ABSTRACT
The primary cilium (PC), a plasma membrane microtubule-based structure, is a sensor of extracellular chemical and mechanical stress stimuli. Upon ciliogenesis, the autophagy protein ATG16L1 and the ciliary protein IFT20 are co-transported to the PC. We demonstrated in a recent study that IFT20 and ATG16L1 interact in a multiprotein complex. This interaction is mediated by the ATG16L1 WD40 domain and an ATG16L1-binding motif newly identified in IFT20. ATG16L1-deficient cells are decorated by giant ciliary structures hallmarked by defects in PC-associated signaling. These structures uncommonly accumulate phosphatidylinositol-4,5-bisphosphate (PtdIns[4,5]P2) while phosphatidylinositol-4-phosphate (PtdIns4P), a lipid normally concentrated in the PC, is excluded. We show that INPP5E, a phosphoinositide-associated phosphatase responsible for PtdIns4P generation, is a partner of ATG16L1 in this context. Perturbation of the ATG16L1-IFT20 complex alters INPP5E trafficking and proper function at the ciliary membrane. Altogether, these results reveal a novel autophagy-independent function of ATG16L1 that contributes to proper PC dynamics and function.
A complex crosstalk exists between macroautophagy (hereafter referred to as autophagy) and PC, a microtubule-based protrusion present at the surface of many cell types. The PC stimulates autophagy in response to various stimuli such as growth factor deprivation and mechanical stress. In turn autophagy controls PC elongation by degrading ciliary proteins that stimulate or repress ciliogenesis.
Upon induction of PC-dependent autophagy many ATG (autophagy related) proteins are recruited at the PC, such as ATG16L1 that colocalizes at the site of PC assembly with the ciliary protein IFT20 on transport vesicles moving toward the plasma membrane. IFT20, primarily associated with the external leaflet of the Golgi membrane, is a component of the intraflagellar transport particles (IFTs) that act in ciliogenesis with microtubules-associated motor proteins and is transported by vesicles to the PC.
The WD40 domain of ATG16L1 is absent from the yeast Atg16 ortholog and not essential for starvation-induced autophagy. We have recently shown [Citation1] that ATG16L1 binds to a newly identified ATG16L1-binding Y-E-F-I motif (Tyr111 [Y], Glu118 [E], Phe124 [F] Ile129 [I]) in IFT20 ().
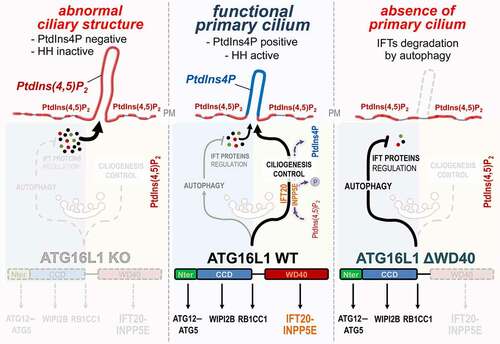
Figure 1. ATG16L1 at the crossroad of autophagy and ciliogenesis regulation. Center: In response to serum deprivation, ATG16L1 participates in IFT proteins turnover by promoting their autophagic degradation, notably via its N-terminal and coiled coil (CCD) domains. Conversely, ATG16L1 enables the proper trafficking of IFT20, via its WD40 domain, as well as the PtdIns(4,5)P2-associated phosphatase INPP5E at the site of ciliogenesis. This allows the proper conversion of PtdIns(4,5)P2 into PtdIns4P which accumulates in the axoneme of the PC. Right: In contrast, the ΔWD40-ATG16L1 mutant is not able to drive IFT20 and INPP5E to the plasma membrane, but autophagy capacities of ATG16L1 are not affected, thus resulting in massive degradation of IFT proteins and abolition of ciliogenesis. Left: in the absence of ATG16L1, autophagy is inhibited, IFT proteins accumulate and INPP5E is not targeted to the plasma membrane. Consequently, an abnormal ciliary-like structure is observed which is devoided of PtdIns4P and key functional markers like the Hedgehog pathway (HH) signaling components
ATG16L1 interacts with other regulators of ciliogenesis such as INPP5E, a phosphatase that hydrolyzes the phosphoinositide PtdIns(4,5)P2 to produce PtdIns4P, and the exocyst component EXOC4/SEC8. Interestingly, ATG16L1 directly binds to PtdIns4P and PtdIns(4,5)P2 in vitro. The binding site(s) for these lipids and their relation(s) to the PtdIns3P-binding site, located in the coiled-coil domain of ATG16L1, are however not yet described.
In atg16−/- MEFs several abnormalities are observed in ciliary-like giant structures, such as the mislocalization of some IFT proteins and of signaling molecules classically associated with the PC. The membrane of these giant ciliary structures is abnormally enriched in PtdIns(4,5)P2 and in the G-coupled receptor GPR161, an inhibitor of the Hedgehog pathway. This is a consequence of the absence INPP5E at the PC. In WT cells, INPP5E is co-transported with IFT20 and ATG16L1 to the PC to generate PtdIns4P which accumulates in the axoneme (the part of the PC that protrudes at the cell surface), limiting the recruitment of GPR161 ().
The function of ATG16L1 in ciliogenesis is independent of its role in autophagosome formation via other ATGs. This is illustrated by the fact that the interaction of ATG16L1 with ITF20 is not altered in the absence of ATG5 and ATG3.
These findings raise questions concerning 1) the cell distribution and trafficking of ATG16L1 and IFT20, 2) the regulation of the interaction between ATG16L1 and IFT20, and 3) the role of ATG16L1 in modulating the function of IFT20.
Where do ATG16L1 and IFT20 meet?
IFT20 is transported to the PC on vesicles emanating from the Golgi complex, whereas ATG16L1 is present in endocytic structures where it interacts with the clathrin adaptor AP2. IFT20 and ATG16L1 co-distribute on uncoated vesicles, as observed by electron microscopy and subcellular fractionation. Thus, it is tempting to hypothesize that the fusion of IFT20-containing vesicles and ATG16L1-containing vesicles is a post Golgi event. However, recent findings obtained in non-ciliated T cells show that IFT20 participates in autophagosome biogenesis by favoring the association of ATG16L1 with early endosomes, which are dynamically connected to Golgi trafficking.
How is the interaction between ATG16L1 and IFT20 regulated?
The YEFI motif of IFT20, which is essential for its interaction with the Golgi protein TMEM59, is also required for its interaction with the WD40 domain of ATG16L1. The latter interacts with several proteins acting in inflammation and xenophagy (TMEM59, TNFAIP3/A20, NOD1 and NOD2), with the lysosomal V-ATPase complex and with GJA1/connexin-43. It is also required for ATG16L1-mediated recruitment of LC3 to single-membrane compartments. How the activity of this multiple-functions domain is regulated is an open question. The T300A variant of ATG16L1, which carries a mutation located upstream of the WD40 domain and is associated with Crohn disease, modifies the interaction of ATG16L1 with the transmembrane protein TMEM59, thus affecting the TMEM59-mediated endosome trafficking. The T300A variant is also associated with an increase in the expression of many small-GTPases RAB proteins and with changes in vesicular trafficking in urothelial cells. Whether polymorphism(s) and/or posttranslational modification(s) in ATG16L1 alter interaction between ATG16L1 and IFT20 is still to be investigated.
Does ATG16L1 modulate IFT20 function?
ATG16L1 is important for the proper trafficking of IFT20 to the PC and for the local delivery of INPP5E to ensure the balance between PtdIns(4,5)P2 and PtdIns4P at the axonemal ciliary membrane. ATG16L1 and IFT20 are both detected in the axoneme, although it contains no vesicles. Thus, how ATG16L1 is transported across the transition zone into the axoneme remains an open question.
Many IFTs including IFT20 are involved in vesicular trafficking unrelated to ciliogenesis, such as endosome recycling during the building of the immunological synapses between T-lymphocytes and dendritic cells, and synaptic transport in non-ciliated neurons. IFT20 is also important for integrin recycling during cell migration. Interestingly the T300A variant of ATG16L1 induces a hyper-stabilization of the immunological synapse in T cells. Whether ATG16L1 can modulate IFT20-dependent activities in addition to its transport is a stimulating question for further studies.
ATG16L1 is dimerized when associated with ATG12–ATG5. Deciphering whether ATG16L1 acts as a monomer or as a dimer in IFT20-dependent mechanisms would help to better understand the role of ATG16L1 during post-Golgi sorting, ciliogenesis and other processes involving IFT20.
In conclusion our work contributes to shedding a new light on the fine but complex molecular crosstalk between autophagy and ciliary proteins. Work by others helps as well to build up a more accurate view of this interplay. The basal body protein OFD1, involved in ciliogenesis, is also a player in selective autophagy by inducing the degradation of ATG13.
The bacterial effector CT622/TaiP produced by Chlamydia trachomatis prevents interaction between ATG16L1 and TMEM59 to ablate the bacterial restriction activity of ATG16L1.
These findings showing the role of ATG16L1 in receptor recycling, protein secretion, regulation of inflammation, acidification of multivesicular bodies and in limiting lethal infection by influenza A virus stress the importance of the non-canonical role of ATG16L1 to maintain cell homeostasis.
Acknowledgments
We thank Dr Zeina Chamoun for critical reading of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Reference
- Boukhalfa A, Roccio F, et al. The autophagy protein ATG16L1 cooperates with IFT20 and INPP5E to regulate the turnover of phosphoinositides at the primary cilium. Cell Rep. 2021;35(4):109045.
