ABSTRACT
AMBRA1 (autophagy/beclin 1 regulator 1) is a multifunctional scaffold protein involved in several cellular processes spanning from cell proliferation to apoptosis and to regulation of macroautophagy/autophagy. Our recent publication revealed that Ambra1 has an antitumorigenic role in melanoma, the most aggressive and deadly skin cancer. We have indeed collected data indicating that the increased proliferative and invasive/metastatic features that we observed in ambra1-ablated melanomas are related to a remarkable regulation by Ambra1 on cellular processes which are beyond autophagy. Our study therefore sheds light on intriguing processes affected by Ambra1 which can be exploited as therapeutic targets in AMBRA1 low-expressing melanoma.
In recent years, the use of genetically engineered mouse models (GEMMs) and of syngeneic mouse models has been of valuable applicability for the better understanding of aspects related to tumor development and progression in different cancer types. Among these is cutaneous melanoma (hereafter referred to as melanoma), which can be considered the deadliest skin cancer, with high heterogeneity and metastatic potential, resistance to therapy as well as high mutational burden. Melanoma can indeed develop from the malignant transformation of melanocytes as a result of alterations in key genes implicated in cell proliferation signaling and, among many, mutations in BRAF (e.g., the V600E substitution) and loss of PTEN, conceivably the best-characterized players in the human disease. Therefore, pre-clinical GEMMs of melanoma in which re-arrangement of Braf to its mutant variant BrafV600E are coupled to loss of Pten have been developed and extensively employed to unknot details about melanoma growth and progression. Hence, it is not surprising that the implications of the autophagy process in melanoma biology have been explored by this means as well, specifically via combined re-arrangements of autophagy core genes, particularly Atg5 and Atg7, with the expression of BrafV600E and Pten loss. In this milieu, the complete loss of either gene – and subsequent compromised autophagy machinery – has been convincingly pinpointed to impair melanoma growth and progression. Though this may suggest that autophagy wields oncogenic functions in melanoma – as also more widely assessed by the use of syngeneic models of melanoma – tables are seemingly turned in early-stage cutaneous melanoma. Here indeed, impairment of autophagy in melanocytes has been interrelated with BRAFV600E-driven tumorigenesis, reasonably pointing to a tumor suppressor function of autophagy instead. Albeit this dual role inevitably adds another layer of complexity to the overall scenario, autophagy clearly plays a significant role in melanoma biology.
To benefit from a better understanding of the role of other autophagy genes in melanoma, we have combined the BrafV600E/+;pten−/− GEMM of melanoma with melanocyte-specific loss of Ambra1 [Citation1]. AMBRA1 has indeed been considerably investigated over the last years in the context of autophagy, and its pro-autophagic functions have been revealed to span from regulation of autophagy initiation by controlling BECN1 and ULK1 (unc-51 like autophagy activating kinase 1) activity to maintenance of mitochondrial clearance by interaction with LC3. However thoroughly described, neither direct implications of AMBRA1 nor its autophagy functions have been disclosed so far in melanoma biology. Our results have undoubtedly shed light on a noteworthy role of AMBRA1 in melanoma development (). Loss of Ambra1 indeed confers an accelerated rate of tumor growth leading to reduced overall survival of mice and remarkably increased melanoma aggressiveness and metastatic burden. We have indeed shown that the proliferative advantage conferred by loss of Ambra1 is ascribable to the recent discovery of the involvement of AMBRA1 in the control of the stability of CCND1 (cyclin D1), a phenomenon which is related to AMBRA1-mediated regulation of proteostasis rather than autophagy. Conversely, the invasive phenotype that we detected upon Ambra1 loss is attributed to the hyperactivation of PTK2/FAK1 (PTK2 protein tyrosine kinase 2) and to the loss of PTK2/FAK1 interaction with AMBRA1. This process as well, together with the increased invasive and migration capacity that we observed in human melanoma cells, displays no commitment to the autophagy functions of AMBRA1. Indeed, the growth of Ambra1-deficient Braf- and Pten-driven melanomas, as well as the propensity of null or low-expressing AMBRA1 melanoma cells to migrate and invade, are compromised by the inhibition of PTK2/FAK1 signaling, highlighting PTK2/FAK1 inhibitors as novel therapeutic approach in melanoma with low expression of AMBRA1.
Figure 1. Loss of Ambra1 promotes melanoma growth and metastasis independently of autophagy. For melanoma induction, ambra1flox/flox mice were crossed with mice carrying the joint BrafV600E substitution and pten deletion (BrafV600E/+;ptenflox/flox), a genetic combination with 100% tumor penetrance in mice. When melanoma is formed, the absence of Ambra1 does not affect the autophagy flux in vivo. Instead, in vivo ablation of Ambra1 in melanoma results in boosted tumor growth and metastatic capacity by means of increased stability of CCND1 and enhanced activation of PTK2/FAK1 signaling, respectively
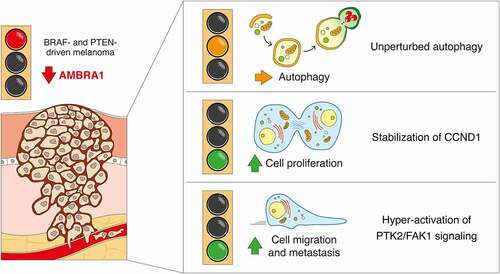
Such independence from autophagy is also true in BrafV600E-mutated melanocytes (in a mouse model used for studying melanoma initiation), in which the increased pigmentation and hyperplasia that we observed upon ablation of Ambra1 are attributed to other functions of the protein. If it is, however, true that autophagy might exert an oncogenic role in melanoma development, our results are likely not in line with this aspect if only the pro-autophagic function of AMBRA1 is taken into account. Most certainly, despite demonstrating that depletion of AMBRA1 impairs autophagy flux in human melanoma cells, our in vivo analyses on bulk tumors revealed that autophagy flux is not altered in ambra1 KO tumors. This scenario seemingly implies that other events and/or processes unrelated to the autophagy function of AMBRA1 are of higher relevance in ambra1 KO tumors.
Overall, and contrary to other autophagy genes, we have shown that Ambra1 rather exerts a tumor suppressive function in melanoma. However complex, the many-sided role for autophagy genes has already been described in melanoma. Such is the case for Atg5, the single-allele loss of which is correlated with the worst prognosis, with further implications on resistance to targeted therapy (such as BRAF inhibitors). This may raise caution regarding the actual role of autophagy genes in melanoma, as other – likely undiscovered – functions of these genes may be involved, as speculated also by other researchers. Ambra1 may certainly and yet play a unique role in this scenario. This is clearly and tightly related to the multifaceted and versatile functions of the protein in essential processes of a cell’s life. This is particularly true in the case of melanoma, where processes such as proliferation and invasiveness, which have now been thoroughly demonstrated to be finely regulated by Ambra1, are highly needed for the progression of this very tumor, and are likely to overshadow the functions of AMBRA1 in autophagy.
Disclosure Statement
The authors declare no competing interests.
Additional information
Funding
Reference
- Di Leo L, Bodemeyer V, Bosisio FM, et al. Loss of Ambra1 promotes melanoma growth and invasion. Nat Commun. 2021;12:2550.
