Abstract
Bioarchaeological studies of human remains from the Marshall Islands have reported dental, aDNA, and some biological profile data, but no behavioral reconstructions have been conducted. In this case study, histology was examined in a fragmented set of long bone and rib samples to test whether strenuous arm use, linked to traditional Marshallese gardening, food collection, and fishing activities, can be inferred from markers of bone remodeling. Cortical bone samples from the right posterior midshaft femur, left proximal radius, right posterior distal humerus, and an unsided and unnumbered rib shaft were examined in a middle-aged adult male excavated from a village site (MLEb-5) on Ebon Islet, Ebon Atoll. The interment is associated with a 1720 BP date making it the oldest burial in the Marshall Islands. Haversian canal area and density were recorded and compared intra-skeletally. The humerus and radius had denser and smaller canals compared to the femur. This suggests that the upper limb bones in this individual might have experienced frequent, strain suppressed, remodeling events. Bone adaptation to rigorous arm loading is inferred, demonstrating value in histological sampling of fragmented human remains for lifestyle interpretations in the ancient Pacific.
Keywords:
Introduction
Archaeological excavations in the Marshall Islands, eastern Micronesia, have yielded human skeletons successfully examined for viable mitochondrial DNA (mtDNA) (Weisler et al. Citation2000), incidence of rocker jaw phenotypes (Weisler and Swindler Citation2002), and variation in dental morphologies such as incisor shoveling and non-metric traits (Swindler and Weisler Citation2000), to indicate complex occupation and settlement histories. While various analytical approaches were used in these studies, including aDNA extraction and gross anatomy, lifestyle reconstructions using microstructural methods have not been attempted. Over the past few decades, contemporary Marshallese, and Pacific Islanders in general (Hawley and McGarvey Citation2015), have become increasingly sedentary and reliant on imported foods of poor nutritional quality—eschewing their traditional diet of locally cultivated produce and marine resources, primarily fish and shellfish (Deenik and Yost Citation2006; Englberger et al. Citation2014). However, some of the traditional plant food preparation practices and fishing activities still exist in parts of the Marshall Islands today (Campbell Citation2015). These typically involve rigorous arm use (). This case study tests whether similar activities might have occurred in the Marshall Islands when the archipelago was initially settled. The oldest (ca. 1720 BP) fragmented human remains known from the islands, representing an adult male, are examined histologically to evaluate remodeling markers in upper and lower limb bones. The utility of histology in Pacific bioarchaeology is highlighted.
Figure 1. Examples of modern day food preparation practices on the Marshall Islands. Ebon villagers husking a coconut (A) and grating giant swamp taro (iaraj, Cyrtosperma merkusii) (B) (images by M. Weisler, with permission).

Bioarchaeology of behavior and histological techniques
The relationship between bone size and shape and physical activity is understood through the principles of bone functional adaptation (Ruff, Holt, and Trinkaus Citation2006). In adult bone, dynamic and weight-bearing physical activity induces a tissue re-distribution response, which ensures bone strength and competence (Robling, Castillo, and Turner Citation2006). In bioarchaeology, this premise has been used to reconstruct human behavior from bone macro-morphology and morphometry combined with the relevant archaeological context (see Meyer et al. Citation2011 for review). A respectful and minimally invasive study design (Mays et al. Citation2013) can also involve a histological examination of samples, assessing bone physiological response to mechanical load and strain (Miszkiewicz and Mahoney Citation2016). When cortical bone is repeatedly over-loaded, it develops micro-damage that is fixed through targeted remodeling executed by basic multicellular units (BMUs) (Martin Citation2007). The activity of a BMU, a three-dimensional longitudinal group of osteoblasts and osteoclasts, can be reconstructed from histological slides by examining the density and geometric properties of its resulting secondary osteon features such as the Haversian canals (Stout, Cole, and Agnew Citation2019). A strained bone region can experience more remodeling, along with a strain suppressed BMU, which results in smaller cross-sections of the osteon products (Miszkiewicz and Mahoney Citation2019; Skedros, Mason, and Bloebaum Citation1994; van Oers et al. Citation2008). Bioarchaeological interpretations can thus be made from bone biological variables such as Haversian canal area (H.Ar) or density (H.Dn) (Miszkiewicz and Mahoney Citation2019; Miszkiewicz et al. Citation2020).
Because bones vary in function (Currey Citation1979), remodeling can be investigated using one bone inter-skeletally (e.g., Burr, Ruff, and Thompson Citation1990), or several bones intra-skeletally (e.g., Eleazer and Jankauskas Citation2016; Fahy et al. Citation2017). For example, femur H.Ar was previously shown to be smaller in English Medieval individuals who engaged in labor-intensive low status occupations (Miszkiewicz and Mahoney Citation2016), or in a physically active fourteenth to nineteenth century Pecos sample from New Mexico when compared to a modern US sedentary group (Burr, Ruff, and Thompson Citation1990). Similarly, femur H.Dn (Haversian canal number/mm2) was higher in nineteenth century Canadian individuals from Baffin Island when also contrasted against modern US groups (Thompson, Salter, and Laughlin Citation1981). Intra-skeletally, the rib, which receives less variable and low strain loading, can be incorporated into histology analyses of limb bones which are loaded more intensely (Eleazer and Jankauskas Citation2016). However, because bone remodeling is sensitive to more than just biomechanical load (Pfeiffer et al. Citation2006), variables such as age, sex, genetics, diet, and disease complicate behavioral interpretations, and thus data should be contextualized as much as possible.
Histological techniques can also help in cases of fragmented skeletal remains, such as our case study. In combination with shaft segment robusticity, localized bone remodeling can be reconstructed using small fragments (Pfeiffer and Zehr Citation1996). Implementing measures of H.Ar and H.Dn accounts for inconsistent preservation of secondary osteon cement lines occurring in archaeological samples (see Miszkiewicz et al. Citation2020) meaning that bone vascularization facilitating remodeling can still be approximated (Marenzana and Arnett Citation2013).
Bioarchaeology of the Marshall Islands
Compared to many Pacific Islands, the Marshalls have been subject to fewer bioarchaeological investigations. Human remains recovered there prior to the 1990s were poorly provenanced (Pietrusewsky Citation1990). However, since the 2000s, some key findings from this region have emerged, providing clues to the complexity of human migration and occupation in the Marshall Islands, and in the Pacific more broadly (Matisoo-Smith Citation2015; Swindler and Weisler Citation2000; Weisler et al. Citation2000; Weisler and Swindler Citation2002).
MtDNA extracted from a calcaneus in a high status middle-aged male adult recovered from a burial on Kwajalein Atoll, dated to AD 1486 (mean date), suggested a recent expansion of the most common genetic Micronesian lineage, referred to as Lineage Group I (Weisler et al. Citation2000). Lineage Group I was defined by a particular 9 base pair deletion, which describes mtDNA lineages belonging to haplogroup B4 and derived lineages—likely B4a1a1 or B4b lineages, which are common in Micronesia today (Vilar et al. Citation2013). An odontometric study using human teeth from a series of sites—the Utrōk (1860–380 BP), Majuro (second to seventh centuries AD), Ujae (third to sixth centuries AD), Kwajalein (date as above), Maloelap (2000 BP), and Ebon (first to fifteenth centuries AD) atolls—recorded tooth morphology (e.g., incisor shoveling) and morphometry to find that the dentition of pre-contact eastern Micronesians resembled Polynesians rather than Melanesians (Swindler and Weisler Citation2000). This further confirmed episodes of mixing and isolation of inter-island contact in the Pacific. A later study examining 27 human mandibulae recovered from three pre-contact sites on Utrōk (AD 20–1638), Majuro (AD 4–713), and Ebon (AD 1042–1294) atolls reported that almost half (49%) of the assemblage presented with rocker jaws (Weisler and Swindler Citation2002). Because rocker jaws are typically attributed to Polynesians, Weisler and Swindler (Citation2002) inferred that a high frequency of this trait in ancient Marshallese was evidence for contact between pre-contact Micronesians and Polynesians. While these bioarchaeological analyses filled major gaps in the prehistory of the Marshall Islands, a lack of ancient behavioral reconstructions is apparent for the region.
The modern Marshallese society outside of urbanized regions has a social structure of hereditary chiefs who determine land allocation (Gittelsohn et al. Citation2003), and is characterized by a traditional gender division of labor (Tuara et al. Citation2008; Wedgwood Citation1942). Males typically engage in food production and collection on land, and capture and transportation of marine resources through fishing, whereas females undertake domestic work involving gardening and food preparation (Tuara et al. Citation2008; Wedgwood Citation1942). These activities are still commonly practiced on the atolls, though they are becoming less commonplace (Thaman, Elevitch, and Kennedy Citation2006). Zooarchaeological evidence indicates that wild foods consumed in the prehistoric Marshall Islands were fish, shellfish, crustaceans, sea birds (Lambrides and Weisler Citation2018; Weisler Citation2001), and domesticated animals, including dogs (Harris and Weisler Citation2017, Citation2018; Rainbird Citation1994; see Fitzpatrick et al. Citation2016 for a discussion about island size and subsistence strategies). Archaeobotanical data point to arboriculture of produce such as coconut (ni, Cocos nucifera), taro (iaraj, primarily giant swamp taro, Cyrtosperma merkusii), breadfruit (mā, Artocarpus altilis), banana (keeprañ, Musa sp.), screwpine (bōb, Pandanus spp.), and arrowroot (makmōk, Tacca leontopetaloides), local cultivation of which continues to present day (Loeak, Kiluwe, and Crowl Citation2004; Weisler Citation1999).
Fishing, canoeing, seafaring, and cultivation of endemic plants require the exertion of energy in the upper limbs as a canoe needs to be propelled through water in a sitting position, and resources need to be harvested through collection, cutting, carrying, and subsequent modification for consumption. These practices result in a combination of repetitive movements alongside weight-bearing and well-developed musculature of the arms (). Archaeological evidence for pit cultivation systems for growing giant swamp taro, fishing, and mollusk foraging in the Marshall Islands has been described (Harris, Weisler, and Faulkner Citation2015; Harris and Weisler Citation2018; Lambrides and Weisler Citation2018; Weisler Citation1999, Citation2001). Inter-island seafaring of the prehistoric Marshallese has been deduced from linguistic analyses of oral traditions (Lum Citation1998; Marck Citation1986). We hypothesized that these strenuous activities would lead to relatively smaller localized H.Ar and higher H.Dn in the arm bone samples compared to the femur.
Materials and methods
The skeletal remains were exhumed and examined with permission from the local landholder, the local Mayor, and the Historic Preservation Office of the Republic of the Marshall Islands. The remains were excavated from a village site (MLEb-5) on Ebon Islet, Ebon Atoll, Marshall Islands (). Located on a low hill ∼120 m inland from the lagoon shore, near an extensive aroid pit cultivation system, transect excavations revealed an extended human burial below a ∼95 cm thick cultural layer in TP6. The skeletal remains were partially disturbed and fragmented from prehistoric residential activities. The remains of five partial individuals were encountered, including material in nearby excavations. The burial in TP6, the subject of this study, was the only individual whose long bone shaft segments were preserved for a histological examination (). The osteological inventory of this individual consisted of identifiable, but heavily fragmented pieces of all the major bones except the tibia, along with loose teeth. Age-at-death and biological sex were estimated following standard methods (Buikstra and Ubelaker Citation1994) applied to identifiable bone fragments. This included dental wear (Miles Citation2001) and cranial suture closure (Meindl and Lovejoy Citation1985) assessment for age-at-death estimation, and evaluation of the robusticity of the cranial (Keen Citation1950) and pelvic bones (Bruzek Citation2002).
Figure 2. Map of Ebon Atoll showing the location of MLEb-5 archaeological site (modified from Harris and Weisler Citation2018, 43, reprinted with permission from Taylor & Francis).
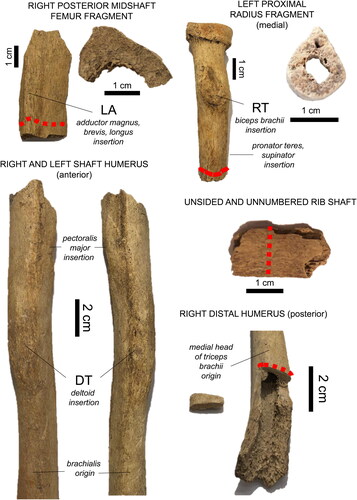
Figure 3. Bone fragments examined in the present case study. The red dashed lines indicate sampling regions for histology, with the extracted samples shown on the right (femur, radius) and left (right distal humerus). RT: radial tuberosity, DT: deltoid tuberosity, LA: linea aspera. Muscle insertions and origin are italicized.
Given the limited skeletal material, our invasive methods were restricted to only one sample per bone. This followed the ethical guidelines for destructive sampling of skeletal remains in archaeology, which also stipulate sampling away from important anatomical landmarks (Mays et al. Citation2013, 5). Bearing this in mind, samples were either loose fragments or removed from the already taphonomically exposed bone surfaces. However, we still ensured the sampling location fitted within our biomechanical study design (). We sampled the distal humerus and proximal radius (part of an elbow complex), and a posterior femur fragment (linea aspera). An unsided and un-numbered loose rib fragment was included as the human rib has been previously suggested as a suitable “control” for comparing intra-skeletal remodeling (Eleazer and Jankauskas Citation2016; Robling and Stout Citation2003). While compact bone formation varies throughout the skeleton, long bones should be within ±3 years of the rib (Frost and Wu Citation1967; Robling and Stout Citation2003).
Prior to sectioning, bones were visually examined for entheseal prominence (Weiss Citation2015) across the humeral deltoid and radial tuberosities, and femoral linea aspera (see for muscle insertions and origins). Those were only used as a general reference given prior research raising concerns over entheseal links to bone remodeling (e.g., Rabey et al. Citation2015). To gain an overview of size variation intra-skeletally, shaft diameter (in antero-posterior and medio-lateral planes in all bone fragments, and additionally in both the left and right humerus), and circumference were measured using digital calipers and a soft measuring tape, respectively. Midshaft was located via deltoid muscle insertions, but the radius and rib could only be measured where feasible (, red dashed line). Only the thickness of the cortical wall (endosteal to the periosteal border) could be measured in the femoral fragment. Reported dimensions are an average of three measurements. Samples were removed using a Dremel 4000 Rotary Tool 175 W equipped with a cutting disk (e.g., Miszkiewicz and Mahoney Citation2016).
Histological preparation and analysis
Standard methods for preparing thin sections of archaeological human bone were followed (Miszkiewicz and Mahoney Citation2017). Samples were embedded in Buehler® epoxy resin and cut on a Kemet Micracut® 151 saw to reveal histological surfaces. Samples were glued onto glass slides, and further reduced on the saw, creating ‘thick’ sections. These sections were subsequently ground down to ∼100 μm and polished using a Buehler® micro-polish powder paste to remove grinding scratches from the sample surface. The sections were placed in an ultrasonic bath for cleaning, dehydrated in a series of ethanol baths, cleared with xylene, and cover-slipped.
An Olympus BX53 microscope operated through Olympus CellSens® software, and equipped with an Olympus DP74 high resolution camera, was used to examine each section under normal transmitted and linearly polarized light (LPL). An overview of each section was recorded using a 4× objective and an ‘auto-stitched’ image was saved. The image was imported into ImageJ® v. 1.52, and a complete area of the section was measured in mm2 (using the “Polygon” tool). Next, Haversian canals seen in the entirety of each section were counted using the “Multi-point” tool, and the total number was divided by section area in mm2 to create H.Dn (, Miszkiewicz et al. Citation2020; Jordan et al. Citation2000). Because cement lines of secondary osteons were not consistently preserved to provide an estimate of partially remodeled osteon densities, H.Dn was used as a proxy for bone vascularity resulting from bone remodeling executed by BMUs (Miszkiewicz et al. Citation2020). Because the samples were viewed under LPL, it was possible to confidently identify all canals as Haversian (e.g., remnants of adult lamellar layers were seen surrounding each canal, ).
Figure 4. A summary of histological procedures (A and B) and results (C) of the present study. Examples of bone cross-sections are shown for the rib under normal transmitted light (A) and the radius under linearly polarized light (LPL) (B). The cross-section marked with red dots (A) indicates Haversian canals counted from the entirety of the cortical surface for the purpose of density calculations. The radius cross-section (B) shows a grid applied in ImageJ® v. 1.52 from which H.Ar regions of interest were identified. The magnified images (A1 and B1) show Haversian canals (white arrows, scale bars = 50 μm), viewed under LPL. The simple bar chart in C visually illustrates that the radius and humerus samples had higher canal densities of lower areas compared to the femur. The rib, as predicted, had the highest densities and smallest canal areas.
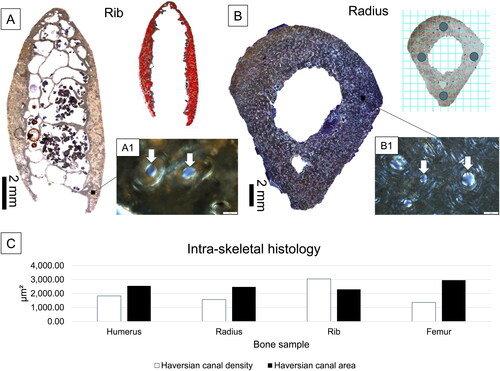
To further understand the size of BMU features, H.Ar was measured using the “Freehand” tool from selected regions of interest (ROIs) (e.g., Miszkiewicz and Mahoney Citation2016, Citation2019). The ROIs were from the anatomical direction midpoints of each sample identified by rotating each image in ImageJ® v. 1.52. A grid of 2 mm2 per ROI was applied and canals seen within were measured: three ROIs through the posterior midpoint of the femur (6 mm2), two ROIs through the posterior midpoint of the humerus (4 mm2), and four ROIs in the radius corresponding to anterior, posterior, medial, lateral anatomical locations (8 mm2) (). The entire cortical area (excluding trabecular space) was included in the H.Ar measurements of the rib. The average H.Ar was computed from all measured canals.
Given that this is a case study, no inferential statistical analysis was attempted. Descriptive data are presented by reporting median, mean, minimum, maximum, and standard deviation data for all the samples, focusing on a comparison between the upper and lower limb bones.
Results
The osteological examination indicated that this individual was a middle-aged (30–49 years old) ‘probable’ male. The deltoid and radial tuberosities were strongly developed, and the linea aspera was somewhat raised (). The right midshaft humerus () was wider in the antero-posterior plane compared to the left, and of a more pronounced robusticity, possibly expressing bilateral differences.
Table 1. Bone shaft measures of size in the middle-aged male from the Marshall Islands. Left humerus was not included in the histological analysis and is only presented here for illustrative purposes.
The cortical bone areas were: 117.29 mm2 in the femur (a patch of bone measuring 17.56 mm2 had to be excluded where no distinctive histology could be identified), 64.42 mm2 in the humerus, 86.70 mm2 in the radius, and 17.73 mm2 in the rib. The raw Haversian canal counts were 1603 in the femur, 1177 in the humerus, 1362 in the radius, and 540 in the rib. The rib H.Dn was 3045.69 which was the highest out of all the samples analyzed (). The humerus had the highest H.Dn (1827.07), followed by the radius (1570.93). The femoral sample had the lowest H.Dn (1366.70), confirming our prediction.
Table 2. Descriptive data for the measurements of Haversian canal area (H.Ar) in μm2 compared between cortical samples from different bones.
An average of 26.33 (femur), 27.5 (humerus), and 18.75 (radius) canals per a 2 mm2 ROI were available for the measurement of H.Ar in the samples. Total data per each bone sample are presented in . When considering each ROI, the H.Ar in the femur ranged from 2515.00 μm2 to 3873.40 μm2. In the humerus, the range was 1567.91 μm2 to 4140.81 μm2, whereas the radius H.Ar ranged from 1958.49 μm2 to 3053.74 μm2. These data demonstrate that the humerus had the widest variability in the H.Ar values, but all data fall into a similar range. Once averaged per bone sample, the H.Ar data followed the same, and as predicted, intra-skeletal trend as the H.Dn data, except the trend was in the opposite direction (). The rib, as expected, showed the smallest overall H.Ar (mean = 2300.65 μm2, median = 1571.98 μm2), which confirmed its use as control bone experiencing relatively stable remodeling. The upper limb bones showed smaller H.Ar (radius mean = 2477.17 μm2; median = 2161.49 μm2; humerus mean = 2550.29 μm2, median = 1688.48 μm2) compared to the lower limb (femur mean = 2958.84 μm2, median = 2232.69 μm2), confirming our predictions.
Discussion and conclusions
The histology data indicate that the upper limb bones of an adult male recovered from an archaeological site in the Marshall Islands showed relatively high bone canal densities of smaller areas compared to the femur. One explanation is that these results suggest experiences of frequent localized remodeling in the arm bones stimulated through weight-bearing. The bioarchaeological interpretation proposed is that this individual might have engaged in food production, fishing, and seafaring behaviors, similar to those observed in modern day Marshallese.
The humerus and radius data suggest experiences of more frequent, but possibly shorter (strain suppressed), bone secondary remodeling events as BMUs would have been targeting biomechanically strained regions of the cortical bone in the individual’s arms (Mori and Burr Citation1993). A similar finding was previously noted in a sample of archaeological English juveniles whose humeri adapted to Medieval tasks requiring high force (Pitfield, Deter, and Mahoney Citation2019), and in a fourteenth to nineteenth century Lithuanian sample where the humerus was more vascularized than the femur (Eleazer and Jankauskas Citation2016). One study where human radial histology was examined in an English Medieval archaeological sample (Walker et al. Citation2019) reported higher variability and larger H.Ar compared to our data. This could suggest that our sample reflects relatively higher experiences of bone remodeling, though we cannot exclude inter-population differences in bone remodeling, or a possibility that our sample is an outlier.
In our study, the humeral external morphology showed well-developed deltoid tuberosities, and a bilateral difference in the antero-posterior midshaft diameter, indicating preferential arm use (e.g., Stirland Citation1993). The proximal end of the radius is associated with the biceps, which contracts during carrying and lifting, pulling the forearm during flexion, and pushing it away during extension, with the other elbow muscles forming the arm complex (Hutchinson, Gloystein, and Gillespie Citation2008; Murray, Buchanan, and Delp Citation2002). We cannot attribute a direct correlation between the entheses and muscle size (Rabey et al. Citation2015), but we can infer a repertoire of activities that go beyond hand manipulation. Experimental research into the effect of hand activity on muscle load shows that the deltoid muscle is only minimally impacted during hand manipulation tasks (Roman-Liu, Tokarski, and Kamińska Citation2001). A hypertrophic deltoid tuberosity was previously shown to relate to weight-bearing and habitual activities such as food production or fishing, where the arm is strained by pulling (Lai and Lovell Citation1992; Weiss Citation2003). Repeated contraction and extension of the arm during climbing of trees (for example when harvesting coconuts) can also be proposed (Veeger and Van Der Helm Citation2007).
The femur data suggest relatively lower localized remodeling, possibly due to sitting activities such as canoeing. The posterior femur adductors move the leg in and out during crossover steps or shifting the leg sideways, yet the individual’s linea aspera was not well developed in our study. As the individual derives from an atoll of small size, low elevation and level topography, his habitual mobility was likely moderate, and between-island sea travel a regular mode of transport. Prior studies have demonstrated that bone remodeling can alter if the lower limb is subject to recumbency or sedentism (e.g., Eleazer and Jankauskas Citation2016, Miszkiewicz et al. Citation2020; Schlecht et al. Citation2012). The femur carries load from the upper body weight in addition to that resulting from mobility (Drapeau and Streeter Citation2006). An alternative interpretation is that the lower H.Dn are due to expanded lamellar space accommodating loads from the upper body weight, but adapted to habitual mobility that does not cross effective strain thresholds needed to evoke targeted remodeling (Frost Citation2000).
Building upon prior bioarchaeological evidence indicating social status division (Weisler et al. Citation2000) in the Marshall Islands, our results contribute data suggesting that typically ‘male’ labor might have also occurred there ca. 2000 years ago. Further comparisons with adult females will expand our findings. We acknowledge that intra-skeletal biomechanics cannot be the only variable explaining the histological data we provide, given the complexity of factors underlying bone remodeling (see Pfeiffer et al. Citation2006). Future data will facilitate more comparisons to entail diet, age, and sex. The fragmented preservation means we could not sample more bones or ROIs, yet having access to the tibia would shed more light on the functional adaptation in the lower leg (Drapeau and Streeter Citation2006). Without experimental validation, which is impossible with the materials at hand, this study will remain interpretive in nature. However, the utility of histological sampling was clearly beneficial as prior non-macroscopic methods were unsuccessful (e.g., viable aDNA extraction) in this individual. Future research in Pacific bioarchaeology may benefit from incorporating microscopic techniques into its methodological toolkit.
Acknowledgements
We are indebted to Josepha Maddison from the Historic Preservation Office, Republic of the Marshall Islands, and Mayor Lajan Kabua for permission to conduct research on Ebon Atoll. Financial support was received from the Australian Research Council under grant DE190100068 (to Miszkiewicz). The fieldwork was supported by a grant to Weisler from the Deputy Vice Chancellor-Research, University of Queensland. The article was improved following feedback from three reviewers and the journal’s Co-Editor, Scott M. Fitzpatrick.
References
- Bruzek, J. 2002. A method for visual determination of sex, using the human hip bone. American Journal of Physical Anthropology 117 (2):157–68. doi:10.1002/ajpa.10012
- Buikstra, J. E., and D. H. Ubelaker. 1994. Standards for data collection from human skeletal remains. Fayetteville: Arkansas Archaeological Survey Research Series No. 44.
- Burr, D. B., C. B. Ruff, and D. D. Thompson. 1990. Patterns of skeletal histologic change through time: Comparison of an archaic Native American population with modern populations. The Anatomical Record 226 (3):307–13. doi:10.1002/ar.1092260306
- Campbell, J. R. 2015. Development, global change and traditional food security in Pacific Island countries. Regional Environmental Change 15 (7):1313–24. doi:10.1007/s10113-014-0697-6
- Currey, J. D. 1979. Mechanical properties of bone tissues with greatly differing functions. Journal of Biomechanics 12 (4):313–9. doi:10.1016/0021-9290(79)90073-3
- Deenik, J. L., and R. S. Yost. 2006. Chemical properties of atoll soils in the Marshall Islands and constraints to crop production. Geoderma 136 (3-4):666–81. doi:10.1016/j.geoderma.2006.05.005
- Drapeau, M. S., and M. A. Streeter. 2006. Modeling and remodeling responses to normal loading in the human lower limb. American Journal of Physical Anthropology 129 (3):403–9. doi:10.1002/ajpa.20336
- Eleazer, C. D., and R. Jankauskas. 2016. Mechanical and metabolic interactions in cortical bone development. American Journal of Physical Anthropology 160 (2):317–33. doi:10.1002/ajpa.22967
- Englberger, L., R. Lorennij, M. Taylor, V. S. Tuia, W. Aalbersberg, U. Dolodolotawake, L. Tibon, J. Tibon, and J. Alfred. 2014. Carotenoid content and traditional knowledge of breadfruit cultivars of the Republic of the Marshall Islands. Journal of Food Composition and Analysis 34 (2):192–9. doi:10.1016/j.jfca.2012.05.002
- Fahy, G. E., C. Deter, R. Pitfield, J. J. Miszkiewicz, and P. Mahoney. 2017. Bone deep: Variation in stable isotope ratios and histomorphometric measurements of bone remodelling within adult humans. Journal of Archaeological Science 87:10–6. doi:10.1016/j.jas.2017.09.009
- Fitzpatrick, S. M., V. D. Thompson, A. S. Poteate, M. F. Napolitano, and J. M. Erlandson. 2016. Marginalization of the margins: The importance of smaller islands in human prehistory. The Journal of Island and Coastal Archaeology 11 (2):155–70. doi:10.1080/15564894.2016.1192568
- Frost, H. M. 2000. The Utah paradigm of skeletal physiology: An overview of its insights for bone, cartilage and collagenous tissue organs. Journal of Bone and Mineral Metabolism 18 (6):305–16. doi:10.1007/s007740070001
- Frost, H. M., and K. Wu. 1967. Histological measurements of bone formation rates in contemporary archaeological and paleontological compact bone. In Miscellaneous papers in paleopathology, ed. W. D. Woode, 9–22. Flagstaff: University of Arizona.
- Gittelsohn, J., H. Haberle, A. E. Vastine, W. Dyckman, and N. A. Palafox. 2003. Macro- and microlevel processes affect food choice and nutritional status in the republic of the Marshall Islands. The Journal of Nutrition 133 (1):310S–13S. doi:10.1093/jn/133.1.310S
- Harris, M., and M. Weisler. 2017. Intertidal foraging on atolls: Prehistoric forager decision-making at Ebon Atoll, Marshall Islands. The Journal of Island and Coastal Archaeology 12 (2):200–23. doi:10.1080/15564894.2016.1167140
- Harris, M., and M. Weisler. 2018. Two millennia of mollusc foraging on Ebon Atoll, Marshall Islands: Sustained marine resource use on a Pacific atoll. Archaeology in Oceania 53 (1):41–57. doi:10.1002/arco.5134
- Harris, M., M. Weisler, and P. Faulkner. 2015. A refined protocol for calculating MNI in archaeological molluscan shell assemblages: A Marshall Islands case study. Journal of Archaeological Science 57:168–79. doi:10.1016/j.jas.2015.01.017
- Hawley, N. L., and S. T. McGarvey. 2015. Obesity and diabetes in Pacific Islanders: The current burden and the need for urgent action. Current Diabetes Reports 15 (5):29. doi:10.1007/s11892-015-0594-5
- Hutchinson, H. L., D. Gloystein, and M. Gillespie. 2008. Distal biceps tendon insertion: An anatomic study. Journal of Shoulder and Elbow Surgery 17 (2):342–6. doi:10.1016/j.jse.2007.05.005
- Jordan, G. R., N. Loveridge, K. L. Bell, J. Power, N. Rushton, and J. Reeve. 2000. Spatial clustering of remodeling osteons in the femoral neck cortex: A cause of weakness in hip fracture? Bone 26 (3):305–13. doi:10.1016/S8756-3282(99)00272-0
- Keen, J. A. 1950. A study of the differences between male and female skulls. American Journal of Physical Anthropology 8 (1):65–80. doi:10.1002/ajpa.1330080113
- Lai, P., and N. C. Lovell. 1992. Skeletal markers of occupational stress in the fur trade: A case study from a Hudson's Bay Company fur trade post. International Journal of Osteoarchaeology 2 (3):221–34. doi:10.1002/oa.1390020306
- Lambrides, A. B. J., and M. I. Weisler. 2018. Late Holocene Marshall Islands archaeological tuna records provide proxy evidence for ENSO variability in the western and central Pacific Ocean. The Journal of Island and Coastal Archaeology 13 (4):531–62. doi:10.1080/15564894.2017.1315350
- Loeak, A. L., V. C. Kiluwe, and L. Crowl. 2004. Life in the Republic of the Marshall Islands. Majuro: University of the South Pacific Centre.
- Lum, J. K. 1998. Central and eastern Micronesia: Genetics, the overnight voyage, and linguistic divergence. Man and Culture in Oceania 14:69–80.
- Marck, J. C. 1986. Micronesian dialects and the overnight voyage. Journal of the Polynesian Society 95:253–8.
- Marenzana, M., and T. R. Arnett. 2013. The key role of the blood supply to bone. Bone Research 1 (3):203–15. doi:10.4248/BR201303001
- Martin, R. B. 2007. Targeted bone remodeling involves BMU steering as well as activation. Bone 40 (6):1574–80. doi:10.1016/j.bone.2007.02.023
- Matisoo-Smith, E. 2015. Ancient DNA and the human settlement of the Pacific: A review. Journal of Human Evolution 79:93–104. doi:10.1016/j.jhevol.2014.10.017
- Mays, S., J. Elders, L. Humphrey, W. White, and P. Marshall. 2013. Science and the dead: A guideline for the destructive sampling of archaeological human remains for scientific analysis. London: English Heritage Publishing with the Advisory Panel on the Archaeology of Burials in England.
- Meindl, R. S., and C. O. Lovejoy. 1985. Ectocranial suture closure: A revised method for the determination of skeletal age at death based on the lateral-anterior sutures. American Journal of Physical Anthropology 68 (1):57–66. doi:10.1002/ajpa.1330680106
- Meyer, C., N. Nicklisch, P. Held, B. Fritsch, and K. W. Alt. 2011. Tracing patterns of activity in the human skeleton: An overview of methods, problems, and limits of interpretation. Homo 62 (3):202–17. doi:10.1016/j.jchb.2011.03.003
- Miles, A. E. W. 2001. The Miles method of assessing age from tooth wear revisited. Journal of Archaeological Science 28 (9):973–82. doi:10.1006/jasc.2000.0652
- Miszkiewicz, J. J., and P. Mahoney. 2016. Ancient human bone microstructure in medieval England: Comparisons between two socio-economic groups. Anatomical Record 299 (1):42–59. doi:10.1002/ar.23285
- Miszkiewicz, J. J., and P. Mahoney. 2017. Human bone and dental histology in an archaeological context. In Human remains: Another dimension, ed. T. Thompson and D. Erricksen, 29–43. Amsterdam: Elsevier Academic Press. doi:10.1016/B978-0-12-804602-9.00004-7
- Miszkiewicz, J. J., and P. Mahoney. 2019. Histomorphometry and cortical robusticity of the adult human femur. Journal of Bone and Mineral Metabolism 37 (1):90–104. doi:10.1007/s00774-017-0899-3
- Miszkiewicz, J. J., C. Rider, S. Kealy, C. Vrahnas, N. A. Sims, J. Vongsvivut, M. J. Tobin, M. J. L. A. Bolunia, A. S. De Leon, A. L. Peñalosa, et al. 2020. Asymmetric midshaft femur remodeling in an adult male with left sided hip joint ankylosis, Metal Period Nagsabaran, Philippines. International Journal of Paleopathology 31:14–22. doi:10.1016/j.ijpp.2020.07.003
- Mori, S., and D. B. Burr. 1993. Increased intracortical remodeling following fatigue damage. Bone 14 (2):103–9. doi:10.1016/8756-3282(93)90235-3
- Murray, W. M., T. S. Buchanan, and S. L. Delp. 2002. Scaling of peak moment arms of elbow muscles with upper extremity bone dimensions. Journal of Biomechanics 35 (1):19–26. doi:10.1016/S0021-9290(01)00173-7
- Pfeiffer, S., C. Crowder, L. Harrington, and M. Brown. 2006. Secondary osteon and Haversian canal dimensions as behavioral indicators. American Journal of Physical Anthropology 131 (4):460–8. doi:10.1002/ajpa.20454
- Pfeiffer, S., and M. K. Zehr. 1996. A morphological and histological study of the human humerus from Border Cave. Journal of Human Evolution 31 (1):49–59. doi:10.1006/jhev.1996.0048
- Pietrusewsky, M. 1990. The physical anthropology of Micronesia: A brief overview. Micronesia Supplement 2:273–402.
- Pitfield, R., C. Deter, and P. Mahoney. 2019. Bone histomorphometric measures of physical activity in children from medieval England. American Journal of Physical Anthropology 169 (4):730–46. doi:10.1002/ajpa.23853
- Rabey, K. N., D. J. Green, A. B. Taylor, D. R. Begun, B. G. Richmond, and S. C. McFarlin. 2015. Locomotor activity influences muscle architecture and bone growth but not muscle attachment site morphology. Journal of Human Evolution 78:91–102. doi:10.1016/j.jhevol.2014.10.010
- Rainbird, P. 1994. Prehistory in the northwest tropical Pacific: The Caroline, Mariana, and Marshall Islands. Journal of World Prehistory 8 (3):293–349. doi:10.1007/BF02221052
- Robling, A. G., A. B. Castillo, and C. H. Turner. 2006. Biomechanical and molecular regulation of bone remodeling. Annual Review of Biomedical Engineering 8:455–98. doi:10.1146/annurev.bioeng.8.061505.095721
- Robling, A. G., and S. D. Stout. 2003. Histomorphology, geometry, and mechanical loading in past populations. In Bone loss and osteoporosis, ed. S. Agarwal and S. Stout, 189–205. Boston: Springer.
- Roman-Liu, D., T. Tokarski, and J. Kamińska. 2001. Assessment of the musculoskeletal load of the trapezius and deltoid muscles during hand activity. International Journal of Occupational Safety and Ergonomics 7 (2):179–93. doi:10.1080/10803548.2001.11076485
- Ruff, C., B. Holt, and E. Trinkaus. 2006. Who's afraid of the big bad Wolff?: “Wolff's law” and bone functional adaptation. American Journal of Physical Anthropology 129 (4):484–98. doi:10.1002/ajpa.20371
- Schlecht, S. H., D. C. Pinto, A. M. Agnew, and S. D. Stout. 2012. Brief communication: The effects of disuse on the mechanical properties of bone: What unloading tells us about the adaptive nature of skeletal tissue. American Journal of Physical Anthropology 149 (4):599–605. doi:10.1002/ajpa.22150
- Skedros, J. G., M. W. Mason, and R. D. Bloebaum. 1994. Differences in osteonal micromorphology between tensile and compressive cortices of a bending skeletal system: Indications of potential strain‐specific differences in bone microstructure. The Anatomical Record 239 (4):405–13. doi:10.1002/ar.1092390407
- Stirland, A. J. 1993. Asymmetry and activity‐related change in the male humerus. International Journal of Osteoarchaeology 3 (2):105–13. doi:10.1002/oa.1390030207
- Stout, S. D., M. E. Cole, and A. M. Agnew. 2019. Histomorphology: Deciphering the metabolic record. In Ortner's identification of pathological conditions in human skeletal remains, ed. J. E. Buikstra, 91–167. Amsterdam: Elsevier Academic Press.
- Swindler, D. R., and M. I. Weisler. 2000. Dental size and morphology of precontact Marshall Islanders (Micronesia) compared with other Pacific Islanders. Anthropological Science 108 (3):261–82. doi:10.1537/ase.108.261
- Thaman, R. R., C. R. Elevitch, and J. Kennedy. 2006. Urban and homegarden agroforestry in the Pacific Islands: Current status and future prospects. In Tropical homegardens, B. M. Kumar and P. K. R. Nair, 25–41. Dordrecht: Springer.
- Thompson, D. D., E. M. Salter, and W. S. Laughlin. 1981. Bone core analysis of Baffin Island skeletons. Arctic Anthropology 18:87–96.
- Tuara, P., K. Passfield, M. J. Williams, M. Williams, Z. Hilly, A. M. Schwarz, D. Boso, I. Novaczek, D. M. Solomona, V. C. Vuki, et al. 2008. Issues on gender in oceanic and coastal fisheries science and management in the Pacific Islands: Case studies from Solomon Islands, Marshall Islands and Tonga. SPC Women in Fisheries Information Bulletin 22:3–16.
- Walker, M. M., E. M. Street, R. Pitfield, J. J. Miszkiewicz, S. L. Brennan-Olsen, and P. Mahoney. 2019. Ancient human bone microstructure case studies from medieval England. In Bone health, ed. J. J. Miszkiewicz, S. L. Brennan-Olsen, and J. A. Riancho, 35–52. Singapore: Springer. doi:10.1007/978-981-13-7256-8_3
- Wedgwood, C. H. 1942. Notes on the Marshall Islands. Oceania 13 (1):1–23. doi:10.1002/j.1834-4461.1942.tb00366.x
- Weisler, M. I. 1999. The antiquity of aroid pit agriculture and significance of buried a horizons on Pacific atolls. Geoarchaeology 14 (7):621–54. doi:10.1002/(SICI)1520-6548(199910)14:7 < 621::AID-GEA2 > 3.0.CO;2-2
- Weisler, M. I. 2001. On the margins of sustainability: Prehistoric settlement of Utrōk Atoll, Northern Marshall Islands. British Archaeological Reports International Series 967. Oxford: Archaeopress.
- Weisler, M. I., J. K. Lum, S. L. Collins, and W. S. Kimoto. 2000. Status, health, and ancestry of a late prehistoric burial from Kwajalein Atoll, Marshall Islands. Micronesica 32 (2):191–220.
- Weisler, M. I., and D. Swindler. 2002. Rocker jaws from the Marshall Islands: Evidence for interaction between eastern Micronesia and west Polynesia. People and Culture in Oceania 18:23–33.
- Weiss, E. 2003. Effects of rowing on humeral strength. American Journal of Physical Anthropology 121 (4):293–302. doi:10.1002/ajpa.10240
- Weiss, E. 2015. The surface of bones: Methods of recording entheseal changes. Surface Topography: Metrology and Properties 3 (3):034003. doi:10.1088/2051-672X/3/3/034003
- van Oers, R. F., R. Ruimerman, B. van Rietbergen, P. A. Hilbers, and R. Huiskes. 2008. Relating osteon diameter to strain. Bone 43 (3):476–82. doi:10.1016/j.bone.2008.05.015
- Veeger, H. E. J., and F. C. T. Van Der Helm. 2007. Shoulder function: The perfect compromise between mobility and stability. Journal of Biomechanics 40 (10):2119–29. doi:10.1016/j.jbiomech.2006.10.016
- Vilar, M. G., C. W. Chan, D. R. Santos, D. Lynch, R. Spathis, R. M. Garruto, and J. K. Lum. 2013. The origins and genetic distinctiveness of the Chamorros of the Marianas Islands: An mtDNA perspective. American Journal of Human Biology 25 (1):116–22. doi:10.1002/ajhb.22349
