ABSTRACT
SQSTM1/p62 (sequestosome 1) is a macroautophagy/autophagy receptor protein that is degraded by selective autophagy. Intracellular accumulation of SQSTM1 activates multiple cell survival signaling pathways including NFΚB/NF-κB (nuclear factor kappa B), MTOR (mechanistic target of rapamycin kinase) and NFE2L2/Nrf2 (nuclear factor, erythroid derived 2, like 2). Both SQSTM1 and NFE2L2 have been considered as oncogenic, and increased accumulation of SQSTM1 and NFE2L2 activation have been frequently observed in various cancers including hepatocellular carcinoma. In a recent study, we found that deletion of Sqstm1 improved hepatic metabolic reprogramming and cell repopulation resulting in the attenuation of liver injury in mice with liver-specific deletion of Atg5 and Tsc1 that have defective hepatic autophagy and persistent MTOR complex 1 (MTORC1) activation. To our surprise, hepatocytic deletion of Sqstm1 promotes liver tumorigenesis in liver-specific atg5 and tsc1 double-knockout mice. Overall, these findings reveal a complex interplay among autophagy, SQSTM1 and MTORC1 and their differential roles either as oncogenic or tumor suppressor in liver tumorigenesis depending on the disease stage and context.
The role of autophagy in cancer is complex and context dependent. Autophagy serves as a tumor suppressor in the tumor initiation stage, which is achieved by selective autophagic removal of damaged organelles (i.e., mitochondria) and misfolded proteins to maintain cellular homeostasis and prevent oxidative and endoplasmic reticulum (ER) stress as well as genome instability. However, once cells are transformed, autophagy may promote tumor cell growth by supplying energy metabolites and nutrients to favor cancer cell survival against the harsh tumor microenvironment. In addition, autophagy in the tumor stroma, especially in cancer-associated fibroblasts and macrophages may also provide fuels and cytokines as well as growth factors to promote tumor cell growth and invasion.
Hepatocellular carcinoma (HCC) is currently the third leading cause of cancer-related tumor death worldwide. The most common HCC risk factors including aflatoxin B1 exposure, hepatitis B or C virus infection, diabetes, obesity, alcohol and nonalcoholic fatty liver diseases as well as genetic disorders such as mutations in tumor suppressors or oncogenes. Interestingly, all the above HCC risk factors impair hepatic autophagy. The role of autophagy as a liver tumor suppressor is further supported by the observation that liver-specific deletion of either Atg5 or Atg7 results in the development of spontaneous liver tumors. In addition, mice with liver-specific deletion of Tsc1, encoding a negative regulator of MTORC1, have impaired autophagy and spontaneous HCC. Because mice with genetic deletion of either Atg5 or Tsc1 develop spontaneous liver tumors, we hypothesized that simultaneous deletion of both Atg5 and Tsc1 would exacerbate liver tumorigenesis. In a recent study [Citation1], we tested this hypothesis by generating liver-specific atg5 and tsc1 double-knockout mice (referred to as DKO mice hereafter). Mice with liver-specific deletion of Atg5 develop hepatomegaly, inflammation and fibrosis with mild ductular reaction, which is exacerbated by further deletion of Tsc1 in DKO mice. Increased liver pathogenesis in DKO mice compared with the single KO mice is not surprising, and is likely due to increased protein synthesis and ER stress as a result of MTORC1 activation in DKO mice. Perhaps, one of the most intriguing findings is that DKO mice die around 8-months old without any noticeable tumors. The death of the DKO mice is likely due to liver failure as there are markedly decreased levels of amino acids (except cysteine) and dipeptides as well as free fatty acids in DKO mice because of the lack of autophagic degradation. Glycolysis and fatty acid beta-oxidation are also decreased in DKO mice at the age of 6-months old. These metabolic changes are accompanied by dramatic liver cell repopulation as DKO mouse livers have decreased hepatocytes with increased non-parenchymal cell populations including cholangiocytes, Kupffer cells/macrophages and hepatic stellate cells. The lack of liver tumors in DKO mice implies that autophagy is required for MTORC1 activation-induced liver tumorigenesis. Conversely, increased MTORC1 activity may also inhibit defective autophagy-induced liver tumorigenesis.
SQSTM1 is a multidomain and multifunction protein that serves as an intracellular signaling hub for various signaling pathways including NFKB, MTORC1 and NFE2L2 activation. Various tumors have increased SQSTM1 protein levels, and accumulation of SQSTM1-positive structures called Mallory-Denk bodies and hyalin bodies are often observed in HCC in humans. As an autophagy substrate protein, SQSTM1 accumulates in liver-specific atg5 or atg7 KO mouse livers resulting in non-canonical NFE2L2 activation by directly interacting with KEAP1 (kelch-like ECH-associated protein 1) to inhibit NFE2L2 degradation. Further deletion of either Sqstm1 or Nfe2l2 markedly blunts liver tumor formation in liver-specific atg5 or atg7 KO mice. Notably, ectopic overexpression of Sqstm1 in mouse livers alone induces HCC in mice. These results from previous studies indicate that Sqstm1 is an oncogene, which is necessary and sufficient for HCC induction in mice. In line with the notion that accumulation of SQSTM1 is detrimental in the liver, deletion of hepatic Sqstm1 in DKO mice improves liver injury, hepatomegaly, ductular reaction, and fibrosis as well as metabolic reprogramming and cell repopulation, and rescues the lethality of DKO mice. Despite the improvement, to our surprise, further deletion of hepatic Sqstm1 promotes HCC in the DKO mice, suggesting hepatic SQSTM1 suppresses liver tumor formation in DKO mice. Strikingly, there are massive increased infiltrated immune cells (macrophages and neutrophils) surrounding the tumors in liver-specific sqstm1 atg5 tsc1 triple-KO (TKO) mice. When compared with isolated hepatocytes, the levels of SQSTM1 in isolated macrophages are almost one hundred-fold higher than hepatocytes. Whether macrophage SQSTM1 would promote HCC in the sqstm1 atg5 tsc1 TKO mice remains unclear. It will be interesting to see the changes of liver tumors when whole body Sqstm1 is deleted in DKO mice.
How does the loss of hepatocytic SQSTM1 promote liver tumor formation in DKO mice? As discussed above, hepatocytic accumulation of SQSTM1 activates NFE2L2, and persistent NFE2L2 activation promotes hepatic metabolic reprogramming (altered amino acid, carbohydrate and fatty acid metabolism) and liver cell repopulation in DKO mice resulting in the death of DKO mice before the tumor development at a relatively old age. Therefore, it seems that the magnitude of NFE2L2 activation is critical for the metabolic programming and liver pathogenesis of DKO mice. Further deletion of Nfe2l2 in DKO mice rescues the metabolic reprogramming and liver cell repopulation and blunts liver tumor formation. Deletion of hepatocytic Sqstm1 eliminates the non-canonical NFE2L2 activation and subsequently improves the metabolic reprogramming to promote the survival of DKO mice. However, in contrast to the complete deletion of Nfe2l2 in DKO mice, the canonic NFE2L2 activation remains intact in liver-specific sqstm1 atg5 tsc1 TKO mice. It is likely that the basal level of NFE2L2 activation is necessary and sufficient to promote cancer cell growth and survival in these TKO mice.
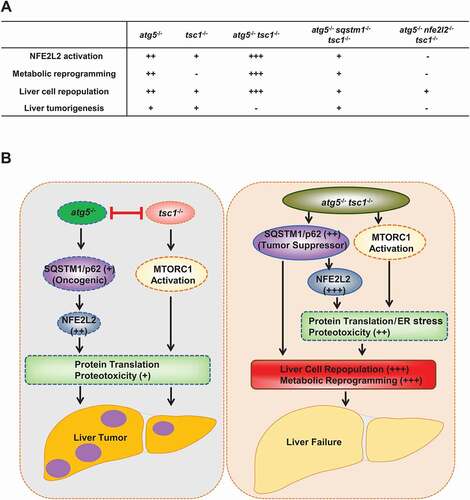
Taken together, the interplay among autophagy, SQSTM1 and MTORC1 in liver pathogenesis and tumorigenesis is very complex, and each of them may act as oncogenic or tumor suppressor depending on the disease stage and context. The molecular events among SQSTM1, autophagy, NFE2L2 and MTORC1 in liver pathogenesis and liver cancer are summarized in .
Figure 1. Hypothesis and molecular events of defective hepatic autophagy, SQSTM1/p62, MTORC1 and NFE2L2/Nrf2 in liver pathogenesis. (A). NFE2L2 activation, metabolic reprogramming, cell repopulation and liver tumorigenesis in genetically engineered mice for Atg5, Tsc1, Nfe2l2 and Sqstm1. (B) L-atg5 KO (atg5 −/−) mice have impaired hepatic autophagy resulting in the accumulation of SQSTM1/p62 and persistent NFE2L2 activation. Deletion of Tsc1 in mouse liver leads to persistent activation of MTORC1. Persistent NFE2L2 and MTORC1 activation leads to increased protein translation, proteotoxicity, liver injury and liver tumorigenesis. However, deletion of Atg5 inhibits liver tumorigenesis caused by deletion of Tsc1 and vice versa. Deletion of both Atg5 and Tsc1 leads to hyperaccumulation of SQSTM1, and persistent activation of NFE2L2 and MTORC1, resulting in liver cell repopulation, metabolic reprogramming and liver failure without liver tumor formation. SQSTM1 functions as a tumor suppressor to inhibit tumorigenesis in mice with deletion of Atg5 and Tsc1.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Reference
- Chao X, Wang S, Fulte S, et al. Hepatocytic p62 suppresses ductular reaction and tumorigenesis in mouse livers with mTORC1 activation and defective autophagy. J Hepatol. 2021. DOI:https://doi.org/10.1016/j.jhep.2021.10.014.
