ABSTRACT
After its discovery in the 1950 s, the autophagy research field has seen its annual number of publications climb from tens to thousands. The ever-growing number of autophagy publications is a wealth of information but presents a challenge to researchers, especially those new to the field, who are looking for a general overview of the field to, for example, determine current topics of the field or formulate new hypotheses. Here, we employed text mining tools to extract research trends in the autophagy field, including those of genes, terms, and topics. The publication trend of the field can be separated into three phases. The exponential rise in publication number began in the last phase and is most likely spurred by a series of highly cited research papers published in previous phases. The exponential increase in papers has resulted in a larger variety of research topics, with the majority involving those that are directly physiologically relevant, such as disease and modulating autophagy. Our findings provide researchers a summary of the history of the autophagy research field and perhaps hints of what is to come.
Abbreviations: 5Y-IF: 5-year impact factor; AIS: article influence score; EM: electron microscopy; HGNC: HUGO gene nomenclature committee; LDA: latent Dirichlet allocation; MeSH: medical subject headings; ncRNA: non-coding RNA
Introduction
Autophagy refers to a set of lysosome-based pathways that the cell employs to “eat itself”. Following a few observations in the 1950 s, it was formally named in 1963 by Christian de Duve [Citation1]. Autophagy is now widely accepted to occur in 3 forms: macroautophagy, microautophagy, and chaperone-mediated autophagy. Macroautophagy is characterized by the “consumption” of cytosolic materials by the autophagosome, an organelle that is generated de novo by the action of numerous autophagy-related proteins (ATGs) [Citation2,Citation3]. Cytosolic materials can be taken up into lysosomes directly by microautophagy, which involves the invagination of lysosomal membranes [Citation4] or through the lysosomal membrane protein LAMP2A, the characteristic protein of chaperone-mediated autophagy [Citation5].
After almost 60 years since the first observation was reported, autophagy has developed into a research field in its own right. Its development was arguably spurred by the identification of macroautophagy yeast mutants in the early 1990 s [Citation6–8]. The discovery of macroautophagy genes opened numerous avenues of investigation, including the study of autophagy in other organisms, the characterization of autophagy-related proteins and the physiological roles of autophagy. Efforts over the years have led to the discovery of more autophagy proteins, more autophagy pathways, and a deeper understanding of its physiological and pathological roles [Citation9–11].
The autophagy research field has seen a tremendous increase in publications. With over 60,000 results for the search term “autophagy” on PubMed, a researcher interested in autophagy can no longer read, much less digest, all the available information. This problem is faced by all genres of science and can be tackled by text mining, the process of extracting knowledge from large quantities of text [Citation12–14].
Thus far, autophagy studies that employed text mining identified genes involved in autophagy [Citation15,Citation16], associations between diseases and autophagy genes [Citation17,Citation18], and autophagy-related drug targets for treating age-related macular degeneration [Citation19]. As these studies were carried out to extract specific information, the text mining done was similarly precise: only specific elements, such as gene names and disease names, were searched for. However, text can also be analyzed in general, without specifying search queries. This is achieved by natural language processing, which converts natural language to structured data that can then be quantitatively analyzed [Citation13,Citation20]. Examples of information extracted by general text mining include global mood patterns [Citation21] and public health surveillance from social media posts [Citation22], predictions on market changes from news headlines [Citation23], and key points from the large number of studies on the COVID-19 pandemic [Citation24]. Text mining can thus be used to make observations and obtain trends from large quantities in a non-biased manner.
In this study, we sought to obtain a general overview of the autophagy field based on data obtained with readily accessible text mining tools. We compiled all autophagy articles (specifically, the title and abstract) on PubMed (https://pubmed.ncbi.nlm.nih.gov/) published before 2020 and carried out targeted searches to determine the prevalence of autophagy genes, non-autophagy genes, and disease terms. Additionally, we identified the topics autophagy researchers have been studying since the discovery of autophagy. We find that the studies on selective autophagy are becoming more popular and that studies in the field are trending toward diseases. Our findings provide an overview of the autophagy research field and offer hints at what is to come.
Results & discussion
Rapid expansion of the autophagy research field within the past decade
We downloaded PubMed records of publications that matched the search term “autophag*” (the asterisk ensuring that all words beginning with “autophag” will be returned in the results) and kept those that were published by 2019 (i.e., publications that were accepted and “available as early access” were excluded). We chose not to include publications released in 2020 as many of them were “early access” publications with unclear publication dates. The total dataset comprises 42,065 publications (Data S1), with the earliest publication appearing in 1966.
According to the rate of publishing, the years of autophagy research can be divided into 3 phases (). In phase 1, from 1966 to 1975, an average of 10.33 autophagy publications were released per year. This average jumped to 73.6 in phase 2, which lasted from 1976 to 2000 and saw a consistent number of autophagy publications being released each year. The rate of publishing increased exponentially from 2001, the beginning of phase 3 (). We will explore the features of each phase in the following sections of this article.
Figure 1. The rapidly expanding autophagy research field. (A) The number of autophagy publications (blue) and last authors (red) from 1965–2020 are shown with a linear scale (left) and a logarithmic scale (right). With a logarithmic scale, the publication history of autophagy can be loosely classified into 3 phases according to the gradient of the graph. (B) A stacked bar plot of publications in the autophagy research field from 1965–2020 showing the percentage of last authors (taken to represent laboratories) publishing in the field for the first time. (C) Stacked bar plots of percentages of autophagy publications from 1965–2020 with the indicated citation counts (obtained from Web of Science in January 2021). (D) Stacked bar plots of percentages of autophagy publications that were published in journals with the indicated 5-Year journal impact factors (left) or article influence scores (right; see Figure S1 for normalized eigenfactor score; all scores obtained from incites journal citation report 2019). The data can be found in Data S2.
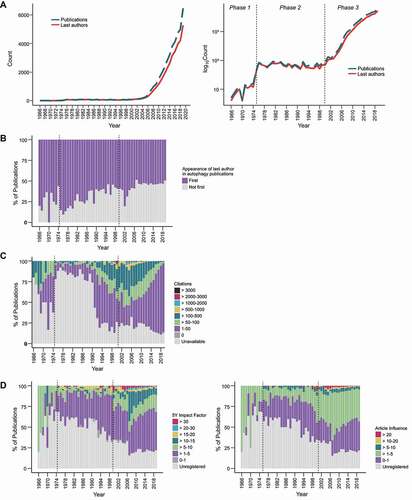
The exponential increase in publications is matched by the increase in last authors, which we consider to represent laboratories (; Data S2). Every year, more than half of the last authors of autophagy publications were publishing in the field for the first time (), suggesting that awareness of and interest in the field has been spreading. Continued efforts of researchers with experience in this field and the steady influx of new researchers are major drivers of the rapid expansion of the field.
We then explored the academic metrics of the autophagy field in an attempt to gauge the “impact” of autophagy publications as the field grew. “Impact” here refers to citation count, which does not necessarily reflect actual scientific impact and quality of the publication but is easier to quantify (metrics such as article views and article downloads are also available but only for more recent publications. For example, records in the Altmetric database (Digital Science) start from 2011). However, citation counts require time to accumulate, which inevitably results in more recent articles having lower numbers. To estimate the potential impact of more recent autophagy papers, we also included the journal-level citation-based metrics, 5-Year Impact Factor (5Y-IF) and Article Influence Score (AIS) in our analysis (see Figure S1 for Eigenfactor score).
The trends of article-level citation counts, 5Y-IF, and AIS suggest that many highly impactful studies (with high citation counts and/or published in journals with a 5Y-IF exceeding 30 or an AIS more than 20) were published during a period of 1998–2008 (, when autophagy transitioned from phase 2 to phase 3 (). The highly cited (>500 times) articles published before this period proposed that autophagosomes are formed from the endoplasmic reticulum [Citation25], identified the yeast as a model organism to study autophagy [Citation26], identified the first of autophagy genes [Citation6–8], reported the observation of autophagosomes in neurons [Citation27,Citation28], and identified drugs that could modulate autophagy [Citation29,Citation30] (Data S2). These studies, together with the highly cited publications released in 1998–2008 ( are likely to have precipitated the dramatic increase in autophagy publications during phase 3 ().
Despite the exponential increase in publications after 2008 (), the distribution of journal metrics remained relatively unchanged (). There is a gradual decrease in the percentage of papers published in journals with 5Y-IFs and AIS’s from 10–30 but the decrease is slight considering the number of publications released during this period. The decrease for papers published in journals with 5Y-IFs and AIS’s above 30 and 20, respectively, is more apparent () but this is due to the increase in overall publication count rather than a drop in absolute number (Figure S1B and S1C). Taken together, these data indicate that after the foundation for the field was laid by the first wave of ground-breaking studies, the autophagy field may be stabilizing, with autophagy researchers steadily adding to our knowledge of this cellular process.
Genetic elements in autophagy publications
We started our analysis on the language of autophagy publications by tallying the frequency of genetic elements in the titles and abstracts (collectively referred to as “documents”) of primary research articles (30,417 of 42,065). Gene symbols from the HUGO Gene Nomenclature Committee (HGNC) database and the Saccharomyces Genome Database (SGD) were used as search queries. Homologs between the two species in the results were subsequently combined. Nearly 10% of the entries in the HGNC database (4,168 of 42,571) was detected. Adding yeast gene-only hits and combining homologs yielded 4,226 hits. 95% of them are protein-coding genes (genes, for short), with the remaining being non-coding RNAs (ncRNAs), pseudogenes or “other” (; Data S3).
Figure 2. The prevalence of official gene names in autophagy publications. (A) The number of genetic elements detected, categorized by their assigned locus group as found in the HUGO gene nomenclature committee database. 4008 protein-coding genes, 206 non-coding RNAs, 9 pseudogenes and 4 “other” (referring to a fragile site, protocadherin, complex locus constituent, and unknown) were found. (B) For each genetic element, the number of publications it was mentioned in (indicating how frequently it was mentioned) was plotted against the average publication year (sum of publication years divided by number of publications; indicates how recent it was mentioned). Left: linear y-axis; right: log2 y-axis. (C) Top occurring protein-coding genes (left) and non-coding RNAs (right) in the titles and abstracts of autophagy publications.
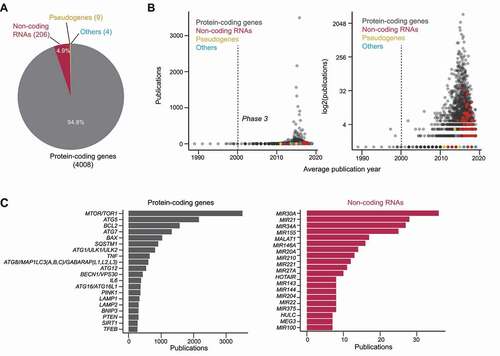
To visualize the “relevance” of each genetic element (how recent and how often it was mentioned), we plotted the total number of documents mentioning the genetic element against the average publication year of the documents (i.e., the sum of the publication years of the relevant documents divided by the total number of relevant documents) [Citation31]. The highly “relevant” (recently and frequently mentioned) genetic elements are genes (), with many of them being recognizable autophagy genes (; Data S3 and S4). The average publication year of many genes falls between 2013–2019, suggesting that they were mentioned in relation to autophagy recently. This is particularly the case for ncRNAs, most of which have an average publication year between 2016–2019 (; Data S3). ncRNAs have been proposed as an alternative way to modulate autophagy and as an alternative autophagy activity indicator [Citation32]. As they were only introduced to the field in the last few years, the extent of ncRNAs’ role in autophagy remains to be established. For the foreseeable future, proteins and genes are likely to remain as the main subjects of autophagy studies.
Autophagy genes for substrate recognition are increasing in frequency
There are now over 100 genes associated with autophagy, four times more than the initial set of genes recognized as “autophagy-related genes” when autophagy gene nomenclature was standardized [Citation33]. Many of them are referred to by alternative names that would not have been detected in our previous search for genetic elements. We thus conducted a more extensive search through documents from primary research articles for autophagy genes (listed in Data S4) by including the names of gene homologs in H. sapiens, S. cerevisiae, D. melanogaster, C. elegans, D. rerio, A. thaliana, and P. falciparum), alternative (e.g., p62 for SQSTM1), and discontinued names. Results for different names of the same gene or of genes encoding the same protein were combined before the genes were ranked by frequency. For the purpose of simplicity, we will be using the nomenclature for gene products instead of gene symbols (e.g., ATG5 instead of ATG5) in this section as many of the alternative names are not gene symbols.
The overall ranking of autophagy genes frequency show that LC3/Atg8 is the most-mentioned autophagy gene by far (). Its predominance is most likely due to it being the most established autophagosome marker in addition to its lipidation state being used as an autophagy activity indicator [Citation19,Citation20]. It is followed by BECN1/Beclin 1, one of the initiation factors. It is highly mentioned because it was the first autophagy gene to be linked to cancer [Citation9] and has been studied extensively in the context of cancer ever since [Citation34]. MTOR/TOR, a major regulator of autophagy, comes 3rd ().
Figure 3. The prevalence of autophagy-associated genes in autophagy publications. (A) Top occurring autophagy genes in the titles and abstracts of autophagy publications (presented in the nomenclature for protein products). (B) The top few most mentioned autophagy genes of selected years of phase 2 and phase 3. Genes within the same table cells have the same frequency. Ellipses indicate the presence of more results (provided in Data S4). (C) The publication trends of the categories of autophagy genes (categorized according to their main function) in total frequency for each category per year (left) and percentage of total number of studies (right).
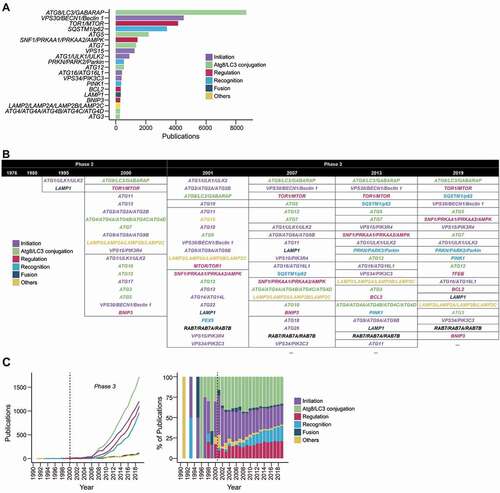
Autophagy genes can be loosely categorized into 6 categories based on their function: (1) initiation of autophagy, (2) autophagy conjugation system, (3) autophagosome-lysosome fusion, (4) autophagy regulation, (5) substrate recognition, (6) others (includes chaperone-mediated autophagy, DNautophagy, etc.) (Data S4). By categorizing the genes, we can generalize the aspects of autophagy that researchers have been studying.
Most of the top 20 mentioned autophagy genes belong to the categories of initiation, LC3/Atg8 conjugation system, regulation, and substrate recognition (). These categories reflect aspects of autophagy that have implications in autophagy modulation: how/when is autophagy activated, how autophagy activity can be measured, how autophagy can be regulated, and how autophagy can be directed to degrade particular substrates. The first three categories have been extensively studied [Citation2,Citation3], having been present in the literature since the late 1990 s (). Nevertheless, discoveries are still being made, such as ncRNA-mediated regulation [Citation32], liquid-liquid phase separation-induced initiation [Citation35], mechanical stress-induced initiation [Citation36], and LC3/Atg8 being conjugated to phosphatidylserine in single membranes during viral infection [Citation37,Citation38]–all of which help us better understand when and how autophagy carries out its physiological functions.
Despite its late start, substrate recognition is becoming one of the major categories (). Substrate recognition enables selective autophagy, which is the pathway by which substrates are recognized and specifically sent for degradation by autophagy [Citation39,Citation40]. The reason for the rapid rise of the substrate recognition category can be mostly attributed to SQSTM1/p62, PRKN/PARK2/Parkin, and PINK1 ( and 3B). p62 was the first soluble receptor to be identified in mammals and its discovery was the first clear demonstration of autophagy’s ability to selectively degrade material (aggregates, in this case) [Citation40,Citation41]. Subsequently, Parkinson disease-associated proteins PRKN and PINK1 were found to bring about the ubiquitination of damaged mitochondria, leading to their engulfment by autophagosomes via ubiquitin-recognizing receptors [Citation42]. The endoplasmic reticulum, pathogens, and other materials have been shown to be cleared by selective autophagy as well [Citation40,Citation43,Citation44]. The ability to clear material in bulk combined with selectivity makes selective autophagy enables the cell to proactively and quickly restore homeostasis. These findings opened up the possibility of modulating autophagy to selectively clear deleterious material, thereby preventing aging and treating neurodegenerative diseases [Citation42,Citation45,Citation46].
Frequently occurring non-autophagy genes are associated with cell death, immunity, and cancer
After reviewing autophagy genes, we turned our attention to the nature of non-autophagy genes frequently mentioned in autophagy documents from primary research articles. These were obtained by removing autophagy genes (Data S4) from the results of the genetic elements search in (Data S3).
Looking at the top 20 mentioned non-autophagy genes (), we realized that they are closely associated with specific processes/functions: (1) growth signaling, (2) cell death, (3) immunity, (4) cell stress response, and (5) others (; Data S3).
Figure 4. Non-autophagy genes in autophagy publications. (A) The top 20 most mentioned non-autophagy genes (of Homo sapiens and Saccharomyces cerevisiae) found in the titles and abstracts of autophagy publications. (B) The top few non-autophagy genes of selected years of phase 2 and phase 3. Genes that are in the same border occurred the same frequency. Genes ending with “’s” are homologs beginning with the same letters and ellipses indicate the presence of more results (see Data S3 for full names and all detected genes).
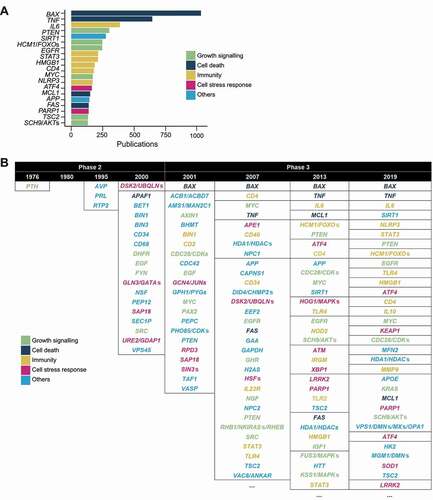
BAX and TNF, genes associated with cell death, are the top 2 mentioned genes in autophagy documents and within phase 3 (; Data S3). The genes that follow BAX and TNF are almost evenly distributed between the categories, growth signaling, immunity, and cell death response, indicating that, together with cell death, these processes/functions are the ones being investigated in the context of autophagy.
When the frequencies are viewed by year, a shift from genes more associated with general cell biology (the “others” category) to stress- and disease-related genes can be observed (; Data S3). At the end of phase 2, following the identification of the “core” autophagy-related genes, the non-autophagy genes mentioned in autophagy studies are associated primarily with protein trafficking (RTP2, BET1, SEC1P, VPS45, and PEP12) (). As these genes encode for proteins that are involved in membrane dynamics, the studies at this stage may have been exploring this aspect of autophagy. They were quickly overtaken by genes not associated with protein trafficking but instead with cellular and organismal homeostasis in phase 3 (), reflecting the rise of physiological studies.
Terms referring to modulating autophagy and disease occur more frequently in recent publications
Next, we examined the general language of autophagy documents. We were interested in the terms commonly used by researchers in the autophagy field. Instead of counting word frequency, we sought to obtain the frequencies of terms, which may consist of more than one word but refer to a single thing or concept (e.g., “reactive oxygen species”). To extract terms from all autophagy documents (including those from review articles), we employed the “en_core_sci_lg” model from the Python package scispaCy [Citation47]. The scispaCy models have been trained to recognize single-and multi-word terms (“entities”) from biomedical text in a process called Named Entity Recognition [Citation12,Citation20] ().
Figure 5. The scientific entities most mentioned in autophagy publications. (A) A scheme illustrating how entities were detected using the Python package scispaCy. Repeats were removed, leaving unique entities for each document (the title and abstract of a publication). (B) The top 20 entities. (C) The top 10 most frequently occurring entities for selected years of the 3 phases are indicated (see Data S5 for more).
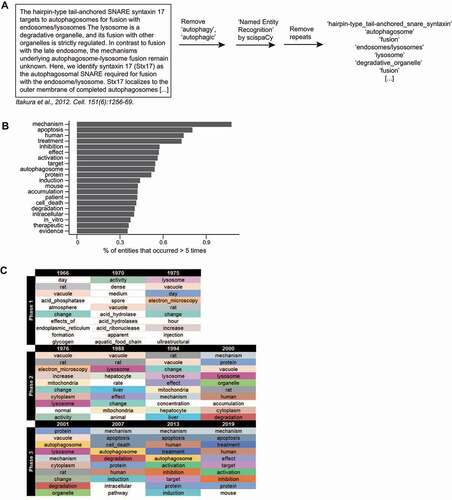
“Mechanism” is the most-mentioned entity (). It is followed by “apoptosis”, which agrees with the prevalence of apoptosis-associated genes. This term could be used to describe an experimental result or to refer to the relationship between autophagy and cell death in a subfield of studies known as “autophagic cell death” or “autophagy-dependent cell death” [Citation48,Citation49]. Other frequently occurring entities (more than 0.5%; namely, “human”, “treatment”, “inhibition”, “effect”, “activation”, “target”) suggest that many studies report effects of autophagy modulation. It is also likely that autophagy has been extensively investigated in mice (“mouse”) and that many studies were carried out with human diseases in mind (“patient”, “therapeutic”; ).
“Mechanism” and the frequently occurring entities now () only became prevalent in autophagy documents toward the end of phase 2 or in phase 3 (; Data S5). This indicates that a large portion of autophagy research shifted from mostly ultrastructural and biochemical studies with yeast or animals (“electron microscopy”, “vacuole”, “lysosome”, “rat”) to investigating molecular mechanisms and physiological functions–a progression that is natural since researchers build their studies on existing knowledge [Citation50,Citation51].
Topic variety has increased in the autophagy research field
The evolution in terms commonly used by autophagy researchers indicate that the major topics being studied in this field has changed over the years (). However, the topics that the terms represent are subject to interpretation. Moreover, summarizing them into groups (“topics”) would be more informative. Therefore, we employed Latent Dirichlet Allocation (LDA), an established topic modeling method used in natural language processing that calculates probability distributions (topics) over a collection of words [Citation50,Citation51] (). Briefly, it considers each document to comprise a mixture of topics, iteratively calculates the probability of words co-occurring together within a document and, by doing so, generates clusters of words i.e., topics. Each document contains several words that are assigned to particular topics. The main topic of a document is the one with the greatest representation amongst the words of the document [Citation50,Citation51].
Figure 6. The topics in the autophagy research field. (A) A scheme illustrating how the LDA model was obtained. The top 20 terms for the topics in each model can be found in Data S6. (B) A t-SNE plot showing the probabilities of each publication assignment and that they are gathered into 17 clusters (“topics”). (C) A table containing the proposed titles of the 17 topics and the top 10 terms of each topic. (D) The publication count of each topic, corresponding to those in (C), was plotted over time as a line graph (left) and as a stacked bar graph, where it is displayed as a percentage of total number of studies for each year (right).
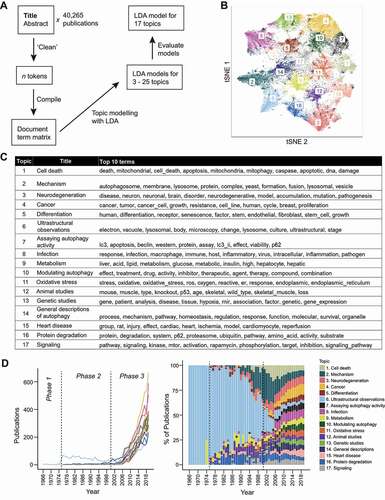
We obtained LDA models for 3–25 topics and found 17 to be the optimal number of topics as it was the largest number of topics with minimal overlap (see Data S6 for the top 20 terms of each model). Our decision also took into account the scores for established LDA evaluation metrics [Citation52–55] (see details in Materials and Methods; Figure S2), the clustering of the t-SNE plot (), and the assignments of autophagy publications from our laboratory (not shown).
Each topic was given a general name based on the top 20 words of each topic (, Data S6). The 17 topics are (in no particular order): “cell death”, “mechanism”, “neurodegeneration”, “cancer”, “differentiation”, “ultrastructural observations”, “assaying autophagy activity”, “infection”, “metabolism”, “modulating autophagy”, “oxidative stress”, “animal studies”, “genetic studies”, “general descriptions of autophagy”, “heart disease”, “protein degradation”, and “signaling”. The distribution of topics is comparable between primary and non-primary (e.g., reviews, commentaries) research articles (Figure S3), suggesting that all topics are being actively studied.
“Ultrastructural observations” (Topic 6) was the main topic of most autophagy studies during phases 1 and 2 (), a testament to the value of electron microscopy (EM) to the development of the field. Autophagy and its function as a cellular degradation pathway were discovered from observations of autophagosomes/autolysosomes made by EM [Citation9,Citation10,Citation56–58]. Studies on physiological topics (“metabolism” (Topic 9), “neurodegeneration” (Topic 3)) conducted during the phase 2 were, more often than not, also conducted with EM [Citation9,Citation10,Citation58]. Notably, the link between autophagy and “metabolism” (Topic 9) was established during this period when early autophagy researchers used EM to show that metabolic hormones, glucagon and insulin, could modulate autophagosome/autolysosome number [Citation59,Citation60]. This laid the foundation to our current understanding of autophagy in maintaining metabolic homeostasis [Citation61,Citation62].
In phase 2, autophagy researchers started exploring other topics, especially “mechanism” (Topic 2), which was the topic of ~25% of the publications at the end of phase 2 (the year 2000). Studies on “modulating autophagy” (Topic 10), “heart disease” (Topic 15), and “signaling” (Topic 17), also appeared occasionally during this period. The topic of cell death appeared in the late 1990 s. Many of these topics started to gain traction during phase 3 () although growth was not sustained for all as seen for cell death and “mechanism” (Topic 2), for which the percentage of studies has been decreasing in recent years. Cancer (Topic 4), assaying autophagic activity (Topic 7), “infection” (Topic 8), and modulating autophagy (Topic 10) are topics that took longer to appear (assaying autophagic activity being the last to do so) but now that they have, they are gradually garnering more publications in a rise that seems likely to continue.
In 2019, publications are nearly evenly distributed across the 17 topics–a stark difference from the end of phase 2. The redistribution of publications across the 17 topics began and is most obvious during the beginning of phase 3, which coincides with the period when many highly cited papers were published (). This was also when the number of laboratories and publications started to increase exponentially (). Taken together, it seems likely that the topics which received more attention in the later years of phase 3 did so because of the highly cited papers published in the beginning of phase 3; the highly cited papers may have introduced ideas that drew more researchers to the field.
Most disease types are represented in the literature on autophagy
As many topics in the field are related to disease, we examined the disease types that occur in autophagy documents. This was achieved by tallying the frequency of disease terms from Category C of the United States National Library of Medicine’s Medical Subject Headings (MeSH), excluding subcategories C22, C23 and C26 (animal diseases, pathological conditions, signs and symptoms and wounds/injuries).
We started by determining the frequencies of MeSH descriptors that could be found in the publication abstracts. MeSH descriptors are organized from most general to most specific in a tree structure that can reach 13 levels. When a document contained more than 1 MeSH descriptor, the one(s) with the most levels was kept as it was a better indicator of the subject of the study. The frequencies of MeSH descriptors were then grouped into their respective MeSH subcategories.
Ranking the frequencies of MeSH subcategories, we found that MeSH descriptors of most disease types could be found in autophagy documents (; Data S7). The top 5 disease types being studied in the autophagy research field are neoplasms (C04), nervous system diseases (C10), infections (C01), cardiovascular diseases (C14), and nutritional & metabolic diseases (C18), which are also represented in the detected autophagy topics ().
Figure 7. The frequency of MeSH disease descriptors in autophagy research field. (A) The ranking of the subcategories of MeSH Category C (“Disease”). (B) Wordclouds of the top 50 MeSH descriptors of each of the top 5 disease types. (See Data S7.).
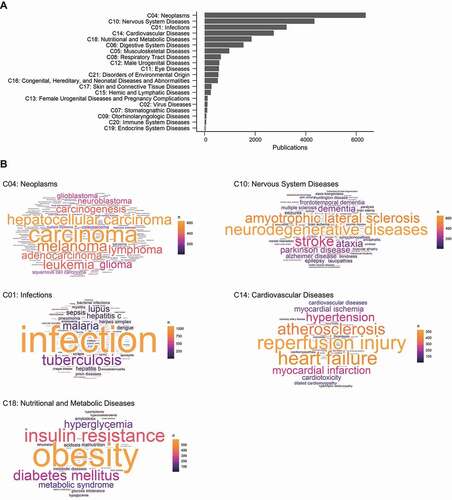
Next, we compiled the frequencies of individual MeSH descriptors from the top 5 occurring disease types to gain insight into the specific diseases autophagy researchers are studying (; Data S7). (It is important to keep in mind that some descriptors may be referring to experimental conditions, material, or phenotypes instead of actual diseases e.g., some counts of “hepatocellular carcinoma” may be referring to cell lines). For nervous system diseases (C10) and infections (C01), the most frequently occurring MeSH descriptor is the most general descriptor (the name of disease type), suggesting that many autophagy documents that came up during the search for these disease types were referring to it in general. The remaining studies, however, may have been concerned with particular diseases such as amyotrophic lateral sclerosis and stroke for the former and tuberculosis and hepatitis C for the latter. Many of the frequently occurring descriptors for neoplasms (C04) involve carcinoma, the most common category of cancer. As for cardiovascular diseases (C14), the main diseases being studied are those that involve ischemia (myocardial ischemia and reperfusion) and those that tend to develop with age (heart failure, atherosclerosis, and hypertension). Obesity is the main disease detected for nutritional and metabolic diseases (C18) but diseases involving the regulation of blood sugar levels (diabetes mellitus, insulin resistance, and hyperglycemia) are also commonly mentioned (; Data S7).
Topics in non-mammalian autophagy research
The findings we have described so far mainly revolve around mammalian physiology despite autophagy being conserved in almost all eukaryotes and autophagy studies having been conducted with non-mammals [Citation63–65]. We wondered what proportion of autophagy publications were conducted with non-mammals and whether the topic distribution for non-mammalian autophagy studies would be different. To this end, we categorized autophagy documents from primary research articles as studies that were conducted with (1) arthropods (including insects, spiders, crustaceans), (2) fungi, (3) mammals, (4) other non-mammals (including fish, birds), (5) nematodes & trematodes, (6) plants (including algae), or (7) protists (amoeba, protozoa), based on the most frequently occurring taxonomy detected in the document. We then tallied the number of publications and the topics occurring in each taxonomic category.
Most autophagy research was conducted with mammalian animals/cell lines () probably in part due to tremendous research interest in human diseases ( and 6). Fungi-based studies began in the 1990 s and its rise preceded the boom in mammalian studies ( inset), corresponding with the history of autophagy [Citation9,Citation10]. The use of other non-mammalian organisms only took off during phase 3 ( inset), which is another instance of the autophagy research field diversifying as it expanded ( and 6).
Figure 8. Topics in non-mammalian autophagy research. (A) The number of publications for each taxonomic category. The top 5 frequently occurring species, where available, are indicated with font size corresponding to their publication count (indicated in parenthesis following each species name). (B) The topics (found in ) present in each taxonomic category. (See Data S1 for classification.).
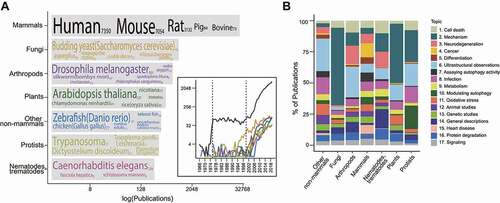
Topic distribution for each category varies (). Although the 17 topics are nearly evenly distributed across mammalian studies, non-mammalian studies are centered around a few topics. “Mechanism” (Topic 2) was the topic of most fungal studies (), highlighting the importance of fungi to the elucidation of many mechanistic details [Citation9,Citation10]. Nearly half of the studies conducted with plants were also on “mechanism” (Topic 2), suggesting that plant autophagy researchers have been mostly focused on clarifying mechanistic details of plant autophagy. Studies with arthropods, nematodes & trematodes, and other non-mammals show greater topic diversity (), which is probably due to them serving as models for mammalian aging and disease pathogenesis. Popular topics of protist studies are “infection” (Topic 8) and “modulating autophagy”, referring to research efforts on developing anti-parasitic treatments. Studying autophagy in non-mammals allows it to be studied from different angles, yielding insights that contribute toward completing our understanding on the mechanisms and functions of autophagy.
Conclusion
Spurred by a series of studies published in the 1990 s and 2000 s, the autophagy research field has grown dramatically in size throughout the past 20 years. The field has been reshaped by the emergence of new topics and appears to be currently at a stage where sufficient understanding of basic mechanism has allowed the rise of physiological studies that investigate the physiological functions of autophagy, the relationship between autophagy and disease, the type of autophagy diseases, and modulation of autophagy with drugs. The extent of autophagy’s contribution to proteostasis [Citation66,Citation67], whether and how autophagy can thus be used to prevent or treat disease are questions many current and future studies are striving to answer [Citation11,Citation68,Citation69]. At the level of basic research, the biophysics of autophagosome formation, the mechanisms of selective autophagy [Citation39,Citation40,Citation70,Citation71], the roles of lipids during a process with such dynamic membrane changes [Citation3,Citation71], non-autophagic functions of autophagy proteins [Citation72], and the evolution of autophagy [Citation63,Citation73] are examples of research directions to pursue. We speculate that the next phase of autophagy research (when the field undergoes another burst of growth) will be triggered by either the development of methods to precisely modulate autophagy in specific tissues of patients or the expansion of non-mammalian autophagy research.
Materials and methods
Data collection
PubMed records were downloaded via the R [Citation74] package easyPubMed R package on 4 October 2020 (https://cran.r-project.org/package=easyPubMed). Articles with the publication status “epublish” or “ppublish” before 2020 were kept for analysis. The title and abstract for each publication were combined and used as the reference material. We will refer to a title-abstract text as a document hereafter. See Data S1 for the table of records.
Citation counts and journal metrics
The number of citations for each article (“Times Cited, All Databases”) was downloaded from the Web of Science database (Clarivate Analytics, 2020) on 30 January 2021. Journal metrics are from the 2019 InCites Journal Citation Report (Clarivate Analytics, 2020) (Data S2).
Genetic elements and MeSH descriptors
Punctuation, standalone numbers, and stop words were removed from each document before conducting the respective searches.
The gene list for Homo sapiens was downloaded from the HUGO Gene Nomenclature Committee (HGNC; downloaded on 24 May 2021 from https://www.genenames.org/), for Saccharomyces cerevisiae gene list from the Saccharomyces Genome Database (SGD; https://www.yeastgenome.org), for Arabidopsis thaliana from The Arabidopsis Information Resource (TAIR; https://www.arabidopsis.org/), for Drosophila melanogaster from FlyBase (https://flybase.org/), for Danio rerio from the Zebrafish Information Network (ZFIN; https://zfin.org/), for Caenorhabditis elegans from WormBase (https://wormbase.org/) and for Plasmodium from Plasmodium Informatics Resources (PlasmoDB; https://plasmodb.org).
Human homologs of yeast genes were identified with YeastMine (https://yeastmine.yeastgenome.org/). Results for yeast and human homologs were combined and treated as a single result.
The autophagy gene list we used is based on the list in Galluzi et al. [Citation75] but expanded to include recently discovered autophagy genes (Data S4). Alternative and discontinued names were also included in the list (e.g., “PARKIN”, “PARK2”, and “PRKN” for PARK2 and “APG1”, “AUT3”, “CVT10”, etc. for ATG1). Although the alternative names were treated as individual search queries, the results for the same gene were later combined and only distinct results were kept (e.g., if PMID 001 appeared in the results for both Gene_Name_Ver-1 and Gene_Name_Ver-2, only one instance will be kept after combining Gene_Name_Ver-1 and Gene_Name_Ver-2).
We allowed 2 characters to be present before the search query to capture non-mammalian matches such as “AtAtg8”. Such results and results of gene names that have alternative meanings were further processed to eliminate false positives. This was achieved by keeping hits that contained words from the full gene name. For example, search hits for “MB” were kept only if they also contained the word “myoglobin”, for “SACS” only if they contained “sacsin”, and so on.
XML files containing Medical Subject Headings (MeSH) descriptors were downloaded from the National Library of Medicine (ftp://nlmpubs.nlm.nih.gov/online/mesh/). Descriptors from all subcategories of Category C were used as search terms, except for those from C22, C23, and C26. When a document contains more than 1 descriptor, the descriptor(s) with the largest number of levels, and thus the most specific, were retained. For example, if a document contained “Anterior Wall Myocardial Infarction” (C14.280.647.500.093), “Inferior Wall Myocardial Infarction” (C14.280.647.500.187), and “Myocardial Infarction” (C14.280.647.500), the last descriptor would be removed. “Starvation” was removed as its presence in autophagy documents typically refers to the condition inducing autophagy rather than the disease.
Entity detection
We considered “autophagy” and “autophagic” to be stop words with regards to autophagy publications and hence removed them from the reference material. Text within brackets ([], (), <>), words that began with a hyphen, and html tags were removed. “-/-“, “-null”, “-knockout”, and “-deficient” were replaced with “knockout”.
The “clean” documents were then processed individually with the scispaCy model “en_core_sci_lg”, which is part of the scispaCy Python package [47]. Verbs and single-character words were removed before entities were extracted. Repeats were removed, leaving only one occurrence of each detected entity. The entities were then combined to produce an overall ranking of frequency or combined per year to produce yearly rankings.
Latent Dirichlet Allocation (LDA)
The documents were processed in the same way as was done for entity detection except that repeated terms were retained. Multi-word entities were split into individual words (e.g., “cell death” to “cell” and “death”). Collocations (words that frequently occurred contiguously) were then detected in each processed document with the udpipe R package (https://cran.r-project.org/web/packages/udpipe). Although most entities are collocations, we wanted to extract collocations that the scispaCy model may have missed or may occur within entities. The detected collocations were then added to the respective documents. Finally, we removed generic terms (namely, “study”, “finding”, “result”, “review”, “level”, “datum”, “expression”, “cell”, “line”, “cellular”, “like”, “related”, “positive”, “analysis”, “type”) that occurred frequently but do not provide insight into the type of research addressed in the document.
LDA was conducted with the LDA function of the topicmodels R package (https://cran.r-project.org/web/packages/topicmodels [Citation76]). The processed documents were first converted into a document-term matrix, which was then subjected to LDA using Gibbs sampling (5 independent runs [nstart = 5] with 5 randomly chosen seeds [122,3,56,890,1234]; the first 1000 iterations were discarded [burnin = 1000], following which every 500th iteration [thin = 500] was returned for 2000 iterations [iter = 2000]).
We used the 4 methods [Citation52–55] of the ldatuning R package (https://cran.r-project.org/web/packages/ldatuning) to evaluate the LDA models generated for 3–25 topics. Briefly, the methods calculate or estimate the overall likelihood [Citation52], distance between pairs of topics [Citation53,Citation55], divergence between pairs of topics [Citation54] for each LDA model.
The tSNE plot was made with the Rtsne R package (https://cran.r-project.org/web/packages/Rtsne).
Taxonomy search
Taxonomic names were detected by phylotastic (https://phylotastic.org/), accessed via the R package rphylotastic (https://cran.r-project.org/web/packages/rphylotastic/index.html), the scispaCy model “en_ner_craft_md”, and a basic dictionary search for common names. Taxonomy was attributed by the taxize R package. The taxonomic category of a document is determined by the most occurring taxonomic name. Non-mammalian categories were further refined by removing documents that mention mammalian cell lines. Documents that did not mention taxonomy were assumed to fall under the “mammals” category. Documents that were classified into more than 1 category were manually assigned.
Supplemental Material
Download Zip (101.4 MB)Acknowledgments
We thank Tomoko Ohshima, Hayashi Yamamoto, Ikuko Honda-Koyama, Jun-ichi Sakamaki, and Fumiya Okawa for their constructive comments. This work was supported by the Japan Society for the Promotion of Science; Exploratory Research for Advanced Technology (ERATO; grant JPMJER1702 to N.M.) from the Japan Science and Technology Agency (JST).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Klionsky DJ. Autophagy revisited: a conversation with Christian de Duve. Autophagy. 2008;4:740–743.
- Nakatogawa H. Mechanisms governing autophagosome biogenesis. Nat Rev Mol Cell Biol. 2020;21:439–458.
- Nishimura T, Tooze SA. Emerging roles of ATG proteins and membrane lipids in autophagosome formation. Cell Discov. 2020;6:32.
- Schuck S. Microautophagy - distinct molecular mechanisms handle cargoes of many sizes. J Cell Sci. 2020;133:jcs246322.
- Kaushik S, Cuervo AM. The coming of age of chaperone-mediated autophagy. Nat Rev Mol Cell Biol. 2018;19:365–381.
- Tsukada M, Ohsumi Y. Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae. FEBS Lett. 1993;333:169–174.
- Thumm M, Egner R, Koch B, et al. Isolation of autophagocytosis mutants of Saccharomyces cerevisiae. FEBS Lett. 1994;349(2):275–280.
- Harding TM, Morano KA, Scott SV, et al. Isolation and characterization of yeast mutants in the cytoplasm to vacuole protein targeting pathway. J Cell Biol. 1995;131:591–602.
- Ohsumi Y. Historical landmarks of autophagy research. Cell Res. 2014;24:9–23.
- Mizushima N. A brief history of autophagy from cell biology to physiology and disease. Nat Cell Biol. 2018;20:521–527.
- Mizushima N, Levine B. Autophagy in human diseases. N Engl J Med. 2020;383:1564–1576.
- Rebholz-Schuhmann D, Oellrich A, Hoehndorf R. Text-mining solutions for biomedical research: enabling integrative biology. Nat Rev Genet. 2012;13:829–839.
- Fleuren WW, Alkema W. Application of text mining in the biomedical domain. Methods. 2015;74:97–106.
- Extance A. How AI technology can tame the scientific literature. Nature. 2018;561:273–274.
- Yang Y, Ma B, Jin Y, et al. Bioinformatic mining of kinase inhibitors that regulate autophagy through kinase signaling pathways. Mol Med Rep. 2014;10:3348–3356.
- Jacomin AC, Samavedam S, Promponas V, et al. iLIR database: a web resource for LIR motif-containing proteins in eukaryotes. Autophagy. 2016;12:1945–1953.
- Wang W, Zhang P, and Li L, et al. ATD: a comprehensive bioinformatics resource for deciphering the association of autophagy and diseases. Database (Oxford). 2018;bay093.
- Chen K, Yang D, and Zhao F, et al. Autophagy and tumor database: ATdb, a novel database connecting autophagy and tumor. Database (Oxford). 2020;baaa052.
- Wang S, Liu C, Ouyang W, et al. Common genes involved in autophagy, cellular senescence and the inflammatory response in AMD and drug discovery identified via biomedical databases. Transl Vis Sci Technol. 2021;10:14.
- Perera N, Dehmer M, Emmert-Streib F. Named entity recognition and relation detection for biomedical information extraction. Front Cell Dev Biol. 2020;8:673.
- Golder SA, Macy MW. Diurnal and seasonal mood vary with work, sleep, and daylength across diverse cultures. Science. 2011;333:1878–1881.
- Jordan SE, Hovet SE, Fung IC-H, et al. Using Twitter for public health surveillance from monitoring and prediction to public response. Data. 2019;4:6.
- Khadjeh Nassirtoussi A, Aghabozorgi S, Ying Wah T, et al. Text mining of news-headlines for FOREX market prediction: a multi-layer dimension reduction Algorithm with semantics and sentiment. Exp Sys with Appl. 2015;42:306–324.
- Brainard J. New tools aim to tame pandemic paper tsunami. Science. 2020;368:924–925.
- Dunn WA Jr. Studies on the mechanisms of autophagy: formation of the autophagic vacuole. J Cell Biol. 1990;10:1923–1933.
- Takeshige K, Baba M, Tsuboi S, et al. Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction. J Cell Biol. 1992;119:301–311.
- Nitatori T, Sato N, Waguri S, et al. Delayed neuronal death in the CA1 pyramidal cell layer of the gerbil hippocampus following transient ischemia is apoptosis. J Neurosci. 1995;15:1001–1011.
- Anglade P, Vyas S, Javoy-Agid F, et al. Apoptosis and autophagy in nigral neurons of patients with Parkinson’s disease. Histol Histopathol. 1997;12:25–31.
- Seglen PO, Gordon PB. 3-Methyladenine: specific inhibitor of autophagic/lysosomal protein degradation in isolated rat hepatocytes. Proc Natl Acad Sci USA. 1982;79:1889–1892.
- Blommaart EF, Krause U, Schellens JP, et al. The phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 inhibit autophagy in isolated rat hepatocytes. Eur J Biochem. 1997;243:240–246.
- Liu RL. Identification of conclusive association entities in biomedical articles. J Biomed Semantics. 2019;10:1.
- Yang L, Wang H, Shen Q, et al. Long non-coding RNAs involved in autophagy regulation. Cell Death Dis. 2017;8:e3073.
- Klionsky DJ, Cregg JM, Dunn WA Jr., et al. A unified nomenclature for yeast autophagy-related genes. Dev Cell. 2003;5:539–545.
- Vega-Rubin-de-Celis S. The role of beclin 1-Dependent autophagy in cancer. Biology (Basel). 2019;9:4.
- Noda NN, Wang Z, and Zhang H. Liquid-liquid phase separation in autophagy. J Cell Biol. 2020;219:e202004062.
- Hernandez-Caceres MP, Munoz L, Pradenas JM, et al. Mechanobiology of autophagy: the unexplored side of cancer. Front Oncol. 2021;11:632956.
- Sou YS, Tanida I, Komatsu M, et al. Phosphatidylserine in addition to phosphatidylethanolamine is an in vitro target of the mammalian Atg8 modifiers, LC3, GABARAP, and GATE-16. J Biol Chem. 2006;281:3017–3024.
- Durgan J, Lystad AH, Sloan K, et al. Non-canonical autophagy drives alternative ATG8 conjugation to phosphatidylserine. Mol Cell. 2021;81:2031–40 e8.
- Johansen T, Lamark T. Selective autophagy: ATG8 family proteins, LIR motifs and cargo receptors. J Mol Biol. 2020;432:80–103.
- Kirkin V. History of the selective autophagy research: how did it begin and where does it stand today? J Mol Biol. 2020;432:3–27.
- Pankiv S, Clausen TH, Lamark T, et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282:24131–24145.
- Killackey SA, Philpott DJ, and Girardin SE. Mitophagy pathways in health and disease. J Cell Biol. 2020;219:e202004029.
- Gatica D, Lahiri V, Klionsky DJ. Cargo recognition and degradation by selective autophagy. Nat Cell Biol. 2018;20:233–242.
- Kirkin V, Rogov VV. A diversity of selective autophagy receptors determines the specificity of the autophagy pathway. Mol Cell. 2019;76:268–285.
- Conway O, Akpinar HA, Rogov VV, et al. Selective autophagy receptors in neuronal health and disease. J Mol Biol. 2020;432:2483–2509.
- Djajadikerta A, Keshri S, Pavel M, et al. Autophagy induction as a therapeutic strategy for neurodegenerative diseases. J Mol Biol. 2020;432:2799–2821.
- Neumann M, King D, and Beltagy I, et al. ScispaCy: fast and robust models for biomedical natural language processing. ArXiv. 2019;abs/1902.07669.
- Tang D, Kang R, Berghe TV, et al. The molecular machinery of regulated cell death. Cell Res. 2019;29:347–364.
- Kist M, and Vucic D. Cell death pathways: intricate connections and disease implications. EMBO J. 2021;40:e106700.
- Blei D, Ng A, and Jordan M. Latent Dirichlet Allocation. Journal of machine learning research. 2003;3:993–1022.
- Asmussen CB, Møller C. Smart literature review: a practical topic modelling approach to exploratory literature review. J Big Data. 2019;6:93.
- Griffiths TL, Steyvers M. Finding scientific topics. Proc Natl Acad Sci U S A. 2004;101(Suppl 1):5228–5235.
- Cao J, Xia T, Li J, et al. A density-based method for adaptive LDA model selection. Neurocomputing. 2009;72:1775–1781.
- Arun R, Suresh V, Veni Madhavan CE, et al. On finding the natural number of topics with latent dirichlet allocation: some observations. In: Zaki MJ, Yu JX, Ravindran B, et al., editors. Advances in knowledge discovery and data mining. Berlin, Heidelberg: Springer Berlin Heidelberg; 2010. p. 391–402.
- Deveaud R, Sanjuan E, and Bellot P. Accurate and effective latent concept modeling for Ad Hoc information retrieval. Document numérique. 2014;17:61–84.
- Novikoff AB, Beaufay H, De Duve C. Electron microscopy of lysosomerich fractions from rat liver. J Biophys Biochem Cytol. 1956;2:179–184.
- Clark SL Jr. Cellular differentiation in the kidneys of newborn mice studies with the electron microscope. J Biophys Biochem Cytol. 1957;3:349–362.
- Eskelinen EL, Reggiori F, Baba M, et al. Seeing is believing: the impact of electron microscopy on autophagy research. Autophagy. 2011;7:935–956.
- Arstila AU, Trump BF. Studies on cellular autophagocytosis. The formation of autophagic vacuoles in the liver after glucagon administration. Am J Pathol. 1968;53:687–733.
- Amherdt M, Harris V, Renold AE, et al. Hepatic autography in uncontrolled experimental diabetes and its relationships to insulin and glucagon. J Clin Invest. 1974;54:188–193.
- Lahiri V, Hawkins WD, Klionsky DJ. Watch what you (Self-) eat: autophagic mechanisms that modulate metabolism. Cell Metab. 2019;29:803–826.
- Madeo F, Zimmermann A, Maiuri MC, et al. Essential role for autophagy in life span extension. J Clin Invest. 2015;125:85–93.
- King JS. Autophagy across the eukaryotes: is S. cerevisiae the odd one out? Autophagy. 2012;8:1159–1162.
- Till A, Saito R, Merkurjev D, et al. Evolutionary trends and functional anatomy of the human expanded autophagy network. Autophagy. 2015;11:1652–1667.
- Zhang S, Hama Y, and Mizushima N. The evolution of autophagy proteins - diversification in eukaryotes and potential ancestors in prokaryotes. J Cell Sci. 2021; 134:jcs233742.
- Hipp MS, Kasturi P, Hartl FU. The proteostasis network and its decline in ageing. Nat Rev Mol Cell Biol. 2019;20:421–435.
- Klaips CL, Jayaraj GG, Hartl FU. Pathways of cellular proteostasis in aging and disease. J Cell Biol. 2018;217:51–63.
- Yang Y, Klionsky DJ. Autophagy and disease: unanswered questions. Cell Death Differ. 2020;27:858–871.
- Mulcahy Levy JM, Thorburn A. Autophagy in cancer: moving from understanding mechanism to improving therapy responses in patients. Cell Death Differ. 2020;27:843–857.
- Stephani M, Dagdas Y. Plant selective autophagy-still an uncharted territory with a lot of hidden gems. J Mol Biol. 2020;432:63–79.
- de la Ballina LR, Munson MJ, Simonsen A. Lipids and lipid-binding proteins in selective autophagy. J Mol Biol. 2020;432:135–159.
- Galluzzi L, Green DR. Autophagy-independent functions of the autophagy machinery. Cell. 2019;177:1682–1699.
- Duszenko M, Ginger ML, Brennand A, et al. Autophagy in protists. Autophagy. 2011;7:127–158.
- Team RC. A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. 2020.
- Galluzzi L, Baehrecke EH, Ballabio A, et al. Molecular definitions of autophagy and related processes. EMBO J. 2017;36:1811–1836.
- Grün B, and Hornik K. topicmodels: an R package for fitting topic models Journal of Statistical Software. 2011;40:1–13.
