Abstract
Context: Zinc chloride (ZnCl2)-based smoke bombs and screens are in use since the Second World War (1939–1945). Many case descriptions on ZnCl2 smoke inhalation incidents appeared since 1945.
Objective: We provide a comprehensive overview of the clinical symptoms and underlying pathophysiology due to exposure to fumes from ZnCl2 smoke producing bombs. In addition, we give a historical overview of treatment regimens and their outcomes.
Methodology: We performed a literature search on Medline, Scopus and Google Scholar databases using combinations of the following search terms “smoke bomb”, “smoke screen”, “ZnCl2”, “intoxication”, “poisoning”, “case report”, “HE smoke”, “hexachloroethane smoke”, “smoke inhalation” and “white smoke”. We retrieved additional reports based on the primary hits. We collected 30 case reports from the last seven decades encompassing 376 patients, 23 of whom died. Of all the patient descriptions, 31 were of sufficient detail for prudent analysis.
Results and conclusions: Intoxication with clinical signs mainly took place in war situations and in military and fire emergency training sessions in enclosed spaces. Symptoms follow a biphasic course mainly characterised by dyspnoea, coughing and lacrimation, related to irritation of the airways in the first six hours, followed by reappearance of early signs complemented with inflammation related signs and tachycardia from 24 h onwards. Acute respiratory stress syndrome developed in severely affected individuals. Chest radiographs did not always correspond with clinical symptoms. Common therapy comprises corticosteroids, antibiotics and supplemental oxygen or positive pressure ventilation in 64% of the cases. Of the 31 patients included, eight died, three had permanent lung damage and 15 showed complete recovery, whereas in five patients outcome was not reported. Early signs likely relate to caustic reactions in the airway lining, whereas inhaled ZnCl2 particles may trigger an inflammatory response and associated delayed fibrotic lung damage. Smoke bomb poisoning is a potentially lethal condition that can occur in large cohorts of victims simultaneously.
Introduction
Smoke screens are in use for many centuries as a tactical aid in military operations. Pre-industrial applications depended on the combustion of natural products, such as damp straw, to generate an opaque cloud. New smoke generating techniques emerged during the First World War (1914–1918) [Citation1]. The French army developed the zinc chloride (ZnCl2)-based smoke screen. Ignition of a mixture of zinc (Zn), carbon tetrachloride (CCl4), zinc oxide (ZnO) and diatomite generated a ZnCl2 aerosol [Citation2]. In the United States prior to the Second World War (1939–1945), an improved mixture of Zn, ammonium perchlorate (NH4ClO4), hexachloroethane (HC) and ammonium chloride (NH4Cl) allowed easier transportation and storage. Further refinements to the mixture occurred later [Citation2]. The final compounds are ZnO, HC and aluminium metal. In a thermochemical reaction these compounds produce heat and an aerosol, from here onwards described as ZnCl2 smoke or fumes, that consists of ZnCl2 (62.5%), ZnO (9.6%), iron oxides (10.7%), aluminium oxides (5.4%) PbO (1.0%) and chlorinated vapours (10.8%), including hydrogen chloride (HCl) [Citation3,Citation4]. Additionally, ethylene tetrachloride (C2Cl4), carbon monoxide dichloride (COCl2) and carbon tetrachloride (CCl4) vapours arise [Citation3,Citation4].
Although a 1943 publication suggested limited health risks [Citation1], three separate reports published in 1945 illustrated the hazards of ZnCl2 smoke exposure [Citation5–7]. Currently, the scientific literature contains nearly 30 reports on subsequent incidents involving ZnCl2 exposure (). Most of these cases involve the use of smoke bombs during military trainings. Only several cases report civilian casualties in a non-military setting. We summarize these reports and discuss their clinical courses, imaging results, treatments and outcomes. We conclude by reviewing the pathophysiological mechanisms involved in ZnCl2 exposure.
Table 1. Overview of ZnCl2 intoxication cases.
Methods
We screened Medline, Scopus and Google Scholar databases using combinations of the following search terms “smoke bomb”, “smoke screen”, “ZnCl2”, “intoxication”, “poisoning”, “case report”, “HE smoke”, “hexachloroethane smoke”, “smoke inhalation” and “white smoke”. We retrieved publications from 1945 through 2015. We excluded reports on non-ZnCl2-based smoke producing devices, i.e., those using other chemical compounds. We retrieved additional case reports on the basis of references in the primary hits. Information on patient cohorts described more than once were combined and cited by the individual reports. Native readers from the Department of Medical Physiology read reports written in non-English languages (Dutch, French, German and Swedish) and discussed these with us in detail.
We subsequently selected those reports on individual patients of ZnCl2 smoke exposure that provided sufficiently detailed information with respect to symptoms, diagnostics, treatment regimens and/or outcomes (, column “cases analysed”). We analysed each of these cases separately and mapped the collected data to identify commonalities and trends across cases. This approach excluded three cohorts that failed to provide details for individual patients [Citation7,Citation8,Citation16]. In the first cohort [Citation7], ZnCl2 fumes in a tunnel intoxicated 70 subjects in 1943. Of these, 35 patients passed through first-aid care facilities and experienced dyspnoea, chest pain/tightness, retrosternal and epigastric pain, stridor and red and running eyes. Ten patients died. In the early 1950s, a thoughtless prank exposed 47 students to ZnCl2 smoke in a fraternity house [Citation8]. Of the 35 hospitalized cases, all experienced cough, approximately two-thirds showed fever and sore throat and one-third displayed vomiting, headache and chest pain. No deaths occurred. In 1979 a detonated smoke bomb exposed 135 individuals to ZnCl2 smoke in an airport disaster drill [Citation16]. Since exposure occurred in open air, only 10 to 20% of the patients experienced clinical signs (cough, sore throat, abnormal taste) and no fatalities occurred.
Results
In total we obtained 30 reports on ZnCl2 smoke exposure, containing 376 patients, for review (). Three papers consisted of large patient groups (47, 70 and 135 individuals), 10 papers described patient groups between 12 and 20 individuals in size, whereas 10 papers described groups between two and seven patients. Finally, seven papers only described an individual case. At least two case reports represent every decade since 1940. Case reports originated from Europe (17 papers), North America (six papers) and Asia (seven papers). Two reports described one incident involving two Danish soldiers [Citation19,Citation20]. Six reports covered the same set of 20 Taiwanese soldiers exposed in a military training exercise [Citation27–32].
The best studied cohort hitherto consists of the Taiwanese soldiers [Citation27–32]. They experienced ZnCl2 fume intoxication during a three to ten minutes exposure to dense smoke in a narrow tunnel during a combat exercise in 2003. All subjects suffered from nausea, sore throat, cough, shortness of breath and chest tightness. Five soldiers developed acute respiratory distress syndrome (ARDS). One patient suffering from ARDS improved upon prolonged treatment with high-dose corticosteroids, methylprednisolone and extracorporeal life support [Citation31]. The majority of patients presented a restrictive type of functional lung impairment, and a combination of functional tests and high-resolution CT scanning may predict its severity [Citation27]. Fumes from ZnCl2 smoke producing bombs caused hepatotoxicity in two patients [Citation28]. A follow-up study among all twenty soldiers and a large control group, indicated that ZnCl2 smoke can induce acute, dose-dependent liver dysfunction [Citation29]. Huang et al. linked systemic inflammation to ZnCl2 exposure in this cohort, in which tumour necrosis factor α concentrations increased and all patients had leucocytosis [Citation30]. Furthermore, temporarily decreased haemoglobin levels, haematocrit and red blood cell counts associated also with ZnCl2 fume exposure in this cohort [Citation32].
Modes of exposure
Exposures resulted mainly from incidents during war situations (four reports, 81 patients) [Citation5–7,Citation34], military trainings (19 reports, 85 patients) [Citation9–11,Citation14,Citation15,Citation19–32] and fire trainings (three reports, 138 patients) [Citation13,Citation16,Citation33]. In general, exposure of the victims to ZnCl2 smoke occurred in confined spaces only, like air-raid shelters, bunkers, tunnels, pipelines, rooms and vehicles. In the case described by Evans, for example, ignition of 79 smoke generators directly exposed 70 of the 116 individuals present in a tunnel to ZnCl2 smoke fumes [Citation7]. Loh et al. also described two cases of ZnCl2 smoke exposure in a tunnel [Citation28].
Several cases of ZnCl2 smoke exposure in confined spaces resulted from negligence or mischievous behaviour. Hoekstra described a case in which a smoke bomb was jokingly thrown in the back of a truck with 17 people in it. Of these individuals, nine were briefly observed at the infirmary whilst four others were hospitalised. One individual eventually succumbed to his injuries [Citation12]. In a similar case reported by Pettilä et al., a group of youngsters threw a smoke bomb into a small cottage with six people in it. Two individuals developed severe symptoms and were hospitalised. Both later died as a result of the exposure [Citation26]. In cases of open air exposure, victims were directly next to the source [Citation11,Citation16,Citation24]. In one case, a soldier ignited a smoke grenade under a poncho to escape from a bee attack [Citation17]. The resulting symptoms in this case were most likely due to ZnCl2 intoxication rather than phosgene [Citation35].
Clinical course
Because the majority of reported exposures occurred in military, police, or fire rescue training, most of the exposed individuals were young men. In fact, all case reports that contained sufficient information for analysis included only males (20 reports, 31 patients) [Citation5,Citation6,Citation9–15,Citation17–20,Citation22–24,Citation26,Citation28,Citation31,Citation33]. The ages of four patients were not reported [Citation12]. The remaining 27 cases had a mean age of 24.1 years. Clinical effects following exposure to ZnCl2 typically had a biphasic pattern. Symptoms occurring immediately after exposure included mucosal irritation, lacrimation, dyspnoea, cough, sore throat, retrosternal pain, nausea and vomiting. Some patients developed fever. These symptoms often subsided within several hours to one day.
Extensively exposed patients developed a second wave of symptoms of respiratory inflammation over the following days. Typical symptoms included dyspnoea, cough, fever, and malaise. It could take up to several weeks for the pyrexia and dyspnoea to abate. In most cases of ZnCl2 exposure, patients fully recovered.
Three papers reported transaminase elevations suggestive for hepatic effects of fume inhalation from ZnCl2 producing bombs [Citation26,Citation28,Citation29]. Alanine aminotransferase (ALT) concentrations peaked between three and five weeks after the exposure, and returned to normal between six and eight weeks following exposure [Citation29]. The median ALT concentration in the group of 20 exposed soldiers from Taiwan was approximately tenfold higher in comparison to a reference group of 64 non-exposed soldiers. The 10 individuals closest to the ZnCl2 source presented significantly higher ALT concentrations than the other 10 patients [Citation29]. The authors reported no occurrences of jaundice, bilirubin elevations or any other signs indicative for liver impairment.
provides an overview of the main symptoms presented in the individual case reports. It specifically shows the number of cases reporting a symptom at a particular point in time after exposure. As illustrated by , many symptoms existed for a brief period immediately after exposure, only to reappear again after one to several days. Extra-pulmonary symptoms, such as subcutaneous crepitus and generalized seizures were uncommon and reported in few cases more than one day after exposure [Citation29]. The clinical outcome depended highly on the dose and length of exposure. Unfortunately, absolute numbers of smoke density and exposure time were lacking. Extensive exposure could lead to a severe disease course culminating in ARDS and death [Citation19,Citation20,Citation25,Citation26]. In addition, a high exposure dose associated with lasting damage to the respiratory system. For example, victims exposed to a dense cloud of ZnCl2 smoke in a confined space developed subglottic stenosis and restricted pulmonary dysfunction [Citation6,Citation26].
Table 2. Frequency of symptoms present after ZnCl2 smoke exposure.
Imaging and functional tests
A total of 23 reports include data from chest radiograph examinations of patients following exposure to ZnCl2 smoke [Citation5,Citation6,Citation8,Citation11–16,Citation18–23,Citation25–28,Citation30–33]. These radiographs showed an interesting pattern when ordered chronologically. In the acute setting, immediately after hospital admission, radiographs remained unremarkable or exhibited signs of pulmonary oedema located around the hilar regions of the lungs. Only radiographs taken more than 48 h after exposure showed more diffuse bilateral infiltrative processes. This may be related to the pattern of ZnCl2 particle diffusion throughout the lungs. It took a relatively long time, i.e., from seven weeks up to 12 months, for the radiographs to return to normal.
Radiographic results did not correlate with the severity of symptoms. This was especially apparent in the first few hours after exposure when patients experienced early symptoms, such as coughing and dyspnoea. Radiographs taken at this stage generally showed no signs of lung infiltrate. Conversely, radiographs may show bilateral consolidations a few days after exposure, at a time when patients begin to recuperate and feel better.
ZnCl2 smoke exposure and spirometry parameters appeared to exhibit an inverse relationship. The amount of smoke a victim was exposed to depended on whether the exposure was in open air as opposed to a confined space, whether the individual was wearing a gas mask and the duration of the exposure. Schenker et al. provided the most extensive account on spirometry results following ZnCl2 smoke exposure in open air [Citation16]. This report described spirometry results for 63 individuals and found no significant effect of exposure in open air on either FEV1 or FVC. However, in cases in which victims were located in an enclosed space and wore ill-fitting gas masks [Citation19,Citation20] or when exposure was less than two minutes [Citation21,Citation23,Citation30], spirometry parameters were aberrant (i.e., restrictive ventilation impairment with mostly reduced TLC, VC and FEV1 values) but normalized within two to seven weeks.
Individuals exposed for longer than two minutes showed a remarkably slower pace of normalization (several months) or incomplete recovery of lung function [Citation18,Citation22,Citation25–27,Citation30,Citation31]. Reports on vital parameters show that virtually all patients exhibited signs of inflammation, such as fever, tachypnoea and tachycardia [Citation6,Citation7,Citation11–14,Citation18–20,Citation22,Citation23,Citation26,Citation27,Citation30,Citation31,Citation33]. Leukocyte counts were elevated in most cases, with higher levels in severely exposed patients [Citation8,Citation12,Citation15,Citation28,Citation30]. The most common acid-base disorder found at acute presentation was respiratory alkalosis, as a consequence of hyperventilation [Citation19,Citation22,Citation26,Citation31]. Unfortunately, the individual case reports lacked functional assessment outcome.
Post-mortem findings
Seven reports discussed post-mortem findings of patients who died as a result of ZnCl2 smoke exposure [Citation7,Citation12–14,Citation19,Citation20,Citation33]. The eight patients from these reports had a median survival of 10 days after exposure (range 4 hours–32 days). Macroscopically, the mucous membranes of the tracheobronchial and upper respiratory tracts were severely damaged [Citation7,Citation12–14,Citation33]. More specifically, mucosae had a red and very oedematous aspect [Citation7]. Additionally, there was necrosis, roughening and a yellow-grey colouring of the mucosae [Citation7,Citation12–14]. The lungs showed a solid homogeneous aspect with oedema, areas of haemorrhage and infarction and widespread fibrosis [Citation7,Citation12–14,Citation19,Citation20,Citation33]. A dry and red surface with prominent vascular septa arose when the lungs were sliced [Citation14]. Two reports described necrosis, numerous cysts and yellowish areas interpreted as foci of pneumonitis [Citation13,Citation33].
The macroscopic observations were congruent with the microscopic findings. Lungs were mostly airless because of intra-alveolar and interstitial oedema and haemorrhage. In addition, intra-alveolar fibrosis with varying degrees of alveolar condensation contributed to this airlessness [Citation7,Citation12–14,Citation19,Citation20,Citation33]. Widespread occlusion of micro vessels developed as a result of endothelial cell proliferation and clots [Citation19,Citation33]. Arterial injection of barium-gelatin sulphate, from which vessels appear white on an arteriogram when properly filled, confirmed the reduced filling of small arteries [Citation19].
Besides the findings in the respiratory system, ZnCl2 exposure associated also with congestion and oedema of liver, kidney, spleen and brain [Citation7,Citation19,Citation33]. One report emphasized the absence of microscopic abnormalities in liver and kidneys of two patients [Citation7], whereas another reported tubular necrosis in one patient [Citation19]. Five patients showed cardiac involvement (right sided dilatation in four, a large right ventricular thrombus and thrombi in epicardic blood vessels in one patient) [Citation7,Citation19,Citation33], whereas the heart of a sixth patient was normal [Citation7].
Treatment
Of the 31 reviewed cases, all but one described treatment regimens [Citation5]. The most frequently applied treatments were supplemental oxygen and positive pressure ventilation, antibiotics, corticosteroids and various combinations of these. Five or fewer patients received other forms of pharmacotherapy, e.g., terpinhydrate, codein, promethazine, aminophylline and calcium. Several cases also mentioned non-pharmacological therapies, such as bed rest, tracheotomy, physical therapy and steam inhalation. Only eight or fewer cases received non pharmacological therapies. In general, treatment regimens followed the prevailing developments in clinical care during the last seven decades.
The first report on the use of corticosteroids in the treatment of ZnCl2 smoke intoxication appeared in 1961 [Citation11]. In subsequent years, different types of corticosteroids became available. The use of corticosteroid therapy as the main treatment for ZnCl2 smoke intoxication, either alone or in combination with antibiotics and supplemental oxygen/positive pressure ventilation, increased during the seven decades covered in this analysis. In the first four decades (1945–1984), physicians used corticosteroids in 44% (7 out of 16) of cases. This number increased to 86% (12 out of 14) in the last three decades (1985–2015).
None of the analysed case reports published prior to 1956 mentioned supplemental oxygen or artificial ventilation. In fact, positive pressure ventilation became widely applied since the 1950s only [Citation36,Citation37]. However, patients in the series reported by Evans received supplemental oxygen through a partial rebreathing mask [Citation7].
The treatment regimen for ZnCl2 smoke-induced injury frequently included antibiotics in order to prevent bacterial infection of damaged lung tissue. In the time period preceding 1985, patients underwent courses of sulfapyridine [Citation6], sulfathiazole [Citation6], penicillin [Citation9,Citation10,Citation12,Citation13], streptomycin [Citation10,Citation12], erythromycin [Citation10], tetracycline [Citation10,Citation11,Citation15], benzylpenicillin [Citation10] and chloramphenicol [Citation15]. After 1985, patients received erythromycin [Citation18], oxacillin [Citation26], gentamicin [Citation26], ceftriaxone [Citation26], ceftazidime [Citation28,Citation31] and levofloxacin [Citation33]. These cases illustrate the wide use of antibiotics in the treatment of ZnCl2 smoke intoxication. Even though the prescribed courses followed the development of antibiotics through history, the courses of antibiotics tended to differ greatly across all cases. This is most likely due to differing antibiotic protocols across countries and hospitals. Generally, physicians prescribed antibiotics as a prophylactic treatment rather than to cure existing bacterial infections. This practice is analogous to the case of aspiration pneumonitis in children with hydrocarbon exposures. It is known for decades that antibiotics and steroids are not useful in hydrocarbon aspiration pneumonitis [Citation38,Citation39], however, they are still prescribed in these children. Similarly, antibiotics are part of the common therapy for ZnCl2 smoke intoxication, however, evidence that they are part of the optimal treatment is absent.
Outcome
provides information on disease outcome for all 31 patients included in this review [Citation5,Citation6,Citation9–15,Citation17–20,Citation22–24,Citation26,Citation28,Citation31,Citation33]. Primary outcome was complete recovery or permanent damage measured by chest radiograph or spirometry. In five patients, no data about complete recovery or permanent damage were given [Citation6,Citation26,Citation28,Citation31]. In two patients, spirometry was performed, however, no data were presented when the patient had fully recovered [Citation26,Citation31]. In three patients, no spirometry or chest radiograph was performed to assess recovery [Citation6,Citation28]. Approximately, 25% of the patients died due to ZnCl2 smoke-induced injury and 50% made a complete recovery.
Figure 1. Clinical outcomes classified as died, “permanent damage” or “complete recovery” of 31 patients with ZnCl2 smoke poisoning. Patient numbers are indicated in bold font. Extent of permanent damage was established by imaging (chest radiograph) or functional (spirometry) techniques, or by a combination of both investigations.
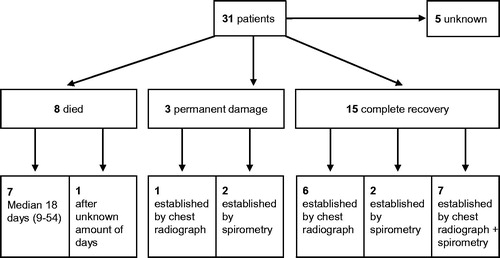
Recovery times varied greatly from several days to 12 months after exposure. Recovery assessment by chest radiography had a median of 69 days (mean 163 days, range 48–366 days). Recovery time based on chest radiograph results was not reported for one patient. In two patients that underwent spirometry only, clinical recovery time was 28 days. For seven patients that both underwent chest radiograph and spirometry assessment, recovery time based on radiograph results varied between 10 and 100 days, with a mean of 31 days and a median of 16 days. Based on spirometry results of four patients, recovery times varied from 10 to 275 days, with a mean of 76 days and a median of 10 days. Spirometry based recovery times were not provided for the other three patients.
Outcome related to treatment
Treatment of ZnCl2-induced smoke injury consisted of monotherapy using supplemental oxygen or positive pressure ventilation, antibiotics or corticosteroids, or co-therapy using combinations of these. The groups are small, except for the patients treated with supplemental oxygen/positive pressure ventilation, corticosteroids and antibiotics. Therefore, we cannot make statements on the optimal treatment regimen. Even in the group that received what is now considered as common care (supplemental oxygen/positive pressure ventilation, corticosteroids and antibiotics), the mortality rate is considerable (6 out of 14 patients died). It is plausible that those who received more treatment were sicker and therefore had a higher mortality rate than those requiring less treatment.
Human pathogenesis and animal studies
The pathophysiological mechanism of ZnCl2 smoke-induced injury was the subject of much speculation in early case reports. Initially, authors attributed the typical biphasic course of symptoms to direct damage to the airways and subsequent lung tissue damage. In fact, Whitaker theorised that early symptoms arose by direct irritation of the airways. This irritation may partially result from hydrochloric acid formed in the explosion. He thought that late symptoms resulted from damage to the alveolar epithelium caused by retained smoke particles in the lungs [Citation5]. Another theory postulated by Evans suggested that both the precipitation of heated smoke particles in the lungs and the hygroscopic and astringent properties of ZnCl2 caused the lung damage [Citation7].
Indeed, initial symptoms, such as coughing and chest tightness, appear to result directly from irritation of tissue structures within the throat, trachea and bronchi [Citation20]. Systemic factors are, however, likely involved in the development of later symptoms. For example, a rapid increase in the pro-inflammatory cytokine TNF-α, haematological changes (i.e., temporary reduction of haemoglobin, red blood cell count and haematocrit) and transaminase elevations associated with fume exposure from ZnCl2 smoke producing bombs [Citation26,Citation28,Citation30,Citation32]. These findings as well as the associated temperature rise in many cases, suggest the involvement of systemic inflammation. ZnCl2 smoke inhalation may in fact induce a form of metal fume fever. In metal fume fever, tissue damage by a heavy metal leads to the discharge of cytokines causing signs of systemic inflammation [Citation20]. The involvement of inflammatory cells may also explain cases in which lung fibrosis developed, as these cells can activate fibroblasts in the lung parenchyma [Citation40].
Animal studies provided useful insights into the pathophysiological mechanisms involved in ZnCl2 smoke induced injury. In particular, several animal studies confirm the positive correlation between exposure dose and severity of symptoms [Citation41–44]. For example, Karlsson et al. found that rats exposed to a relatively high dose of ZnCl2 smoke (200 mg/m3) for five minutes had a 50% mortality rate [Citation41]. In comparison, the US National Institute for Occupational Safety and Health declared a dosage of 50 mg/m3 immediately dangerous to life and health [Citation45]. Rats exposed to a dose of 50 mg/m3 showed minor symptoms, e.g., wet noses and swollen eyelids. These symptoms disappeared gradually and the animals appeared normal 24–48 h after exposure. Rats exposed to higher doses developed severe symptoms in the hours after exposure [Citation41].
In animal studies, diffuse bronchopneumonia and interstitial pneumonitis in the first three days after exposure dominated the histological findings. In the first and second week after exposure, inflammatory infiltrates in the lungs and disseminated confluent atelectasis were found. These findings are consistent with the biphasic course of the disease as observed in the reviewed cases. Interestingly, rats sacrificed three months after exposure still showed residual inflammatory infiltrate and band-like atelectasis [Citation41]. This is also congruent with the extended recovery times observed in multiple cases. In addition, ZnCl2 smoke exposure induced acute inflammation and necrosis of the larynx and bronchi in rats and rabbits [Citation42].
Radiological findings in dogs showed pulmonary oedema with an increased lung volume as soon as two hours after exposure [Citation46]. This is striking, as chest radiographs appeared unremarkable in the acute setting in patients. A possible explanation for these inconsistent findings may be differences in the exposure doses. That is, the dog study may have exposed the subjects to relatively higher concentrations of ZnCl2 smoke, causing more severe symptoms in these subjects. This difference may, however, also result from variation between species.
Extra-pulmonary symptoms of ZnCl2 producing bomb fume inhalation are uncommon, except for hepatotoxicity. A number of reports described raised serum ALT concentrations [Citation26,Citation28,Citation29]. ALT concentrations increased from week one to two after exposure and reached a peak in week three to five. A possible cause of the transaminase elevations is the treatment regimen for pulmonary symptoms. Both steroids and antibiotics are potentially hepatotoxic. A more likely cause, however, are chlorinated compounds present in the smoke, such as CCl4 [Citation44]. These chlorinated compounds are hepatotoxic [Citation47,Citation48]. In the metabolism of CCl4 a trichloromethylperoxyl radical is formed. This compound may attribute to liver injury [Citation47].
There is not much convincing evidence supported by studies in men. Cullumbine exposed volunteers to increasing concentrations of ZnCl2 smoke, 80 mg/m3 and 120 mg/m3 respectively, for two minutes [Citation49]. Subjects exposed to the lower dose showed only minor symptoms, such as slight nausea and an occasional cough. Those exposed to a concentration of 120 mg/m3 clearly had more severe symptoms; all volunteers complained of irritation of the nose, throat and chest. Moreover, all the subjects experienced nausea and had a cough. Cullumbine’s study supports our findings that people who are exposed to a higher concentration of ZnCl2 smoke (e.g., those who were closer to the source of the smoke or who were exposed in a confined space), showed more severe symptoms.
Based on animal studies, Cullumbine estimated a human lethal dose of 50,000 mg min/m3. In a confined room this dosage can be reached within two to three minutes [Citation49].
Limitations
Publication bias likely favours reporting of more severe cases and likely underreports milder exposures.
Information on ZnCl2 exposure dose was in many cases not available or incomparable due to a lack of standardisation. In order to allow for cross-group comparisons, we used the exposure time and space in which the exposure took place, i.e., confined space or open air, as proxies for the exposure dose. We assumed that exposure doses were higher for those that were exposed for a prolonged time period and/or in a confined space.
Several case reports provide no information on the exact constituents of the ZnCl2 smoke producing devices. Therefore, there is a possibility that chemical agents other than ZnCl2 may be responsible for the systemic effects, for instance hepatotoxicity, described. The available published cases primarily included healthy young men in military service. The health effects may differ among women, children, older patients, or patients with other chronic illness (especially pulmonary disease).
Conclusions and recommendations
Smoke screens and smoke bombs containing ZnCl2 may cause serious harm in exposed individuals. Exposures most often occur in military training activities or combat. The primary determinants of health effects are the intensity and duration of exposure. Even brief exposures, especially occurring in confined spaces, result in mucosal irritation of the respiratory tract, eyes, and upper digestive tract with dyspnoea, cough, and lacrimation being the most common symptoms. Airway inflammation resembling metal fume fever often follows one to two days after an intense exposure. Severe cases may progress to ARDS. Chest radiographs may appear normal or mildly abnormal early in the course of disease despite severe symptoms and may remain abnormal while symptoms are resolving. Maintaining adequate oxygen delivery is likely the most important treatment goal. No clear and convincing evidence supports the use of corticosteroids or antibiotics.
Acknowledgements
We thank Malin Jonsson-Boezelman (Genome Institute of Singapore), Jane Synnergren (University of Skövde, Sweden), Hugues Abriel (Bern University, Switzerland), Yuan Ji and Alexandre Bossu (both University Medical Hospital Utrecht, The Netherlands) for their help in retrieving and/or translating non-English case reports.
Disclosure statement
The authors report no declarations of interest.
References
- Nicholson DG. Chemical agents as screening smokes. J Chem Educ. 1943;20:499–505.
- US Patent. Publication number 3329624 A [Internet]. [cited 2016 Jan 19]. Available from: www.google.com/patents/US3329624
- United States. IIT Research Institute. Physical and chemical characterization of fog oil smoke and hexachloroethane smokes. Chicago (IL): IIT Research Institute; 1980.
- National Research Council (US) Subcommittee on Military Smokes and Obscurants. Toxicity of military smokes and obscurants. Vol. 1. Washington (DC): National Academies Press; 1997. Chapter 5, Hexachloroethane smoke; p. 127–159.
- Whitaker PH. Radiological appearances of the chest following partial asphyxiation by a smoke screen. Br J Radiol. 1945;18:396–397.
- Lumsden RB, Weir CD. Subglottic stenosis after high concentration of screening smoke. Br Med J. 1945;1:554–555.
- Evans EH. Casualties following exposure to zinc chloride smoke. Lancet. 1945;246:368–370.
- Fitzpatrick WJ, Yeager LB. Respiratory complications from military screening smoke bomb. Q Bull Northwest Univ Med Sch. 1952;26:313–314.
- Pare CM, Sandler M. Smoke-bomb pneumonitis: description of a case. J R Army Med Corps. 1954;100:320–322.
- Linderholm H, Strandberg O. Lungskador i samband med rökgasexposition [Lung injury associated with smoke explosion]. Tidskr Mil Halsov. 1956; 81:229–241. Swedish.
- Johnson FA, Stonehill RB. Chemical pneumonitis from inhalation of zinc chloride. Dis Chest. 1961;40:619–624.
- Hoekstra JA. De gevolgen van blootstelling aan de inhoud van rookpotten [The effects of smoke bomb exposure]. Nederlands Militair Geneeskundig Tijdschrift. 1963;16:141–147. Dutch.
- Milliken JA, Waugh D, Kadish ME. Acute interstitial pulmonary fibrosis caused by a smoke bomb. Can Med Assoc J. 1963;88:36–39.
- Macaulay MB, Mant AK. Smoke-bomb poisoning. A fatal case following the inhalation of zinc chloride smoke. J R Army Med Corps. 1964;110:27–32.
- Schmahl K. Klinik der Zinknebelvergiftung [Clinical features of zinc fog poisoning]. Pneumonologie. 1974;150:161–169. German.
- Schenker MB, Speizer FE, Taylor JO. Acute upper respiratory symptoms resulting from exposure to zinc chloride aerosol. Environ Res. 1981;25:317–324.
- Wang YT, Lee LK, Poh SC. Phosgene poisoning from a smoke grenade. Eur J Respir Dis. 1987;70:126–128.
- Matarese SL, Matthews JI. Zinc chloride (smoke bomb) inhalational lung injury. Chest. 1986;89:308–309.
- Hjortsø E, Qvist J, Bud MI, et al. ARDS after accidental inhalation of zinc chloride smoke. Intensive Care Med. 1988;14:17–24.
- Homma S, Jones R, Qvist J, et al. Pulmonary vascular lesions in the adult respiratory distress syndrome caused by inhalation of zinc chloride smoke: a morphometric study. Hum Pathol. 1992;23:45–50.
- Harry P, Caubet A, Durand G, et al. Intoxications aiguës par inhalation de chlorures de zinc et d’aluminium (12 observations) [Acute poisoning by inhalation of zinc and aluminum chloride (12 cases)]. J Toxicol Clin Exp. 1989;9:359–362. French.
- Allen MB, Crisp A, Snook N, et al. ‘Smoke-bomb’ pneumonitis. Respir Med. 1992;86:165–616.
- Freitag A, Caduff B. ARDS durch militärische Zinknebelexposition [ARDS resulting from military zinc fog exposure]. Schweiz Med Wochenschr. 1996;126:1006–1010. German.
- Holmes PS. Pneumomediastinum associated with inhalation of white smoke. Mil Med. 1999;164:751–752.
- Zerahn B, Kofoed-Enevoldsen A, Jensen BV, et al. Pulmonary damage after modest exposure to zinc chloride smoke. Respir Med. 1999;93:885–890.
- Pettilä V, Takkunen O, Tukiainen P. Zinc chloride smoke inhalation: a rare cause of severe acute respiratory distress syndrome. Intensive Care Med. 2000;26:215–217.
- Hsu HH, Tzao C, Chang WC, et al. Zinc chloride (smoke bomb) inhalation lung injury: clinical presentations, high-resolution CT findings, and pulmonary function test results. Chest. 2005;127:2064–2071.
- Loh CH, Chang YW, Liou SH, et al. Case report: hexachloroethane smoke inhalation: a rare cause of severe hepatic injuries. Environ Health Perspect. 2006;114:763–765.
- Loh CH, Liou SH, Chang YW, et al. Hepatic injuries of hexachloroethane smoke inhalation: the first analytical epidemiological study. Toxicology. 2008;247:119–122.
- Huang KL, Chen CW, Chu SJ, et al. Systemic inflammation caused by white smoke inhalation in a combat exercise. Chest. 2008;133:722–728.
- Chian CF, Wu CP, Chen CW, et al. Acute respiratory distress syndrome after zinc chloride inhalation: survival after extracorporeal life support and corticosteroid treatment. Am J Crit Care. 2010;19:86–90.
- Chou CH, Kao TW, Liou SH, et al. Hematological abnormalities of acute exposure to hexachloroethane smoke inhalation. Inhal Toxicol. 2010;22:486–492.
- Gil F, Pla A, Hernández AF, et al. A fatal case following exposure to zinc chloride and hexachloroethane from a smoke bomb in a fire simulation at a school. Clin Toxicol. 2008;46:563–565.
- Yilmaz B, Yeşiloğlu N, Firincioğullari R, et al. Awful face of the war-impacted smoke bomb capsule in the face and systemic toxicity: reports from the conflict in Syria. J Craniofac Surg. 2015;26:167–169.
- Karlsson N. Poisoning from smoke grenades is not due to phosgene. Eur Respir J. 1988;1:575.
- Meinesz AF, Wijkstra PJ, Zijlstra JG, et al. Van de poliomyelitisepidemie naar de oprichting van beademingscentra, intensivecareafdelingen en centra voor thuisbeademing [From the poliomyelitisepidemia to establishing ventilation centers, intensive care units and centers for homeventilation]. Ned Tijdschr Geneeskd. 2006;150:444–449. Dutch.
- Slutsky AS. History of mechanical ventilation. from vesalius to ventilator-induced lung injury. Am J Respir Crit Care Med. 2015;191:1106–1115.
- Eade NR, Taussig LM, Marks MI. Hydrocarbon pneumonitis. Pediatrics. 1974;54:351–357.
- Balme KH, Zar H, Swift DK, et al. The efficacy of prophylactic antibiotics in the management of children with kerosene-associated pneumonitis: a double-blind randomised controlled trial. Clin Toxicol. 2015;53:789–796.
- Goldstein RH, Fine A. Fibrotic reactions in the lung: the activation of the lung fibroblast. Exp Lung Res. 1986;11:245–261.
- Karlsson N, Cassel G, Fängmark I, et al. A comparative study of the acute inhalation toxicity of smoke from TiO2-hexachloroethane and Zn-hexachloroethane pyrotechnic mixtures. Arch Toxicol. 1986;59:160–166.
- Marrs TC, Clifford WE, Colgrave HF. Pathological changes produced by exposure of rabbits and rats to smokes from mixtures of hexachloroethane and zinc oxide. Toxicol Lett. 1983;19:247–252.
- Reaves WW, Carlon KG. Comparison of dechlorane and hexachloroethane in smokescreen compositions. CRDL Special Publication, AD 2663645 [Internet]. 1961 [cited 2016 Jan 27]. Available from: http://oai.dtic.mil/oai/oai?verb=getRecord&metadataPrefix=html&identifier=AD026636449
- Marrs TC, Colgrave HF, Edginton JA, et al. The repeated dose toxicity of a zinc oxide/hexachloroethane smoke. Arch Toxicol. 1988;62:123–132.
- Centers for Disease Control and Prevention. Zinc chloride fume [Internet]. [updated 2014 Dec 4; cited 2016 Apr 21]. Available from: http://www.cdc.gov/niosh/idlh/7646857.html
- Ardran GM. The pulmonary effects of toxic gases and smokes; an experimental radiographic investigation. Br J Radiol. 1950;23:107–115.
- Mico BA, Pohl LR. Reductive oxygenation of carbon tetrachloride: trichloromethylperoxyl radical as a possible intermediate in the conversion of carbon tetrachloride to electrophilic chlorine. Arch Biochem Biophys. 1983;225:596–609.
- Farber JL, Gerson RJ. Mechanisms of cell injury with hepatotoxic chemicals. Pharmacol Rev. 1984;36:71S–75S.
- Cullumbine H. The toxicity of screening smokes. J R Army Med Corps. 1957;103:119–122.
