Abstract
Background
FAO specifications for liquid paraquat dichloride SL formulations require the use of an emetic agent to stimulate vomiting within 30 min of ingestion. To date, there is no high-quality evidence of efficacy, despite use of the PP796 emetic since 1979. We first examined the validity of patients’ self-reported dose of paraquat ingested by examining the relationship with blood paraquat concentration and time to death for patients ingesting the standard paraquat SL formulation in a Sri Lankan cohort. As a secondary outcome, we assessed whether ingestion resulted in vomiting within 30 min and whether vomiting was associated with good outcome.
Methods
Patients presenting with paraquat SL self-poisoning were prospectively studied in ten Sri Lankan hospitals in 2003–08. Data on reported dose ingested, incidence and timing of vomiting after ingestion, treatment received, plasma paraquat concentration, and outcome were collected prospectively on presentation to hospital. Time between ingestion and blood sampling was incorporated by covariate adjustment.
Results
441 patients were recruited to the case series, presenting a median (IQR) of 3.0 (1.5–8.1] h post ingestion. Outcome was known for 435 patients of whom 322 (74.0%) died within 42 days, a median of 1.3 (0.6–4.4) days post ingestion. Median reported dose ingested was 15 to <30 mL. There was a highly significant linear trend between log plasma paraquat and reported dose ingested (p < .001); adjustment for the log of the time from ingestion to sampling further improved the model fit. Case fatality and median time to death also showed good agreement with estimated ingestion amount. 347/438 patients (79.2%) were stated to have vomited before reaching the study hospital with 300 (68.5%) vomiting within 30 min of ingestion; time to vomiting was unknown for a further 12 (2.7%). The proportion vomiting was strongly associated with reported dose ingested (p < .001); of note the proportion vomiting within 30 min only increased to 83.3% for the highest ingestion group. Patients vomiting within 30 min had higher plasma paraquat concentrations (p = .008), and higher hazard ratio in the adjusted Cox regression model of 2.01 (95% CI 1.45–2.77) compared to those who did not. Vomiting within 30 min was associated with a higher case fatality (241/295 [81.7%] vs 68/125 [54.4%], p < .001). Forty-three (47.3%) of the 91 patients who did not vomit before reaching hospital died (one had unknown outcome).
Conclusion
Importantly, we found good agreement between reported dose ingested and plasma paraquat concentration, case fatality, and time to death, suggesting that the reported dose is a valid marker for the dose ingested. Vomiting occurred within 30 min for 68.5% of patients, exceeding the characteristics for a purported effective emetic in the FAO specifications. However, vomiting within 30 min was associated with approximately double the risk of death compared to those who did not vomit, larger paraquat ingestions, and higher blood paraquat concentrations. In addition, death occurred in many patients who did not vomit, and the proportion vomiting within 30 min only reached 82.1% for the highest ingested dose group. Overall, we found no evidence of benefit resulting from incorporation of the emetic, suggesting that the current FAO specification is not effective at preventing deaths after ingestion of the paraquat SL formulation.
Keywords:
Introduction
In the mid-1970s, after a series of deaths following unintentional poisonings in which paraquat had been decanted into soft drinks bottles [Citation1–3], the widely used agricultural paraquat SL20 liquid formulation (200 g/L paraquat ion, major brand Gramoxone®) was changed to include a stenching agent, a colouring agent, and an emetic (PP796) with the purpose of reducing unintentional ingestion and stimulating vomiting after ingestion [Citation4,Citation5]. The manufacturer considered it effective at reducing unintentional deaths [Citation6]. However, an emetic’s ability to reduce toxicity from liquid paraquat formulations will be limited by a central location of effect, countered by rapid stomach emptying after fasting, and active uptake of paraquat, meaning that toxic amounts of paraquat may well be absorbed before the emetic can have its effect. Unpublished animal studies, by contrast, indicate that high doses of PP796 (>2 mg/kg) reduce toxicity if stomach emptying is slowed with food [Citation7].
The Food and Agricultural Organization (FAO)’s Committee of Experts on Pesticides in Agriculture (predecessor to the current FAO/WHO Joint Meeting on Pesticide Specifications [JMPS]) subsequently adopted this formulation for its international specifications (Box 1) [Citation8]; it remains the global quality specification for paraquat [Citation9], with some revisions in text [Citation10]. The specifications note that an emetic that causes more than 50% of patients to vomit within 30 min must be included in the formulation; early versions note that only PP796 has been shown to do this (Box 1). Unfortunately, no evidence was provided to support this statement, which has been removed in the most recent version of the specifications [Citation10]. Furthermore, review of the pre-clinical and clinical data suggest that the dose of emetic might be too low to be effective [Citation7].
The key aim of the change in formulation was to prevent unintentional deaths [Citation7]; however, fatal cases continue to occur [Citation11–17]. An effective emetic should also prevent many of the deaths that occur from low-intent self-poisoning since small doses are often ingested in spontaneous acts of self-harm [Citation18,Citation19]. However, we have noted a case fatality of >35% for self-reported ingestions of less than 10 mL [Citation19], a dose not expected to be fatal in most cases [Citation5]. It is unclear whether these deaths occur because the ingested dose was larger than reported or because the lethal dose is actually lower than reported, even in the presence of the PP796 emetic.
Box 1. FAO specifications for paraquat dichloride soluble concentrate (February 2008) [Citation8]
“1 Description
The material shall consist of technical paraquat dichloride, complying with the requirements of FAO specification 56.302/TK (2003), in the form of an aqueous solution (Notes 1 and 3), together with any other necessary formulants, and must contain an effective emetic (Note 2). The material may also include colorants, olfactory alerting agents and thickeners. It shall contain not more than a trace of suspended matter, immiscible solvents and sediment.”
“Note 1. An effective emetic, having the following characteristics, must be incorporated into the SL.
It must be rapidly absorbed (more rapidly than paraquat) and be quick acting. Emesis must occur in about half an hour in at least 50% of cases.
It must be an effective (strong) stimulant of the emetic centre of the brain, to produce effective emesis. The emetic effect should have a limited “action period”, of about two to three hours, to allow effective treatment of poisoning.
It must act centrally on the emetic centre in the brain.
It must not be a gastric irritant because, as paraquat is itself an irritant, this could potentiate the toxicity of paraquat.
It must be toxicologically acceptable. It must have a short half-life in the body (to comply with the need for a limited action period).
It must be compatible with, and stable in, the paraquat formulation and not affect the herbicidal efficacy or occupational use of the product.
“To date, the only compound found to meet these requirements is 2-amino-4,5-dihydro-6-methyl-4-propyl-s-triazole-(1,5a)pyrimidin-5-one (PP796). PP796 must be present in the SL at not less than 0.23% of the paraquat ion content.”
There are currently no high-quality data on whether the emeticised paraquat SL20 formulation induces vomiting within 30 min or the effectiveness of such vomiting at improving outcome [Citation7]. A study was performed by the UK’s National Poisons Information Service with the manufacturer ICI during the 1980s [Citation4,Citation20,Citation21]; however, the published data [Citation4] include both paraquat SL20 and granular low concentration formulations (major brand Weedol®), which has a lower paraquat concentration, higher PP796 to paraquat ratio, and substantially lower case fatality [Citation4]. The two products were not distinguished in the analysis, meaning that the effect of PP796 in the SL formulation could not be identified. A small Malaysian study of 30 cases with paraquat SL20 poisoning noted that vomiting occurred within 15 min for 24/30 (80.0%) cases [Citation22]. Twenty-seven (90.0%) patients died; whether any of the patients who vomited survived was not stated. An unpublished study from Western Samoa did not find any evidence that emeticised paraquat SL20 improved outcome [Citation23].
In 2002, we established a prospective cohort of pesticide acute self-poisoning cases in Sri Lanka [Citation24] that now includes 1,477 patients with paraquat self-poisoning [Citation25], the great majority after ingesting paraquat SL20 [Citation19,Citation26]. In 2003–2008, the cohort was used to study the possible benefits of a new SL20 formulation (called Inteon) that included an alginate, a purgative, and a 3-fold increased concentration of PP796 [Citation19,Citation26,Citation27]. At that time, we did not assess the rates of vomiting amongst these patients.
We now use data from this prospective cohort to assess the validity of the dose of paraquat ingested, as reported by patient and/or relatives, through examining the relationship with blood paraquat concentration and time to death for patients ingesting the standard paraquat SL formulation. As secondary analyses, we looked at the incidence of early vomiting and association of vomiting and of gastric decontamination with outcome. We particularly wished to determine whether the formulation fulfils the FAO quality criteria for >50% vomiting within 30 min and whether such vomiting affected the outcome.
Methods
Ethics approval was received for the original cohort from the Faculty of Medicine Ethics Committee, Colombo, and ethics committees of University of Ruhuna, Anuradhapura General Hospital, Teaching Hospital Kandy, and National Hospital of Sri Lanka. Methods for the prospective cohort in general and for the paraquat patients in particular have been published previously [Citation19,Citation24–26].
Patients
The patients were recruited prospectively from Dec 2003 to Sep 2008 in two studies [Citation19,Citation26] conducted in ten Sri Lankan hospitals (Anuradhapura, Colombo, Embilipitiya, Galle, Gampaha, Hambantota, Kandy, Peradeniya, Polonnaruwa, Ratnapura). The original studies were conducted to compare the outcome of paraquat self-poisoning with either the standard formulation or new (Inteon) formulations using alginate technology. The first alginate-containing formulation became available in Sri Lanka in October 2004. Patients were only included in the current analysis if they had ingested standard formulation paraquat SL20 that did not use alginate technology.
Patients were recruited by study clinicians if they reported ingesting products containing paraquat or, if the pesticide ingested was unknown, the patient had clinical signs typical of paraquat poisoning (mouth lesions and/or blue colouration around the mouth) or a positive urine dithionite test. Oral informed consent to participate in the survey was sought from patients or their relatives in their own language. Two centres, Anuradhapura and Polonnaruwa, collected information on paraquat poisoned patients from 2002 using a slightly different proforma; patients recruited at these centres before October 2004 were missing data on some key variables and were excluded from the study.
Procedures
Data on the exposure, treatment, and outcome of patients ingesting paraquat were collected by trained research assistants using a standardised questionnaire. Upon admission, demographic data (age, sex, and weight) were recorded together with information relating to previous treatments and transfer from a primary hospital. Details relating to the ingestion were taken: time of exposure; circumstances (intentional self-harm, accidental, homicide, or occupational); time to emesis; and number and force of vomiting episodes. The patient was asked to state the ingested volume using a variety of measuring schemes (millilitres, fluid ounces, or various-sized spoon/cup measures). These volumes were then converted into mL and categorised as <5 mL, 5 to <10 mL, 10 to <15 mL, 15 to <30 mL, 30 to <50 mL, 50 to <100 mL, 100 to 150 mL, and >150 mL.
A plasma and/or urine sample was taken soon after admission (where possible), stored frozen, and sent to Syngenta CTL (Alderley Park, Macclesfield, Cheshire, UK) for measurement of paraquat ion concentration and identification of tracer compounds (diquat or diethyl paraquat ions) in alginate technology poisoned patients (allowing cases to be classified as confirmed standard formulation or confirmed alginate cases). Analysis was conducted using HPLC, LC-MS-MS, and LC fluorescence [Citation28]. Standard paraquat 20SL cases were confirmed on the basis of plasma or urine analysis. The plasma and/or urine paraquat concentration had to be >0.04 mg/L for the co-formulated marker compounds (diquat, diethyl paraquat) to be detected, if present. This meant that patients who had ingested low doses (or vomited out the pesticide) after October 2004 could not be accurately assigned to the standard or alginate technology formulations, since the paraquat concentration was too low for the co-formulated markers to be measured (if present), and were therefore excluded from the study.
Details of treatments and clinical observations throughout the patients’ stay in hospital and clinical outcome were prospectively recorded. In the first study [Citation19], if the patient survived to discharge from hospital, study doctors visited their home at least 3 months after exposure to check survival. However, for the second study [Citation26], follow up was performed at only 6 weeks because all deaths in the first study had occurred by 42 days.
Cases were initially recorded on paper and then transferred to a Microsoft Access database. For quality control, a separate database was created from data collected from the medical notes by an auditor (except for two hospitals where access to medical records was refused). The two databases were compared to assess completeness of case ascertainment and to highlight differences in recording of details.
Statistical analysis
The relationship between estimated paraquat ingestion amounts and absorption was assessed using mean plasma paraquat concentrations and mortality outcome. Mortality outcome for all patients was assessed at 42 days. Analysis of variance was used to compare logged plasma paraquat concentrations (logPQ) between different ingestion amount categories. Plasma paraquat concentrations were also adjusted for the log of the post ingestion time to sampling (logTIME) using analysis of covariance and marginal means estimated. Linear trend analysis was also performed. In addition, a simpler adjustment for time from ingestion to sampling was made using the log of the Severity Index for Paraquat Poisoning (SIPP) score [Citation29] i.e., logPQ + logTIME. Survival and median time to death of patients ingesting different amounts was compared using Kaplan Meier survival curves and Mantel-Cox logrank test. Categorical comparisons including those of the proportions of patients vomiting within 30 min were performed using Χ2 and linear trend tests.
Analyses were also performed to examine the effect of vomiting, treatment with charcoal and Fuller’s earth adsorbents, and gastric lavage on plasma concentrations and outcome. Analysis of covariance was used to compare logged plasma concentrations adjusted for logTIME and ingestion amounts. Cox proportional hazards [PH] regression models were also used to estimate adjusted hazard ratios (HR) for the factors of interest. In Cox PH models, the 10 hospitals were included as strata and analyses always included terms for the following covariates: (a) sex, age, and weight of participant; (b) treatments received; (c) use of adsorbent; (d) time from ingestion to presentation at a hospital; (e) standard group (pre-alginate, confirmed standard formulation product in first and second alginate technology studies); (f) vomit before admission (except when assessing the effect of vomiting within 30 min of ingestion) and (g) estimated ingestion amount.
Estimated self-reported ingestion amount was an important factor influencing survival, but information was not available for several cases. Consequently, adjusted hazard ratios (HR) were only derived for the subset of patients who had ingestion information, and ingestion amount was included as a categorical variable with eight levels. Some of the confirmed cases in the second alginate study [Citation26] were included in a randomised controlled trial of immunosuppression with cyclophosphamide and corticosteroids [Citation30]. Cyclophosphamide showed no evidence of effect in confirmed cases in the 2nd study who were allocated the treatment at random whereas the case fatality rate was very high among the small number of patients given the treatment in the first study [Citation19]. Similar results were seen for corticosteroids, and it was decided to exclude these treatments from the Cox models. Evidence of nonproportional hazard functions was assessed by visual methods and by testing the significance of the interaction with the logarithm of survival time. Stratification was used to account for nonproportionality of the hazard functions.
All statistical analyses were performed using IBM SPSS Statistics for Windows version 27.0 (IBM Corp, Armonk, NY).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
The first study of alginate formulation effectiveness [Citation19] included 297 standard formulation cases recruited before 1 October 2004 (when the first alginate formulation was introduced) and 85 confirmed cases recruited after that date (). The second study [Citation24] included a further 126 confirmed standard formulation cases recruited after the introduction of a second alginate formulation in October 2006. After excluding the 67 patients recruited at Anuradhapura and Polonnaruwa hospitals before 01 October 2004 with incomplete data, the study group included 441 patients who had ingested the standard paraquat SL20 formulation, presenting a median (IQR) of 3.0 (1.5 to 8.1) h post ingestion (). Males (n = 349, 79.1%) outnumbered females, median age was 27 (21.0 to 38.8) years, and ingestion was reported as intentional for 416 (94.3%) cases.
Table 1. Patients in the analysis.
Table 2. Demographic and clinical characteristics.
Outcome was known for 435/441 (98.6%) patients, of whom 322 (74.0%, 95% CI 69.6% to 78.0%) died within 42 days, a median of 1.3 (0.6–4.4) days post ingestion. The case fatality was higher for the earliest patients recruited, before the introduction of the first alginate (Inteon) formulation (175/227, 77.1%, 95% CI 71.1% to 82.4% [lost to follow up, n = 3]), than for both the first series of confirmed patients (60/82, 73.2%, 95% CI 62.2% to 82.4% [lost to follow up, n = 3]) and later series (87/126, 69.0%, 95% CI 60.2%–77.0%) despite the likely higher paraquat plasma levels of confirmed patients (as required to exclude co-presence of the markers).
Accuracy of reported dose ingested
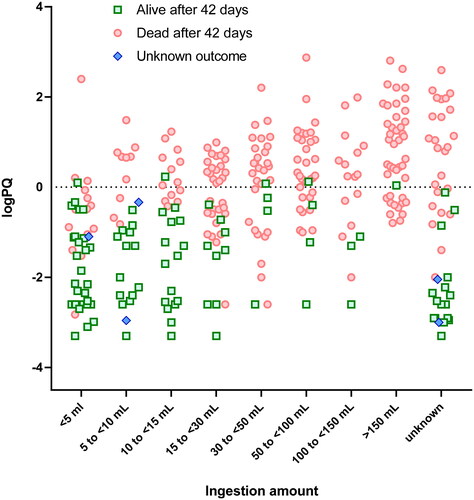
An estimated dose ingested (in eight range categories) was available for 388 (88.0%) patients with a median dose category of 15 to <30 mL of paraquat SL20 (). shows logPQ plotted against the reported dose ingested with outcome at 42 days. Unsurprisingly, there was considerable overlap between ingestion categories; however, there was a highly significant linear trend between logPQ and reported dose ingested (p < .001), with no evidence of deviation from linearity (p = .61). Mean logPQ increased from −1.4 (95%CI − 1.8 to −1.0) to 0.8 (0.4–1.1) with increasing ingestion amounts. Adjusting for logTIME improved the model fit significantly (p < .001). Log SIPP also demonstrated a strong linear relationship with ingestion amount (p < .001) with no evidence of deviation from linearity (p = .84), but the model fit was significantly improved (p < .05) by further adjustment for logTIME. The case fatality at 42 days increased with ingestion amount from 32.2% of the 59 patients ingesting <5 mL of paraquat to 98.2% of 67 patients ingesting >150 mL.
Figure 1. Log plasma paraquat concentration categorised by reported dose ingested.
Key survivors: green squares; fatalities: red circles; unknown outcome: blue diamonds. LogPQ: log plasma paraquat concentration.
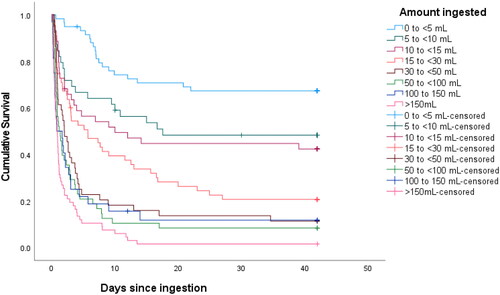
The Kaplan-Meier survival analysis () includes the six patients with unknown outcome at 42 days and indicated a significant difference in survival between the reported dose ranges (log rank test, p < .001). However, there was little difference in survival among patients who reported ingesting 5–10 mL and 10–15 mL, and among those who reported ingesting 30–50 mL, 50–100 mL and 100–150 mL. Median (IQR) time to death showed a similar relation with estimated ingestion amount, decreasing from 6.9 (4.7 to 9.7) days for patients ingesting <5 mL to 0.8 (0.4–1.9) days for those ingesting >150 mL.
Vomiting post ingestion
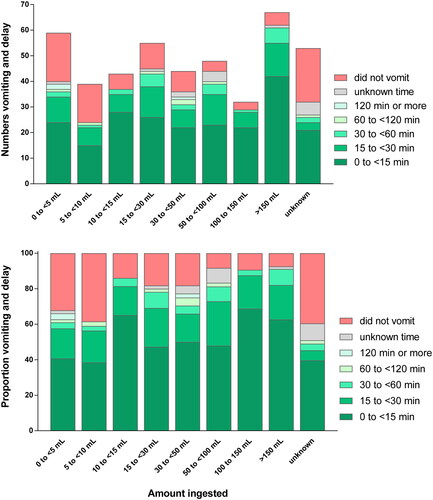
Data on vomiting before hospital presentation was available for 438/441 (99.3%) patients. A total of 347 patients (79.2%) vomited before reaching the study hospital; 300 (68.5%) vomited within 30 min of ingestion, indicating that the formulation fulfilled the FAO criterion (Box 1). A further 12 patients (2.7%) vomited before admission, but timing was not reported.
The proportions vomiting pre-hospital and within 30 min of ingestion were both significantly related to estimated ingestion amount (p trend <0.001). Excluding the 12 patients above, the proportion vomiting within 30 min increased from 58.6% for patients ingesting <5 mL to 83.3% for patients ingesting >150 mL; the corresponding figure for those with unknown ingestion amount was 50.0% (). However, among patients who vomited within 30 min and had known ingestion amount, 202 (73.2%) vomited within 15 min of ingestion. There was no significant difference (no dose response) in this proportion between patients in different ingestion dose categories (p = .58).
Early emesis is not associated with improved outcome
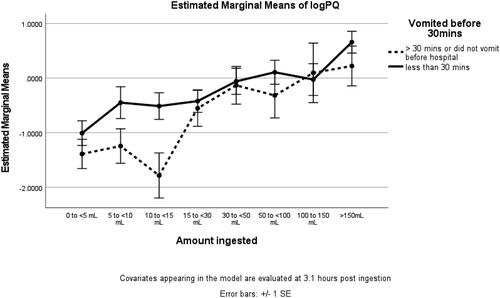
Vomiting within 30 min of ingestion was not associated with improved outcome. In the univariate analysis of covariance, estimated marginal mean plasma paraquat concentrations were significantly higher (p = .008) for patients who vomited within 30 min. The estimated marginal mean logPQ evaluated at 3.1 h post ingestion was −0.64 (85% CI −0.90 to −0.37) for not vomiting within 30 min vs −0.21 (85% CI −0.38 to −0.05) for vomiting within 30 min. The largest differences were seen for ingestions between 5 and 15 mL but the interaction between vomiting and ingestion amount was not statistically significant (p = .55) ().
Figure 4. Estimated marginal means of log plasma paraquat concentration categorised by reported dose ingested and by occurrence or not of vomiting within 30 min of ingestion. Error bars show standard error. Covariates appearing in the model are evaluated at 3.1 h post ingestion.
Among patients with known vomiting status and time and outcome (n = 420), vomiting within 30 min was associated with a higher case fatality (241/295 [81.7%] vs 68/125 [54.4%], p < .001). However, those who vomited within 30 min had much higher ingestion amounts (median ingestion amount 30–50 mL versus just above 10 mL). The KM survival curves show that survival was worse among patients who vomited for each ingestion amount ().
Figure 5. Time to death categorised by reported dose ingested and by vomiting within 30 min of ingestion (Left: vomited <30 min; Right: did not vomit <30 min).
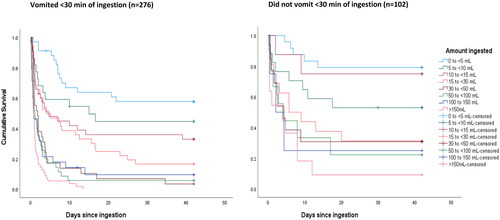
Cox regression analysis adjusted for several factors including estimated ingestion amount confirmed that survival was significantly worse for patients that vomited within 30 min of ingestion (HR = 2.01; 95% CI 1.45–2.77). A further analysis compared those that vomited within 15 min, and those who vomited later than 15 min after ingestion, with those who didn’t vomit. The HR for vomiting within 15 min was 2.16 (85% CI 1.43 to 3.26) but the HR for vomiting later than 15 min after ingestion was not significantly elevated (HR = 1.55; 95% CI 0.99–2.44).
Mortality among patients who did not vomit
Forty-three (47.3%) of the 91 patients who did not vomit before hospital presentation died (one had unknown outcome). Many patients who did not vomit had taken relatively large doses of paraquat - as indicated by the reported dose ingested, blood paraquat concentration, and time to death. Thirty-six patients with known outcome at 42 days ingested >10 mL of paraquat SL20 but did not vomit; 21 (58.3%) died with a median time to death (IQR) of 1.5 (0.5–5.0) days). The case fatality was much higher (84.0%) among 25 patients who ingested >10 mL of paraquat and vomited after 30 min, but surprisingly they survived longer; median time to death (IQR) of 4.1 (1.7–8.0) days. Seven of 20 (35.0%) who did not vomit after ingesting ≥30 mL survived, compared to 6 of 169 (3.6%) who ingested the same amount but vomited.
Effects of medical decontamination
Many of the patients received an adsorbent such as activated charcoal or Fuller’s earth, and/or received gastric lavage (). Adsorbent treatment did not significantly affect plasma paraquat levels (p = .343) and there was no evidence of increased survival (HR = 0.99: 95% CI 0.68–1.45).
Gastric lavage treatment was associated with reported dose ingested: patients reporting higher dose ingestions were more likely to get lavage, but the difference was not statistically significant (p = .29) and only 69.6% of those ingesting ≥30 mL received lavage compared to 64.5% ingesting lower ingestions. The patients receiving lavage had slightly lower paraquat plasma concentrations, especially among those ingesting 30 to 150 mL, but the difference was not significant (p = .082). There was no evidence of increased survival in the Cox regression analysis (HR = 0.97: 95% CI 0.70–1.33) among patients who received lavage in primary and/or secondary hospitals.
Discussion
We found good correlations between reported dose ingested and plasma paraquat concentration, case fatality, and time to death, suggesting that the reported dose is a valid marker for the amount ingested. We also found that vomiting occurred within 30 min for almost 70% of patients, exceeding the FAO specification of 50% within 30 min. However, some patients who drank large volumes did not vomit. We also found no evidence of a benefit from this vomiting within 30 min, despite patients often vomiting on multiple occasions. Early vomiting was associated with approximately double the risk of death and with higher plasma concentrations of paraquat, suggesting that early vomiting is simply a marker of high exposure.
The formulation was designed to provide a dose of emetic that would cause the majority of people ingesting a likely lethal dose (≥10 mL of paraquat SL20) to vomit [Citation5] and to reduce their risk of death [Citation31,Citation32]. Excluding the 12 patients who vomited but for whom timing was not reported, the proportion that vomited within 30 min of ingestion ranged from 58.6% for patients ingesting <5 mL to 83.3% for patients ingesting >150 mL. These data indicate that the dose of PP796 in a lethal dose of paraquat SL20 does not always cause vomiting within 30 min of ingestion. We found no evidence to indicate that PP796 5 mg/L in paraquat SL20 causes vomiting that improves outcome.
The early emesis was probably caused by the PP796. Pre-clinical studies in vomiting species showed vomiting occurring after 1–2 h in paraquat SL20 formulations lacking emetic; addition of PP796 at concentrations >2 mg/kg resulted in much more rapid vomiting occurring within 10–20 min [Citation7]. Clinical studies suggested more rapid vomiting after ingestion of low concentration granular paraquat products with high concentrations of PP796 [Citation4,Citation7]. It seems likely that the early vomiting seen here was due to the PP796, although the concentration was lower than found to be ideal in pre-clinical studies [Citation7].
The study was done both before and during the introduction of a new formulation in Sri Lanka with an increased emetic concentration [Citation27] that was initially associated with greater vomiting and increased survival, especially for patients taking modest doses [Citation19]. The new formulation also contained an alginate designed to gel in the stomach as well as magnesium sulfate [Citation19,Citation26,Citation27], which may have contributed to the overall effect. It is not possible to conclude that higher doses of PP796 alone would be more effective at inducing vomiting that lowers plasma paraquat concentration and improves outcome, although this is suggested by animal studies [Citation7]. It is unclear whether increasing the PP796 concentration would be an effective strategy. Clinical trials would be required to prove the value of such higher concentrations.
An analysis of 586 standard formulation cases and confirmed alginate formulation cases suggested that gastric lavage may have contributed to a poorer outcome for patients who ingested <30 mL, whereas it may have had a small beneficial effect in patients who ingested higher volumes, in particular in those admitted to hospital within one hour of ingestion [Citation33]. However, this was not confirmed in the current study looking only at standard paraquat SL20 products.
Data collection in this study was prospective, occurring on admission a median of 3.25 h post ingestion. It was also collected directly from the patient and/or relative by a researcher. After introduction of the alginate formulations, the identity of the formulation ingested was confirmed by blood analysis where possible (however, see below for limitations). This contrasts with the UK NPIS study performed in the 1980s when data was collected retrospectively by phone from the doctor who had cared for the patient, typically several days after the admission [Citation34]. The specific formulation was not reported in the published abstracts [Citation4], meaning that data for paraquat SL20 formulations has not previously been published (although available in internal company reports [Citation7]).
Limitations
Many of the cases collected before the introduction of Inteon in 2004 had no urine or plasma samples collected; these represent the majority of confirmed standard formulation cases in the 1st study [Citation19]. Therefore, the plasma analyses included only 60% of subjects. The paraquat concentration in plasma and/or urine also had to be >0.04 mg/L for the co-formulated marker compounds (diquat, diethyl paraquat) to be detected, if present. This meant that patients who had ingested low doses (or vomited out the pesticide) were excluded from this analysis. Of note, the case fatality was lower for the patients recruited after the introduction of Inteon, suggesting that excluding low dose ingestions that would have been below the level of detection for the markers did not have a major effect on the analysis.
The relatively low proportion of patients with unknown ingestion amounts known to have vomited within 30 min (46.2%) suggests some inaccuracy in the vomiting information. Many patients claimed to have spat out some or all the concentrated paraquat formulation, making it difficult to estimate how much had been ingested.
Conclusion
Our data indicate that the current paraquat SL20 formulation containing 5 mg/L PP796 fulfils the FAO specification for vomiting with 30 min in its current form; however, we found no evidence that this strategy is effective at preventing deaths after ingestion.
Author contributions
ED, JAT, MW and ME designed the study. FM, IG, NAB, MW and ME were responsible for collecting the original data. JAT performed the analysis with feedback from other authors. ED and ME drafted the first version of the manuscript, redrafting with input from all authors. All authors read and approved the final version.
Acknowledgements
We thank the directors, consultant physicians, and medical and nursing staff of the study hospitals for their support; and SACTRC study doctors and coordinators for their immensely valuable work. The Centre for Pesticide Suicide Prevention is funded by a grant from Open Philanthropy, at the recommendation of GiveWell, USA. For the purpose of open access, the author has applied a CC-BY public copyright licence to any Author Accepted Manuscript version arising from this submission.
Disclosure statement
Syngenta funded the original Inteon studies, built upon an independent Wellcome Trust/NHMRC-funded study infrastructure in Sri Lanka, and funded JAT who is a statistical consultant to Syngenta. MW was an employee of Syngenta at the time of the data collection. ME and NAB were reimbursed travel expenses by Syngenta to attend steering committee meetings for the Inteon studies. IBG led a study of immune-suppression that was supported by Syngenta. ME, NAB and IBG did not receive any personal payments for collaborative work with Syngenta. ED has no conflict of interest.
Additional information
Funding
References
- Bullivant CM. Accidental poisoning by paraquat: report of two cases in man. Br Med J. 1966;1(5498):1272–1273.
- McDonagh BJ, Martin J. Paraquat poisoning in children. Arch Dis Child. 1970;45(241):425–427.
- Smith P, Heath D, Fishman AP. Paraquat. CRC Crit Rev Toxicol. 1976;4(4):411–445.
- Meredith TJ, Vale JA. Treatment of paraquat poisoning in man: methods to prevent absorption. Hum Toxicol. 1987;6(1):49–55.
- Lock EA, Wilks MF. Paraquat. In: Krieger R, editors. Hayes’ handbook of pesticide toxicology. 3rd ed. New York: Elsevier; 2010. p. 1771–1827.
- Brown R, Clapp M, Dyson J, et al. Paraquat in perspective. Outlook Pest Man. 2004;15(6):259–267. Dec 2004:
- Eddleston M. Evidence for the efficacy of the emetic PP796 in paraquat SL20 formulations - a narrative review of published and unpublished evidence. Clin Toxicol. 2022;60(10):1–13.
- Food and Agricultural Organization. FAO specifications and evaluations for agricultural pesticides. Paraquat dichloride. Rome: FAO; 2008; [cited 2021 Apr 4]. Available from: http://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/Specs/Paraquat08.pdf.
- Australian Pesticides and Veterinary Medicines Authority. Standard for paraquat dichloride technical concentrate active constituent. Caberra: Australian Government; 2011; [last reviewed 2021; cited 2022 Jan 06]. Available from: https://apvma.gov.au/node/2594.
- Food and Agricultural Organization. FAO specifications and evaluations for agricultural pesticides. Paraquat dichloride. Rome: FAO; 2021; [cited 2022 Jan 06]. Available from: https://www.fao.org/3/ca9629en/ca9629en.pdf.
- Millard YC, Slaughter RJ, Shieffelbien LM, et al. Poisoning following exposure to chemicals stored in mislabelled or unlabelled containers: a recipe for potential disaster. N Z Med J. 2014;127(1403):17–23.
- Angotti L. Accidental death due to pulmonary fibrosis and multiorgan failure after paraquat ingestion: a case report. Abstract. Chest. 2015;148(4):369A.
- Delirrad M, Majidi M, Boushehri B. Clinical features and prognosis of paraquat poisoning: a review of 41 cases. Int J Clin Exp Med. 2015;8(5):8122–8128.
- Duan Y, Wang Z. To explore the characteristics of fatality in children poisoned by paraquat–with analysis of 146 cases. Int J Artif Organs. 2016;39(2):51–55.
- Fortenberry GZ, Beckman J, Schwartz A, et al. Magnitude and characteristics of acute paraquat- and diquat-related illnesses in the US: 1998–2013. Environ Res. 2016;146:191–199.
- United States Environmental Protection Agency. Paraquat dichloride: one sip can kill. Washington DC, EPA; 2019. Available from: https://www.epa.gov/pesticide-worker-safety/paraquat-dichloride-one-sip-can-kill.
- Carpenter JE, Murray BP, Moran TP, et al. Poisonings due to storage in a secondary container reported to the National Poison Data System, 2007–2017. Clin Toxicol. 2021;59(6):521–527.
- Eddleston M, Karunaratne A, Weerakoon M, et al. Choice of poison for intentional self-poisoning in rural Sri Lanka. Clin Toxicol. 2006;44(3):283–286.
- Wilks MF, Fernando R, Ariyananda PL, et al. Improvement in survival after paraquat ingestion following introduction of a new formulation in Sri Lanka. PLoS Med. 2008;5(2):e49.
- Bramley A, Hart TB. Paraquat poisoning in the United Kingdom. Human Toxicol. 1983;2:417.
- Denduyts-Whitehead AP, Hart TB, Volans GN. Effects of the addition of an emetic to paraquat formulations on acute poisoning in man. J Toxicol Clin Toxicol. 1985;23:422–423.
- Chan KW, Cheong IK. Paraquat poisoning: a clinical and epidemiological review of 30 cases. Med J Malaysia. 1982;37(3):227–230.
- Howard JK. Paraquat poisoning in Western Samoa. A preliminary assessment of the effect of PP796. 1978; 1977–1978; [cited 2022 Jan 06]. Available from: https://usrtk.org/wp-content/uploads/2021/03/1978.09.30-ICI-rpt-PQ-poisonings-in-Western-Samoa-1977-78-preliminary-assessment-of-effect-of-PP796-SYNG-PQ-04263349_R.pdf.
- Dawson AH, Eddleston M, Senarathna L, et al. Acute human lethal toxicity of agricultural pesticides: a prospective cohort study. PLoS Med. 2010;7(10):e1000357.
- Buckley NA, Fahim M, Raubenheimer J, et al. Case fatality of agricultural pesticides after self-poisoning in Sri Lanka: a prospective cohort study. Lancet Glob Health. 2021;9(6):e854–e862.
- Wilks MF, Tomenson JA, Fernando R, et al. Formulation changes and time trends in outcome following paraquat ingestion in Sri Lanka. Clin Toxicol. 2011;49(1):21–28.
- Heylings JR, Farnworth MJ, Swain CM, et al. Identification of an alginate-based formulation of paraquat to reduce the exposure of the herbicide following oral ingestion. Toxicology. 2007;241(1–2):1–10.
- Blake DK, Gallagher RT, Woollen BH. Improved methods for the analysis of paraquat in biological fluids. Chromatographia. 2002;55(S1):S183–S185.
- Sawada Y, Yamamoto I, Hirokane T, et al. Severity index of paraquat poisoning. Lancet. 1988;1(8598):1333.
- Gawarammana I, Buckley NA, Mohamed F, et al. High-dose immunosuppression to prevent death after paraquat self-poisoning – a randomised controlled trial. Clin Toxicol. 2018;56(7):633–639.
- Conso F. Paraquat poisoning: experience of poison control centers in France. Vet Hum Toxicol. 1979;21(Suppl):112–113.
- Foulkes DM. Paraquat/Emetic: points for presentation to the FDA. ICI; 1977. Available from: https://usrtk.org/wp-content/uploads/2021/03/1977.09.06-ICI-PQ-emetic-points-for-presentation-to-FDA-SYNG-PQ-02450812_R.pdf.
- Wilks M, Tomenson JA, Buckley NA, et al. Influence of gastric decontamination on patient outcome after paraquat ingestion. Asia Pacific Association of Medical Toxicology (APAMT) congress Bangkok; 2007.
- Bramley A, Hart TB. Paraquat poisoning in the United Kingdom (Internal Report). Fernhurst, Surrey: plant Protection Division, ICI Ltd; 1982; [cited 2021 Apr 05]. Available from: https://usrtk.org/wp-content/uploads/2021/03/1982.06.xx-ICI-rpt-Bramley-Hart-PQ-poisonings-in-the-UK-SYNG-PQ-03720006_R.pdf.