ABSTRACT
SQSTM1/p62 facilitates responses to various cellular stresses and has been implicated in human diseases. This protein functions as a major cytoplasmic signaling hub and has multiple binding partners, including arginylated (Nt-R) proteins that are recognized by the ZZ domain of SQSTM1/p62 (SQSTM1/p62ZZ). We have determined the molecular mechanism of Nt-R recognition using a combination of biochemical and NMR approaches and obtained the crystal structure of SQSTM1/p62ZZ in complex with Nt-R. We found that binding of SQSTM1/p62ZZ to Nt-R induces SQSTM1/p62 puncta formation and macroautophagy/autophagy and identified a regulatory linker (RL) region of SQSTM1/p62 that associates with SQSTM1/p62ZZ in vitro. Our findings suggest a mechanism for SQSTM1/p62 autoregulation that can be essential in mediating autophagy.
Intracellular signaling networks, such as autophagy and the MTORC1 pathway, control cell growth and proliferation and facilitate responses to environmental cues. Cells use the autophagic recycling machinery to aid in cell survival under various stresses and the MTORC1 pathway to sense nutrients, including amino acids, to modulate metabolism. SQSTM1/p62 is a major autophagic receptor and a positive mediator of the MTORC1 pathway. It links an autophagy-dependent cell detoxification with MTORC1 activation and maintenance of metabolic homeostasis. Recent studies have shown that the ZZ-type zinc-finger of SQSTM1/p62 (SQSTM1/p62ZZ) is capable of recognizing a degradation signal in proteins – the N-terminal arginine residue (Nt-R). The Nt-R signal can be generated either through proteolytic cleavage of the peptide bond at the amino side of arginine or enzymatically added by Arg-tRNA transferases. Despite the importance of SQSTM1/p62 for degradation of arginylated substrates, the molecular mechanism underlying its interaction with Nt-R and subsequent effects on autophagy and MTORC1 activation remain unclear.
To define the structural basis for the selective recognition of Nt-R by SQSTM1/p62, we determined the crystal structure of SQSTM1/p62ZZ in complex with the amino acid sequence commonly found in arginylated substrates, Arg1-Glu2 [1]. In the complex, Arg1 occupies a highly negatively charged groove of the ZZ domain, formed by the residues D129, N132, D147, and D149 of SQSTM1/p62. A set of intermolecular and intramolecular contacts stabilizes the interaction, tightly locking the conformation of the Arg1-Glu2 side chains. Microscale thermophoresis (MST) and fluorescence measurements revealed a 2–6 μM binding affinity of SQSTM1/p62ZZ to the REEE peptide, which is in the range of affinities reported for other known receptors of degradation signals. The importance of Arg1-Glu2 of the substrate and the groove residues D129, D147 and D149 of SQSTM1/p62 for the complex formation was substantiated by mutagenesis, NMR titrations, and affinity isolation experiments. Notably, in contrast to the robust interaction of SQSTM1/p62ZZ with the REEE peptide, we detect no interaction with AEEE or Ac-REEE peptides, and interaction with the RAEE peptide is decreased ~3 fold. These results point to the critical role of Arg1 and Glu2 in coupling the arginylated substrates to SQSTM1/p62ZZ.
What is the biological consequence of binding of the arginylated substrates to SQSTM1/p62ZZ? We examined the effect of a small molecule and known Nt-R mimetic, XIE62-1004, using sqstm1/p62 knockout MEFs reconstituted with either WT SQSTM1/p62 or the D129K mutant of SQSTM1/p62 defective in the Nt-R binding function. Our results show that binding of the Nt-R mimetic to SQSTM1/p62ZZ induces SQSTM1/p62 puncta formation and enhances macroautophagy in cells. In vitro experiments confirm that SQSTM1/p62ZZ mediates SQSTM1/p62 aggregation, a process that also depends on the adjacent Phox and Bem1 (PB1) domain of SQSTM1/p62, and the formation of disulfide conjugates. To characterize the autophagic activity of SQSTM1/p62ZZ in more detail, we employed a tandem fluorescent LC3 assay to analyze autolysosome formation in cells. We found that in starved conditions, adding back WT SQSTM1/p62 but not the ZZ domain loss-of-function mutants to sqstm1/p62-/- MEFs increases the percentage of cells undergoing autophagy. Interestingly, the effect on autophagy induction is selective because SQSTM1/p62 is dispensable in mitophagy but required for autophagy induced by proteasome inhibitor MG132. Collectively, these data underscore that in addition to its well-known function as a ubiquitinated cargo receptor, SQSTM1/p62 can promote autophagy through SQSTM1/p62ZZ.
Considering that only two residues, Arg1-Glu2, are in contact with SQSTM1/p62ZZ in the crystal structure, we tested whether a free arginine amino acid, which is abundant in cells, could be a substrate for SQSTM1/p62. Our NMR and crystallographic analyses show that SQSTM1/p62ZZ indeed recognizes arginine albeit more weakly than it recognizes the REEE peptide, as measured by NMR and MST. However, cell experiments using sqstm1/p62-/- MEFs overexpressing WT SQSTM1/p62 and SQSTM1/p62ZZ mutants indicate that although SQSTM1/p62 is necessary for arginine-mediated MTORC1 activation, this activation is largely independent of the arginine-binding capacity of SQSTM1/p62ZZ. Furthermore, we observed no significant difference in autophagic turnover under these conditions. Together, these data suggest that binding of arginylated substrates to SQSTM1/p62ZZ is necessary for the autophagic activity of SQSTM1/p62, whereas a free arginine is incapable of inducing autophagy.
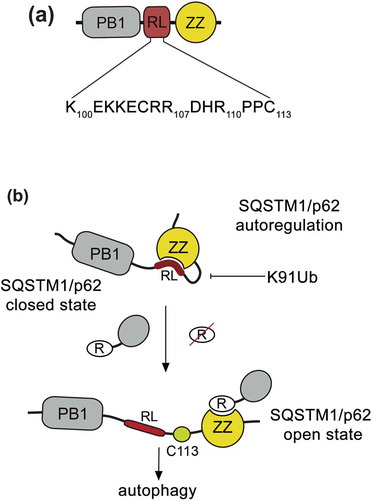
The PB1 and ZZ domains in SQSTM1/p62 are separated by a short, 20-residue linker, which contains 2 positively charged motifs that could mimic arginylated substrates as suggested by modeling of the peptide mimetics (). We used NMR and MST experiments to demonstrate that SQSTM1/p62ZZ binds to the peptide corresponding to the residues 100–110 of SQSTM1/p62 (SQSTM1/p62100-110), which we refer to as a regulatory linker (RL). Interestingly, a similar set of SQSTM1/p62ZZ resonances is perturbed by either REEE or SQSTM1/p62100-110 peptide, implying that the same binding site of SQSTM1/p62ZZ accommodates both ligands. The binding affinity of SQSTM1/p62ZZ to an isolated RL peptide is ~5-fold tighter than to a free arginine, but ~60-fold weaker than to REEE. We note that the SQSTM1/p62ZZ-RL interaction must be tighter in the physiologically relevant condition, as RL and ZZ are naturally linked in SQSTM1/p62. Nevertheless, the difference in binding affinities of SQSTM1/p62ZZ to Nt-R, RL, and arginine may be vital in directing SQSTM1/p62 toward the distinct autophagic and MTORC1 signaling pathways. Furthermore, recognition of the internal SQSTM1/p62 sequence by SQSTM1/p62ZZ can modulate SQSTM1/p62 activities and can in turn be modulated by post-translational modifications of SQSTM1/p62. Although the idea of autoregulation involving RL needs to be explored in cells, our findings provide a new avenue for future studies aimed at better understanding how p62 senses various stimuli and regulates distinct signaling pathways.
Figure 1. ZZ autoregulates SQSTM1/p62 function. (a) Schematic representation of the N-terminal region of SQSTM1/p62 containing the PB1 domain, RL, and the ZZ domain. (b) A model for potential SQSTM1/p62 autoregulation. The ZZ domain binds to the RL region, locking SQSTM1/p62 in a closed state. Upon binding of the ZZ domain to arginylated substrates but not arginine, the autoinhibition is released. Cys113 in RL, which is implicated in the formation of disulfide-linked conjugates, is shown. Future studies will be required to test this idea and to determine whether the SQSTM1/p62ZZ-RL interaction occurs in an intermolecular or intramolecular manner.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Reference
- Zhang Y, Mun SR, Linares JF, et al. ZZ-dependent regulation of p62/SQSTM1 in autophagy. Nat Commun. 2018;9:4373.
