ABSTRACT
Photoreceptor degeneration and damages often lead to blindness, and the underlying molecular mechanisms are largely unknown. There is also a lot of missing information for establishing the role of macroautophagy/autophagy in the retinopathy. We recently generated knockout mouse lines of the essential gene Tubgcp4 (tubulin, gamma complex associated protein 4) and revealed an interplay between essential genes and autophagy regulation. Complete knockout of Tubgcp4 in mice results in early embryonic lethality due to abnormal spindle assembly, whereas heterozygotes are viable through dosage compensation from one wild-type allele, suggesting a dosage effect of the essential gene. However, haploinsufficiency of TUBGCP4 impairs assembly of TUBG/γ-tubulin ring complexes and disturbs autophagy homeostasis of the retina, with pathological phenotypes of photoreceptor degeneration and a decrease of electroretinography responses. TUBGCP4 can inhibit autophagy by competing with ATG3 to interact with ATG7, thus interfering with lipidation of LC3B. Taken together, these findings demonstrate dosage effect of the essential gene Tubgcp4 for viability of mutant mice, and suggest key roles of TUBGCP4 in embryo development and retinal homeostasis by autophagy regulation.
Abbreviations
ATG3: autophagy related 3; ATG7: autophagy related 7; CRISPR: clustered regularly interspaced short palindromic repeats; ERG: electroretinography; HCQ: hydroxychloroquine; LC3B: microtubule-associated protein 1 light chain 3 beta; NFE2L2: nuclear factor, erythroid 2 like 2; ONL: outer nuclear layer; PPARGC1A: peroxisome proliferator-activated receptor gamma coactivator-1 alpha; RB1CC1: RB1 inducible coiled-coil 1; SQSTM1: sequestosome 1; TUBGCP: tubulin, gamma complex associated protein; TUBGRC/γ: TuRCs gamma-tubulin ring complexes
In humans, early embryonic death, followed by miscarriage, is a major cause of infertility. Miscarriages occur in 15–20% of clinically recognized pregnancies every year in the United States [Citation1], most of which are due to gene mutations and/or chromosomal abnormalities. Accordingly, there are a group of key genes in the human genome, which are essential for survival of early embryos. However, our understanding of the essential genome in humans is very limited. Accumulating evidence supports the concept that a set of core genes are essential for cell/organism survival in genomes, which are referred to as essential genes. Gene essentiality is typically defined as the indispensability of the core genes for survival or reproductive success, on the basis of the viability of the corresponding mutant cells/organisms [Citation2]. Essential genes exist in a variety of species from prokaryotes to vertebrates. Currently, over 53,700 essential genes have been compiled by in silico prediction, including bacteria (18,806), archaea (519) and eukaryotes (34,430) (www.essentialgene.org). However, functional analysis in mice and comparison with exome sequence data in humans revealed an overlap of 932 essential genes between humans and mice [Citation3]. Thus, a vast majority of essential genes remain to be identified by loss‑of‑function variants in human diseases. Gene essentiality analysis for cell survival in the Burkitt’s lymphoma cell line by CRISPR-Cas9 identified 1878 candidate essential genes, including TUBGCP4 (tubulin, gamma complex associated protein 4) [Citation4]. In human, Scheidecker and coauthors identified TUBGCP4 mutations in individuals with autosomal-recessive microcephaly and chorioretinopathy from three families in France and the UK, and determined the role of TUBGCP6 in the pathogenesis of the disease [Citation5].
TUBGCP4 associates with TUBGCP5 and TUBGCP6. Together with the TUBG/γ-tubulin small complex (TUBGCP2, TUBGCP3 and TUBG/γ-tubulin), they form a large TUBG/γ-tubulin ring complex (TUBGRC/γTuRC), which has an essential function in microtubule nucleation. Maintaining cellular homeostasis needs dynamic regulation of microtubule nucleation, which is consistent with autophagy in the sense of regulation of cellular homeostasis. Numerous studies have shown that dynamics and nucleation of actin filaments are involved in the regulation of autophagosome formation [Citation6], suggesting that cytoskeleton proteins are associated with autophagy. However, the interplay between the tubulin TUBG proteins and autophagy regulation remain largely unknown.
To explore a possible link between autophagy and the essential genes in vivo, we recently generated two lines of heterozygous mutant mice (Tubgcp4+/-) by gene targeting in ES cells [Citation7]. However, no tubgcp4−/- homozygous mutant mouse was detected by genotype analysis of progenies from heterozygote intercrosses, indicating that the homozygous mutation results in embryonic lethality. Further genotyping of different stage of embryos indicated that embryonic death occurred at E6.5. Histological analysis showed that the death was due to obviously developmental defects with a short embryonic span. These data demonstrate that Tubgcp4 is an essential gene for embryo survival. In heterozygotes, embryo survival can be rescued by the wild-type allele through dosage compensation. Thus, gene essentiality should be assessed as a dosage effect. The study demonstrates a novel genetic cause for embryonic lethality, at least partially explains the etiology of infertility, and suggests that essential genes can be used as candidate markers in genetic counseling.
Nevertheless, haploid dosage is insufficient for optimal health, and Tubgcp4+/- heterozygous mice display a slightly smaller head size in comparison with their wild-type littermates, an phenotype mimicking TUBGCP4 mutant autosomal-recessive microcephaly in humans [Citation5]. Further detection reveals a severe phenotype of retinopathy in heterozygotes. Electroretinography (ERG) showed that a-wave, b-wave, and photopic ERG responses decrease approximately 40–50% compared with wild-type mice, suggesting that the rod- and cone-driven circuits are significantly affected in the retinas of Tubgcp4+/- mice. Thus, haploinsufficiency of TUBGCP4 leads to photoreceptor damage in retina.
To further explore the underlying molecular mechanism for the photoreceptor degeneration, morphology of the retina in Tubgcp4+/- mice was first assessed. Histological analysis showed a decrease in the thickness of the outer nuclear layer, and electron microscopy indicated a disorganized outer segment morphology and disrupted lamellar structure. The retina is structured of several layers of specialized and multilayered neural tissue for initial capture and processing of visual signals, and links the optic nerve to the brain. Disrupted photoreceptor segment in Tubgcp4+/- mice will disturb delivery of visual signals to the brain.
In the heterozygous mice, we then determined that TUBGCP4 is depleted. Sucrose gradient sedimentation and western blot analysis can detect a series of TUBGRC components. Indeed, lower-molecular weight fractions are detected in Tubgcp4+/- retinas, and co-immunoprecipitation determined the disassembly of the TUBGRC. Further Tubgcp4 knockdown in cell lines confirmed abnormal spindle assembly in a dose-dependent manner. These data suggested that the haploinsufficiency of TUBGCP4 affects the assembly of the TUBGRC and leads to photoreceptor degeneration in retina.
Autophagy is an essential mechanism for cellular renovation in maintaining cellular homeostasis [Citation8]. To further investigate the molecular mechanisms of photoreceptor degeneration in the Tubgcp4+/- retina, autophagy was assessed in the retinas of mice at different ages. Western blot analysis showed that LC3B-II is upregulated, and its downstream binding partner, SQSTM1, is downregulated in the heterozygous retinas in comparison with the wild-type retinas. Overexpression of TUBGCP4 inhibits autophagy in the 293T cell line. The results suggested that the decrease in TUBGCP4 protein levels is associated with the upregulation of autophagy in the heterozygous retinas. Furthermore, TUBGCP4-involved autophagic flux analysis in the retinal cells showed that hydroxychloroquine treatment results in accumulation of both LC3B-II and SQSTM1 proteins in both wild-type and heterozygous retinas. LC3B puncta are mainly detected at photoreceptor inner segments and near the nuclei, indicating autophagosome formation in the segment. Transmission electron microscopy confirmed the formation of autophagosomes in the photoreceptor inner segment in Tubgcp4+/- retinas. This observation is consistent with the expression pattern of relevant genes. Both LC3B and TUBGCP4 are mainly expressed in photoreceptor segment in retina, not in retinal pigment epithelial cells (RPE), indicating that RPE is relatively stable in case of haploinsufficiency of TUBGCP4. However, loss of functions of other genes, such as NFE2L2, PPARGC1A, and RB1CC1 caused RPE damage, and further led to photoreceptor degeneration and vision loss [Citation9,Citation10].
Interestingly, nuclear autophagy has been observed in photoreceptor cell segments. Three major processes of nuclear autophagy were observed, including nuclear membrane protrusion, contact, and fusion with autolysosomes. When a nucleus begins to be degraded, the nuclear membrane shrinks and becomes irregular. The nuclear membrane protrudes, contacts, and fuses with autolysosomes. Since the discovery of autophagy in the 1950s, most studies in this field have focused on the degradation of cytoplasmic components. Our recent review emphasizes the importance of nuclear autophagy [Citation11]. As an essential part of the cell, the nuclei of photoreceptors encounter various physiological and pathological types of stress, such as genetic mutations and aging. Thus, haploinsufficiency of TUBGCP4 induces both cytoplasmic and nuclear autophagy. Further rescue of the retinopathy phenotypes by autophagy inhibition via administering the heterozygous mice with HCQ via the drinking water for 2 months revealed an obvious increase of ONL thickness in both the superior and inferior retina, and a significant improvement of retinal function in the HCQ-treated mice. Thus, inhibition of autophagy activity in the Tubgcp4+/- retinas can increase photoreceptor survival and rescue retinal function.
Further insight into molecular mechanisms of TUBGCP4 in regulation of autophagy will provide new understanding of maintaining of retinal functions. TUBGCP4-interacting proteins involved in autophagy initiation were screened through co-immunoprecipitation and colocalization analysis. Interestingly, ATG7, a key protein for autophagy initiation, can interact with TUBGCP4 via its N-terminal domain. The direct association by GST affinity-isolation assays was observed between TUBGCP4 and ATG7, particularly at the N terminus. In addition, immunofluorescence analysis of the retinal sections showed a colocalization of TUBGCP4 and ATG7 in the retina. Further co-immunoprecipitation analysis indicated that TUBGCP4 can compete with ATG3 for interaction with ATG7, suggesting that this is the mechanism through which TUBGCP4 inhibits autophagy. When Tubgcp4 is depleted, ATG3 interacts with ATG7 to promote lipidation of LC3B and autophagy. In wild-type cells, TUBGCP4 can compete with ATG3 for binding to ATG7, which inhibits LC3B lipidation and autophagy. Thus, TUBGCP4 establishes a balance between ATG3 and ATG7 proteins in a dose-dependent manner in autophagy regulation (). However, understanding how retinal homeostasis is maintained remains a great challenge. The retina transmits signals through the optic nerve to the brain. Both physiological and pathological insults impair retinal functions and lead to retinal diseases. Accumulating evidence shows that autophagy plays an important role in maintaining retinal functions; for example, depletion of BECN1/Beclin1 leads to light-induced retinal degeneration [Citation12]. However, excessive upregulation of autophagy also disrupts functions of photoreceptor cells. Thus, homeostasis of autophagic activity is essential to supporting retinal function. The pathway of ATG3-TUBGCP4-ATG7-LC3B in the regulation of autophagy in the retina has both physiological and pathological implications in understanding the molecular mechanisms underlying retina degeneration. Our results also have clinical significance in the potential treatment of retinopathy through the dosage effect of the essential gene Tubgcp4.
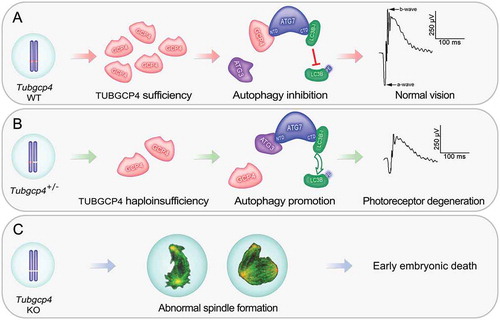
Figure 1. TUBGCP4 plays essential roles in early embryo development and retina homeostasis. (A) Two copies of Tubgcp4 are sufficient for embryo viability and normal retinal function. GCP4, TUBGCP4; PE, phosphatidylethanolamine. (B) Haploinsufficiency of TUBGCP4 affects TUBGRC/γ-TuRC assembly, disrupts autophagy homeostasis, and leads to photoreceptor degeneration. (C) Homozygous mutation of Tubgcp4 results in early embryonic lethality owing to abnormal spindle assembly. ATG7 can interact with either ATG3 or TUBGCP4 through its N-terminal domain (NTD). When Tubgcp4 is knocked out, ATG3 interacts with ATG7 to promote lipidation of LC3B and autophagy. Thus, TUBGCP4 can inhibit autophagy by competing with ATG3 to interact with ATG7, and interfere with lipidation of LC3B. TUBGCP4 regulates autophagy through an ATG3-TUBGCP4-ATG7-LC3B pathway, which plays key roles in maintaining retina homeostasis in a dose-dependent manner.
Ethics statement
All animal experiments and methods were performed in accordance with the relevant approved guidelines and regulations, as well as under the approval of the Ethics Committee of Wuhan University.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bardos J, Hercz D, Friedenthal J, et al. A national survey on public perceptions of miscarriage. Obstet Gynecol. 2015;125:1313–1320.
- Rancati G, Moffat J, Typas A, et al. Emerging and evolving concepts in gene essentiality. Nat Rev Genet. 2018;19:34–49.
- Bartha I, di Iulio J, Venter JC, et al. Human gene essentiality. Nat Rev Genet. 2018;19:51–62.
- Wang T, Birsoy K, Hughes NW, et al. Identification and characterization of essential genes in the human genome. Science. 2015;350:1096–1101.
- Scheidecker S, Etard C, Haren L, et al. Mutations in TUBGCP4 alter microtubule organization via the gamma-tubulin ring complex in autosomal-recessive microcephaly with chorioretinopathy. Am J Hum Genet. 2015;96:666–674.
- Coutts AS, La Thangue NB. Regulation of actin nucleation and autophagosome formation. Cell Mol Life Sci. 2016;73:3249–3263.
- Li Z, Li H, Xu X, et al. Haploinsufficiency of GCP4 induces autophagy and leads to photoreceptor degeneration due to defective spindle assembly in retina. Cell Death Differ. 2019;26.
- Yang Z, Klionsky DJ. Eaten alive: a history of macroautophagy. Nat Cell Biol. 2010;12:814–822.
- Felszeghy S, Viiri J, Paterno JJ, et al. Loss of NRF-2 and PGC-1alpha genes leads to retinal pigment epithelium damage resembling dry age-related macular degeneration. Redox Biol. 2019;20:1–12.
- Yao J, Jia L, Khan N, et al. Deletion of autophagy inducer RB1CC1 results in degeneration of the retinal pigment epithelium. Autophagy. 2015;11:939–953.
- Luo M, Zhao X, Song Y, et al. Nuclear autophagy: an evolutionarily conserved mechanism of nuclear degradation in the cytoplasm. Autophagy. 2016;12:1973–1983.
- Chen Y, Sawada O, Kohno H, et al. Autophagy protects the retina from light-induced degeneration. J Biol Chem. 2013;288:7506–7518.
