Abstract
Objective: Although anecdotal reports suggest that intravenous lipid emulsion (ILE) therapy is effective in a large variety of overdoses, the few controlled human trials published to date yielded disappointing results. Because of potential publication biases, there are few reports concerning the failure of ILE. The primary aim of this study was to identify fatal poisoning cases in the American Association of Poison Control Centers (AAPCC) National Poison Data System (NPDS) in which ILE was administered.
Methods: We obtained an approved release of data from NPDS for years 2010–2015 in which the words “lipid,” “ILE,” or “fat” appeared in the narrative. Duplicate cases were excluded as were cases in which ILE was not clearly given. Case data were extracted by one author using a predetermined tool, and the information was confirmed by a second author. The timing of ILE administration was characterized into one of four categories: cardiac arrest, first line, last resort, or part of multiple therapies given simultaneously. Response to ILE and adverse events was recorded.
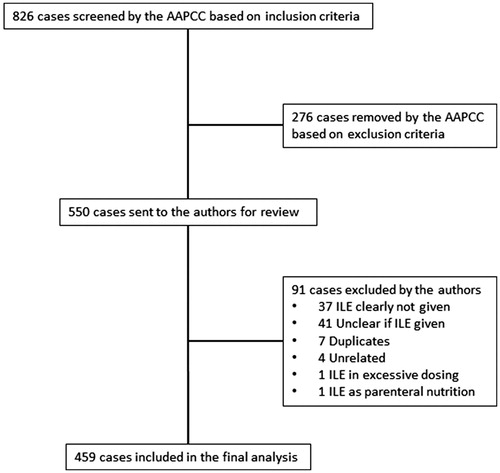
Results: Of the 826 cases retrieved from NPDS, 459 met final inclusion criteria. Over 50% of included cases involved either a calcium channel blocker or a beta-adrenergic antagonist. Of note, less than 25% of cases involved a substance for which the Lipid Emulsion Working Group found evidence to support its use. Most often, ILE was given along with multiple therapies (277 cases) or as a last resort (137 cases). In 127 cases, ILE was given during cardiac arrest. ILE was used as first line therapy in 34 cases. Response rates were reported as follows: no response (45%), unknown response (38%), transient/minimal response (7%), ROSC (7%), and immediate worsening (3%). Possible adverse reactions included: ARDS in 39 patients, lipemia causing a delay in laboratory evaluation in three cases, lipemia causing failure of a CRRT filter in two cases, worsening or new onset seizure in two cases, asystole immediately after administration in two cases, and fat embolism in one case.
Conclusion: Within the Association of Poison Control Centers (AAPCC) National Poison Data System (NPDS), hundreds of cases exist in which ILE therapy was given and death occurred. In many of these cases, ILE was given prior to cardiovascular collapse. Although there is some suggestion of transient improvement in a small subset of cases, adverse effects are also reported. When taken in totality, the number of published cases of failed lipid emulsion therapy outnumbers the published instances of ILE success. Given all the uncertainty generated by case reports, the evaluation of the role and efficacy of ILE therapy in non-local anesthetic poisoning needs robust controlled clinical trials.
Introduction
Although anecdotal reports suggest that intravenous lipid emulsion (ILE) therapy is effective in a large variety of overdoses, the few controlled human trials published to date yield disappointing results [Citation1–4]. Additionally, because of potential publication biases, less publications address the failure of ILE, either when used as a last resort or as primary therapy for local anesthetic systemic toxicity (LAST). There are recent published recommendations from the Lipid Emulsion Workgroup on the use of ILE therapy in poisonings derived from systematic reviews of ILE in local-anesthetic toxicity, non-local anesthetic overdoses, clinical adverse events reported, and effect on laboratory analyses [Citation5]. These recommendations draw attention to limitations and points of concern in the numerous reports in medical journals over the previous decade regarding the off-label use of ILE both in LAST and overdoses from a large variety of other substances. Currently, several formulations of ILE are approved for parenteral nutrition only. The understanding of the efficacy of ILE, its mechanism of action, and safety remain incomplete, and the true numbers of positive and negative outcomes of ILE therapy remain unreported. Most published cases lack information on adverse events or drug interactions with other treatments. Unsuccessful or adverse outcomes likely remain under-reported. Data on the effect of analytical interferences on frequently monitored critical clinical biochemical analyses were well reported. Recommendations from the workgroup described limitations due to a lack of controlled studies with models sufficiently resembling clinical poisonings.
The primary aim of this study was to identify fatal poisoning cases in the American Association of Poison Control Centers (AAPCC) National Poison Data System (NPDS) in which patients received ILE. Secondary aims included demographic data of cases, description of substances and routes involved, the timing of ILE in relation to other therapies, response to ILE therapy, and adverse effects of ILE. We examined fatal cases because the NPDS only includes case narratives for fatal cases and until 2018 has had no mechanism or treatment code to identify non-fatal cases involving ILE
Materials and methods
The National Poison Data System (NPDS), maintained by the AAPCC, captures data on all calls to poison centers serving the entire United States and its territories. In addition to capturing demographic and substance data, there is a meticulous process to describe cases with fatal outcomes [Citation6].
We obtained an approved release of data from NPDS for years 2010–2015 in which the words “lipid,” “ILE,” or “fat” appeared in the narrative. We included cases if they clearly received ILE and the relative contribution to fatality (RCF) was 1 or 2, meaning that the substance(s) were undoubtedly or probably responsible for the death [Citation6]. The NPDS team conducted a preliminary review of each narrative and removed any cases in which patients did not receive ILE. As the NPDS team did not review for duplicates and may have missed cases in which there was uncertainty about ILE administration, the authors divided up cases for further review to exclude cases in which either patient did not receive ILE, the case was a duplicate, or in which the substance responsible for the death was coded as ILE. Duplicate cases occur when two poison centers inadvertently create a narrative on the same patient. This can happen when the patient is treated in two adjacent states, or when there is a consultation with a toxicologist from another state. We defined duplicates as cases in which there was an exact match of the date of exposure, date of death, age, sex, and substance list and where the narrative was substantially similar. We excluded cases in which the narrative review did not explicitly confirm ILE administration.
One author determined the timing of ILE administration in relation to other therapies and a second author confirmed it. We created four possible categories to distinguish the timing of administration with more than one category possible for each case. Cardiac arrest included cases in which patients received ILE a short time after or in response to a cardiac arrest. First line included cases in which patients receive ILE prior to other aggressive therapies. Last resort included cases in which it was clear that the patient received other aggressive therapies before ILE. Cocktail represented cases in which ILE was one of the multiple therapies in the narrative and the order of administration of each therapy could not be determined. We categorized the response to ILE therapy as ROSC (return of spontaneous circulation) if there was a clear statement in the narrative of a temporal relationship. We classified cases with clearly described relapse soon after ILE or clearly described minimal response to ILE as transient/minimal response. We coded “no response” if there was a definitive statement of a total lack of response and “immediate worsening” if the patient rapidly deteriorated after ILE administration.
We considered adverse reactions attributable or related to ILE therapy if the narrative clearly stated as such, if the effect was previously reported from ILE therapy [Citation7], or if the author concluded that the reaction was more likely related to the ILE than to the primary poison.
We obtained institutional review board exemptions from the University of New Mexico Health Sciences Center and the NYC Department of Health and Mental Hygiene. We used an Excel spreadsheet to evaluate demographic data and followed the STROBE guidelines for observational studies [Citation8].
Results
The AAPCC initially retrieved 826 cases. They excluded 276 cases who did not receive ILE. We further excluded 91 cases. In 37 of those, ILE was not administered. The narrative did not specify if patients received ILE in 41 cases. There were seven duplicates. Four cases contained the search term “lipid” within a term not related to ILE (e.g., hyperlipidemia). One case was a primary excessive dosing of ILE. Another case was a primary exposure to lipid as part of total parenteral nutrition therapy ().
We conducted the final analysis on 459 unduplicated cases. The mean age of patients included was 46 years, with a median age of 48 years, and range 6 months–96 years. There were 20 children under the age of 18 included. There were more females than males (260 (57%) vs. 199 (43%)).
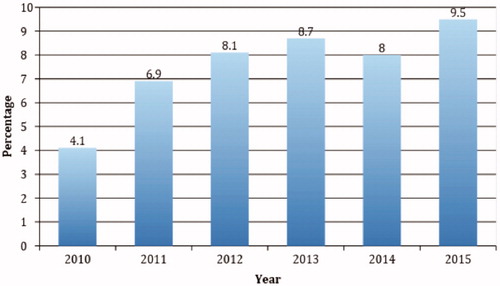
A trend analysis of the 6 years of data showed increased mention of ILE in fatal narratives over time. There were 40 mentions in 2010 and 102 mentions in 2015 in which ILE was used. By comparison, there were 965 total fatal cases with RCF 1 or 2 in NPDS in 2010 and 1070 cases in 2015. This represents a 155% increase in ILE mentions compared to an 11% increase in all reported fatalities with RCF of 1 or 2 over the same timeframe. During the 6 years of the study, there were 6026 fatalities in NPDS with RCF of 1 (undoubtedly responsible) or 2 (probably responsible). Of these, 459 case narratives (7.6%) included ILE administration. shows the relationship between ILE use in RCF 1 and 2 fatalities to the total RCF 1 and 2 fatalities over time.
Figure 2. Annual trends in intravenous lipid emulsion use in cases of fatal poisoning. The graph demonstrates the number RCF category 1 or 2 fatal cases in which intravenous lipid emulsion therapy was used, represented as a percentage of all RCF category 1 or 2 cases in that year.
We evaluated the substances involved in these exposures regardless of their causality ranking by AAPCC. Of the 459 cases, 183 (39.9%) involved overdose with a calcium channel blocker and 102 (22.2%) of cases involved a beta-adrenergic antagonist. Substances involved for which the Lipid Emulsion Working Group provided a recommendation for use of ILE include bupropion, tricyclic antidepressants, and local anesthetics [Citation5]. The database reported these substances in only 109 cases (23.7%); therefore, the majority of cases involved the use of a therapy that the systematic review found no evidence to support (). The timing of ILE administration in relation to other therapies is indicated in .
Figure 3. Timing of intravenous lipid emulsion administration. Some cases have more than one timing situation because of multiple administrations of intravenous lipid emulsion.
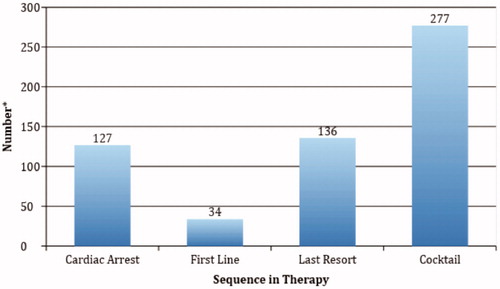
Table 1. List of the most common substances involved in the exposure.
The most frequent category of response to therapy was “no response” (45%), followed by “unknown response” (38%), “transient/minimal response” (7%), ROSC (7.4%), and “immediate worsening” (3%). Of the 34 cases achieving ROSC, five involved flecainide (out of 21 cases), five involved bupropion (out of 53 cases), and eight involved a calcium channel antagonist (out of 183 cases). Possible adverse reactions attributed to ILE by the narrative authors included ARDS with hypoxemia and/or fluid overload in 39 patients (8.5%), lipemia causing delay in laboratory evaluation in three cases, lipemia causing failure of a CRRT filter in two cases, worsening or new onset seizure in two cases (nortriptyline), asystole immediately after administration in two cases (flecainide, bupropion), and fat embolism (propranolol) in one case. Of the total cohort, 49 (10.7%) experienced a possible adverse effect.
provides detailed information about the eight cases in which LAST cases received ILE. Many cases lacked detailed descriptions of dose and exact timing of ILE administration. Only one case had ROSC and transient survival thereafter. For comparison, a separate search of NPDS data covering the same study period identified 113 cases of major toxicity associated with lidocaine and an additional 13 lidocaine related deaths. It is unknown how many of the lidocaine cases with major outcomes included the antidotal use of ILE.
Table 2. Detailed information on the use of intravenous lipid emulsion (ILE) for patients with local anesthetic systemic toxicity (LAST).Table Footnotea
Discussion
Following the first reported success in a patient with parenteral LAST [Citation9], the use of ILE blossomed to include oral and parenteral poisonings from a vast array of substances. Supported largely by case reports, in-vitro studies, and animal models with very limited applicability to clinical care, widespread use of ILE occurred in humans long before the mechanism of action, pharmacokinetics and safety were sufficiently elucidated. Suffering from a lack of properly designed human randomized controlled trials, the early literature is flawed by both a publication bias favoring positive outcomes and inferences of efficacy made without sufficient evidence for causation given that cases often received multiple other therapies simultaneously. These limitations were highlighted in systematic reviews of safety [Citation7] as well as toxicity from local anesthetics [Citation10] and non-local anesthetics [Citation11]. As these shortcomings became evident, another body of literature, also of heterogeneous quality, consisting of animal models of gastrointestinal poisoning [Citation12], human case reports [Citation13], and a few human observational [Citation14] or controlled trials [Citation2,Citation4] reported not only the failure of ILE but also its potential for harm. Despite this, advocates of ILE continue to cite case data as evidence of efficacy and, on that basis, an FDA application for a specific commercial product was submitted in 2015.
We undertook the present study with the goal of reviewing the NPDS database to find human clinical evidence of ILE use, effect, and failure as a therapy for poisoning as a counterbalance to case reports of positive outcomes to distinguish variables that might explain failures and successes in either time of administration, substances, or toxic load. As there is no specific field code for ILE, the only way to find data for ILE use was in the narrative sections of the database. Additionally, given the expanse of the NPDS data and the variable use of the notes field we chose to limit our investigation to the fatality database, which contains the most robust and verified notes sections [6]. Fortunately, ILE will be a therapy field choice as of 2018, which will enable researchers to distinguish better if ILE was recommended, given, or not given but recommended, and also to provide the ability to have a comparison group with non-fatal cases.
Although we knew a-priori that all of the patients in the present study died, however, this did not exclude the possibility of finding evidence of clinical benefit of ILE as many patients survive their acute overdose only to die later of complications such as aspiration, anoxia, etc. In fact, we noted evidence of benefit in a small heterogenous group of patients, with 7.4% developing ROSC in close proximity to ILE administration. The substance represented in this database with the highest percent achieving ROSC was flecainide followed by bupropion. This finding suggests that a clinical trial of ILE in overdose of these substances may be worthy. However, the vast majority of cases did not have any clearly defined clinical response to ILE administration. Interestingly, the clinical presentation and management of these ILE failures resemble those of the ILE successes described in the recent systematic reviews [Citation10,Citation11], other than that the outcome was negative. We were unable to distinguish successes from failures by the timing of administration, toxic doses, or other features. This relationship appears to hold true even for patients with LAST.
The role of ILE therapy in poisoning would be best determined by controlled clinical trials. However, as a first step, a phase I escalating dose trial to determine maximum tolerated dose and safety is still warranted based upon the high rate of adverse reactions to ILE in this series (10.7%) as well as exploration of laboratory analyses and extracorporeal techniques that are affected by ILE use [Citation15].
Limitations
The study limitations are the same as those with any study using the NPDS database. The narratives are written by a large pool of authors and vary greatly in quality and completeness. Some narratives have a single author/reviewer while others have a second person at the poison center to verify data. Most cases do not have autopsy confirmation. There is a potential for reporting bias in that ILE may have been given in some fatalities not included in this review. Additionally, the exact timing and dosing of ILE were not provided for the majority of cases. Although it is possible that larger, smaller doses, or differently timed ILE administrations would have been associated with different outcomes, this data set represents the largest series of ILE administrations known to date.
ILE was not a standardized therapy category in NPDS, which limited the search strategy to manual text searching, and prevented the ability to identify non-fatal cases who received ILE. Although those cases would likely be coded as a major outcome, they are too numerous to be searched as part of this study. We recognize that by only evaluating fatal cases we were unlikely to find potential benefits of ILE although we did find 34 cases (7.4%) with transient ROSC. Furthermore, the case report literature (with its inherent publication bias mentioned above) was extensively reviewed elsewhere [Citation10,Citation11]. Finally, although we cannot say with certainty that the cases in our data set would have died regardless of ILE therapy, the same is true of the published case reports with positive outcomes, in that the authors cannot say with certainty that those patients would not have survived without ILE therapy.
Conclusions
Within the Association of Poison Control Centers (AAPCC) National Poison Data System (NPDS), hundreds of cases exist in which ILE therapy was given and death occurred. In many of these cases, ILE was given prior to cardiovascular collapse. Although there is some suggestion of transient improvement in a small subset of cases, adverse effects are also reported. When taken in totality, the number of published cases of failed lipid emulsion therapy outnumbers the published instances of ILE success. Given all the uncertainty generated by case reports, the evaluation of the role and efficacy of ILE therapy in non-local anesthetic poisoning needs robust controlled clinical trials.
Acknowledgments
We are grateful for all those who cared for these patients, all the Specialists in Poison Information who collected the data, and all the toxicologists who reviewed and edited the fatality abstracts.
Disclosure statement
None of the authors have any conflicts of interest or funding to disclose.
References
- Kasnavieh MH, Nodoushan SJ, Ghafori HB, et al. Intravenous lipid emulsion for the treatment of tricyclic antidepressant toxicity a randomized controlled trial. VIIth Mediterranean Emergency Medicine Congress 2013; September 8–11; Marseille, France.
- Taftachi F, Sanaei-Zadeh H, Sepehrian B, et al. Lipid emulsion improves Glasgow coma scale and decreases blood glucose level in the setting of acute non-local anesthetic drug poisoning-a randomized controlled trial. Eur Rev Med Pharmacol Sci. 2012;16(Suppl 1):38–42.
- Heinonen JA, Litonius E, Salmi T, et al. Intravenous lipid emulsion given to volunteers does not affect symptoms of lidocaine brain toxicity. Basic Clin Pharmacol Toxicol. 2015;116:378–383.
- Dureau P, Charbit B, Nicolas N, et al. Effect of Intralipid® on the dose of ropivacaine or levobupivacaine tolerated by volunteers: a clinical and pharmacokinetic study. Anesthesiology. 2016;125:474–483.
- Gosselin S, Hoegberg LC, Hoffman RS, et al. Evidence-based recommendations on the use of intravenous lipid emulsion therapy in poisoning. Clin Toxicol. 2016;54:899–923.
- Bronstein AC, Spyker DA, Cantilena LR, Jr, et al. 2008 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 26th Annual Report. Clin Toxicol. 2009;47:911–1084.
- Hayes BD, Gosselin S, Calello DP, et al. Systematic review of clinical adverse events reported after acute intravenous lipid emulsion administration. Clin Toxicol. 2016;54:365–404.
- Benchimol EI, Smeeth L, Guttmann A, et al. The REporting of studies conducted using observational routinely-collected health data (RECORD) statement. PLoS Med. 2015;12:e1001885.
- Rosenblatt MA, Abel M, Fischer GW, et al. Successful use of a 20% lipid emulsion to resuscitate a patient after a presumed bupivacaine-related cardiac arrest. Anesthesiology. 2006;105:217–218.
- Hoegberg LC, Bania TC, Lavergne V, et al. Systematic review of the effect of intravenous lipid emulsion therapy for local anesthetic toxicity. Clin Toxicol. 2016;54:167–193.
- Levine M, Hoffman RS, Lavergne V, et al. Systematic review of the effect of intravenous lipid emulsion therapy for non-local anesthetics toxicity. Clin Toxicol. 2016;54:194–221.
- Perichon D, Turfus S, Gerostamoulos D, et al. An assessment of the in vivo effects of intravenous lipid emulsion on blood drug concentration and haemodynamics following oro-gastric amitriptyline overdose. Clin Toxicol. 2013;51:208–215.
- Cole JB, Stellpflug SJ, Engebretsen KM. Asystole immediately following intravenous fat emulsion for overdose. J Med Toxicol. 2014;10:307–310.
- Mithani S, Dong K, Wilmott A, et al. A cohort study of unstable overdose patients treated with intravenous lipid emulsion therapy. CJEM. 2017;19:256–264.
- Grunbaum AM, Gilfix BM, Hoffman RS, et al. Review of the effect of intravenous lipid emulsion on laboratory analyses. Clin Toxicol. 2016;54:92–102.