity to mobilize essential metabolites from complex energy stores in conditions of stress. As a result, genetic or pharmacological interventions able to activate or reestablish proficient autophagy are now in the spotlight for their potential to extend lifespan and improve healthspan by reducing the incidence of age-associated disorders. Based on these premises, it is not surprising that cell biologists are testing novel autophagy inducers for their therapeutic effects on age-related disorders such as metabolic syndromes and cardiovascular diseases. Such new autophagy inducers can be identified by screening libraries of compounds that have not yet undergone any in-depth characterization or by screening collections of already known drugs. This latter repurposing strategy may accelerate the bench-to-bedside translation, because pharmacodynamics and adverse events tied to the administration of these agents have already been investigated in detail. Supporting this strategy, anti-diabetic biguanides (such as metformin) are now being tested for their anticancer and anti-aging actions in human studies.
Aspirin (acetylsalicylic acid), the prodrug of salicylate, ranks among the pharmacological agents that have the most positive impact on human health. The beneficial effects linked to the administration of aspirin encompass broad anti-arteriosclerotic and anti-inflammatory actions, prevention of different types of human neoplasia (in particular gastrointestinal cancers but also lung and breast carcinomas), reduction in metabolic syndrome-associated phenotypes and extended lifespan in animal models. This pleiotropic action likely reflects the multitude of molecular targets that are modulated by aspirin, well beyond the inhibition of prostaglandin synthesis by PTGS/cyclooxygenase. For example, aspirin and salicylate suppress the activity of IKBKB (inhibitor of nuclear factor kappa B kinase subunit beta) – and the consequent translocation of the NFKB complex to the nucleus – and allosterically promote the activation of 5ʹ-AMP-activated protein kinase (AMPK). Notwithstanding these observations, the detailed mechanism through which aspirin induces autophagy has been elusive.
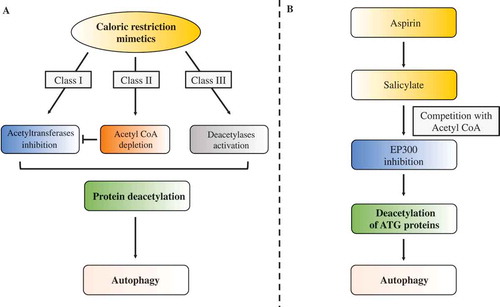
Recently, our group proposed a revisited molecular definition of compounds that are able to recapitulate the major biochemical feature of caloric restriction, namely the deacetylation of cytoplasmic proteins, resulting in the induction of autophagy. Depending on their mode of action, caloric-restriction mimetics (CRMs) can be subdivided into 3 distinct classes ():
Direct inhibitors of protein acetyltransferases and notably inhibitors of the master autophagy repressor histone acetyltransferase EP300/p300.
Inhibitors of acetyl coenzyme A (AcCoA) biosynthesis, which indirectly affect the enzymatic activity of acetyltransferases by depleting the sole donor of acetyl moieties.
Activators of protein (de)acetylases, in particular SIRT1 (sirtuin 1).
Irrespective of their precise molecular mechanism, treatment with such agents invariantly culminates in reduced acetylation of several target proteins (including essential ATG proteins) and in the consequent induction of autophagy. In addition, administration of CRMs in vivo is able to faithfully reproduce some of the beneficial effects of CR including improved energy expenditure and anti-obesity effects, enhanced anticancer immunosurveillance and an extended lifespan in model organisms.
Recently, we elucidated the mode of action through which aspirin and its metabolite salicylate induce autophagy, demonstrating that these molecules act as bona fide class I CRMS by virtue of their capacity to inhibit the enzymatic activity of EP300 (). This inhibitory effect is achieved at physiological concentrations of AcCoA, and it is attenuated when AcCoA levels are increased by 10-fold in a cell-free in vitro assay, hence suggesting that salicylate competes with AcCoA for binding to the catalytic domain of EP300. The knockout or siRNA-mediated depletion of EP300 is epistatic to the autophagy-inducing effect of salicylate in human and murine cell lines, as indicated by the fact that salicylate treatment is able to trigger autophagy in cell lines expressing normal levels of EP300 protein, yet fails to further stimulate autophagic flux in their EP300-deficient counterparts. Of note, transfection of EP300-deficient cells with a plasmid expressing functional wild-type EP300, but not with a plasmid carrying a mutated form of EP300 that lacks salicylate binding, is sufficient to restore the pro-autophagic activity of salicylate. The activation of autophagic flux by salicylate is still observed in cells deficient for AMPK, supporting the idea that AMPK activation is not essential for the pro-autophagic effect of salicylate. As a proof of the evolutionarily conserved nature of this mechanism, we found that inhibition of EP300 (or that of its C. elegans ortholog CBP-1) by aspirin or salicylate (the active metabolite of aspirin that is rapidly generated in vivo after aspirin administration to mice) accounts for the pro-autophagic activity of these molecules in mice organs and in the nematode C. elegans. Notably, treatment with aspirin (as previously shown for the CRM spermidine) can promote targeting of mitochondria to lysosomal degradation both in worms and in the in mouse heart, indicating that aspirin stimulates mitophagy in vivo.
Figure 1. Comparison of aspirin with other caloric-restriction mimetics. (A). Caloric-restriction mimetics may directly inhibit acetyltransferases, deplete acetyl coenzyme A (AcCoA) or activate deacetylases to stimulate protein deacetylation and consequent autophagy induction. (B) Salicylate generated from its prodrug, aspirin, directly inhibits the acetyltransferase activity of EP300, thus acting as a class I caloric-restriction mimetic to induce protein deacetylation and autophagy.
Collectively, these data shed new light on the molecular mechanisms that underlie the pro-autophagic activity of aspirin and first describe aspirin as a CRM. As many of the pro-healthy features associated with autophagy activation and aspirin administration in vivo (including anti-inflammatory effects as well as prophylactic or therapeutic effects on cancer, cardiovascular diseases, diabetes and obesity) are convergent, it is tempting to speculate that positive clinical phenotypes tied to aspirin treatment depend on the induction of autophagic flux. To this purpose, it will be important to evaluate the health-improving effects of aspirin in suitable autophagy-deficient animal models.
Acknowledgments
GK is supported by the Ligue contre le Cancer (équipe labellisée); Agence National de la Recherche (ANR) – Projets blancs; ANR under the frame of E-Rare-2, the ERA-Net for Research on Rare Diseases; Association pour la recherche sur le cancer (ARC); Cancéropôle Ile-de-France; Chancelerie des universités de Paris (Legs Poix), Fondation pour la Recherche Médicale (FRM); a donation by Elior; the European Commission (ArtForce); European Research Area Network on Cardiovascular Diseases (ERA-CVD, MINOTAUR); the European Research Council (ERC); Fondation Carrefour; Institut National du Cancer (INCa); Inserm (HTE); Institut Universitaire de France; LeDucq Foundation; the LabEx Immuno-Oncology; the RHU Torino Lumière; the Seerave Foundation; the SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); the SIRIC Cancer Research and Personalized Medicine (CARPEM); and the Paris Alliance of Cancer Research Institutes (PACRI).
