ABSTRACT
Psychosocial stress is a common risk factor for anxiety disorders. The cellular mechanism for the anxiogenic effect of psychosocial stress is largely unclear. We recently showed that chronic social defeat (CSD) stress in mice causes mitochondrial impairment, which triggers the PINK1-PRKN/parkin mitophagy pathway selectively in the amygdala. This mitophagy elevation causes excessive mitochondrial elimination and consequent mitochondrial deficiency. Mitochondrial deficiency in the basolateral amygdalae (BLA) causes weakening of synaptic transmission in the BLA-BNST (bed nucleus of the stria terminalis) anxiolytic pathway and increased anxiety. The CSD-induced increase in anxiety-like behaviors is abolished in pink1−/− and prkn−/− mice and alleviated by optogenetic activation of the BLA-BNST synapse. This study identifies an unsuspected role of mitophagy in psychogenetic-stress-induced anxiety elevation and reveals that mitochondrial deficiency is sufficient to increase anxiety and underlies the psychosocial-stress-induced anxiety increase. Mitochondria and mitophagy, therefore, can be potentially targeted to ameliorate anxiety.
The brain uses around 20% of the body’s glucose despite being only around 2% of its mass. Functional neuronal activity critically depends on the ATP mitochondria produce through oxidative phosphorylation, and alterations to this supply impair short-term synaptic plasticity, neurotransmitter release, and synaptic numbers. Anxiety is a prolonged fear of anticipated threats; while this can be a normal occurrence in human or animals, persistent and chronic anxiety can be highly detrimental. Chronic stress from psychogenic stress is a notable trigger for such severe problems and can be caused by social situations like social defeat and abuse. Chronic stress can cause long-term perturbations in mitochondrial morphology, number, ATP production, and free radical responses and result in mtDNA variations. However, the translation from social-anxiety induced mitochondrial stresses to a behavioral or neurophysiological output is not clear.
In order to link psychogenic stress to a mitochondrial deficit, a chronic social defeat (CSD) paradigm was used to expose transgenic mito-YFP mice of C57BL/6 background to aggressor mice of CD-1 background for 10 or 30 days [Citation1]. CSD for both time frames has no effect on locomotion and increased anxiety-like behaviors, as shown by the light-dark box, elevated plus maze, and open field tests. However, only 30-day CSD mice show increased behavioral despair in the forced swim test. Defeated mice were divided into two groups: susceptible and resilient based on whether or not they exhibit social avoidance. Following these behavioral assays mitochondria were examined in the amygdala, which encodes fearful or threatening stimuli; and in the ventral hippocampus which is thought to process stress responses and emotional reactions. In both susceptible and resilient 30-day CSD mice, mitochondrial size and mass are reduced in the basolateral (BLA) and central nucleus (CeA) of the amygdala. In the hippocampus, the only change found is smaller mitochondria in the dentate gyrus (DG) and no differences in any other subregions (CA1, CA2, CA3). The functionality of mitochondria can be accessed by mitochondrial membrane potential (MMP), which drives ATP production. Tetramethylrhodamine ethyl ester (TMRE) accumulates in mitochondria in proportion to MMP, allowing it to act as an indicator for mitochondrial activity. In all 30-day CSD mice, TMRE is reduced in both amygdala subregions, but again no differences are seen anywhere in the ventral hippocampus. To access whether MMP changes result in altered ATP levels, phosphorylated AMP-activated protein kinase (p-AMPK), which inversely correlates with ATP levels, and an ATP sensor, ATPSnFR1.0, were used to access ATP. Confirming the trend seen, cellular ATP levels are only decreased in the amygdala. No alterations of mitochondrial functional indicators are found in 10-day CSD mice. These data suggest that 30-day CSD causes amygdala-specific mitochondrial changes.
Damaged or functionally impaired mitochondria undergo elimination through mitophagy, and mitochondrial biogenesis produces new mitochondria. To look at biogenesis, mtDNA was stained using EdU to indicate mitochondrial replication. The amygdala of mice defeated for 30 days has elevated mitochondrial production, but a reduction in mtDNA copy number. In addition, using mitoRCA-seq (mitochondria rolling circle sequencing) we found that mtDNA mutations are also increased in the BLA of defeated mice, consistent with mitochondrial biogenesis. Mitophagy removes these damaged, morphologically aberrant mitochondria. Using a lysosomal and autophagosome marker (LC3) or OPTN (optineurin; an autophagy receptor for damaged mitochondria), mitophagy was found to be increased, suggesting increased mitochondrial removal via mitophagy from the BLA in defeated mice. Further analysis using electron microscopy (EM) confirmed increased mitophagy.
Mitophagy has a well-known pathway. To test if this pathway is involved in the mitochondrial BLA impairments pink1 knockout (pink1−/−) and prkn knockout (prkn−/−) mice were given 30-day CSD and mitochondrial assessments. Notably, both PRKN and PINK1 are required for CSD-induced mitophagy increase.
Behaviorally, pink1 and prkn knockout mice that have undergone CSD show an increase in anxiety-like behaviors, but not in behavioral despair. To examine if mitochondrial deficiency in the amygdala can induce anxiety-like behaviors independent of external stress, mtKillerRed, which generates reactive oxygen species (ROS) upon light irradiation, was used in the BLA of mice to damage mitochondria while not killing the residing cell. Photostimulation increases anxiety-like behaviors in mice injected with mtKillerRed but not controls. EM confirmed that irradiation of mtKillerRed increases mitophagosome-like structures and abnormal mitochondria, along with reduced mitochondrial numbers in the BLA, without any additional cellular impairments. Finally, to examine the synaptic connections the BLA deviancies may affect, channelrhodopsin 2 (ChR2-EYFP) was injected into mice 4 weeks prior to undergoing the CSD protocol, and the BLA-adBNST pathway, which is anxiolytic underwent electrophysiological recording. EPSCs in adBNST neurons evoked by stimulating the BLA projections to adBNST show weakened synaptic transmission due to pre-synaptic deficiency. Mice with channelrhodopsin 2 injected in the BLA were then implanted with an optic fiber to enable BLA activation during CSD. Photostimulation of the BLA restores anxiety-like behaviors to pre-CS levels, suggesting the BLA-adBNST pathway is a key driver of CSD-induced impairments.
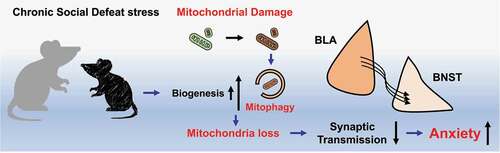
Overall, whether by CSD or specific induction, mitochondrial impairments in the BLA-adBNST pathway drive anxiety-like behaviors (). Mitophagy was seen as the key cellular pathway which drives this pathology and thus therapeutic targeting of mitophagy, or PINK1-PRKN could aid in alleviating the mitochondrial abnormalities that drive pathology.
Figure 1. Chronic social defeat stress in mice induces mitochondrial damage and biogenesis or mitophagy impairments through the PINK1-PRKN/parkin pathway. The stress-induced mitochondrial deficiencies in the basolateral amygdalae (BLA) results in weakened synaptic transmission in the BLA-BNST (bed nucleus of the stria terminalis) pathway, leading to anxiety-like behavior.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Reference
- Golden SA, Covington HE 3rd, Berton O, et al. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 2011;6:1183–1191.
