ABSTRACT
Chimpanzees have consistent individual differences in behaviour, also referred to as personality. Similar to human personality structure, five dimensions are commonly found in chimpanzee studies that show evidence for convergent and predictive validity (Dominance, Openness, Extraversion, Agreeableness, and Reactivity/Undependability). These dimensions are to some extent heritable, indicating a genetic component that explains part of the variation in personality scores, but are also influenced by environmental factors, such as the early social rearing background of the individuals. In this study, we investigated the role of epigenetic modification of the dopamine receptor D2 gene (DRD2) as a potential mechanism underlying personality variation in 51 captive chimpanzees. We used previously collected personality trait rating data and determined levels of DRD2 CpG methylation in peripheral blood samples for these same individuals. Results showed that DRD2 methylation is most strongly associated with Extraversion, and that varying methylation levels at specific DRD2 sites are associated with changes in Extraversion in nursery-reared, but not mother-reared, individuals. These results highlight the role of dopaminergic signalling in chimpanzee personality, and indicate that environmental factors, such as social experiences early in life, can have long-lasting behavioural effects, potentially through modification of the epigenome. These findings add to the growing evidence demonstrating the importance of the experience-dependent methylome for the development of complex social traits like personality.
Introduction
Chimpanzees have consistent individual differences in behaviour that can be quantified as personality dimensions, utilizing methodologies similar to those employed with human subjects [Citation1]. Using personality trait ratings based on the experience of people familiar with the individual animals, studies typically yield six personality dimensions: Reactivity/Undependability, Dominance, Openness, Extraversion, Agreeableness and Methodical [Citation1,Citation2–4]. How individual chimpanzees score on each of these dimensions is determined by a combination of their genetic architecture, the environment, and interaction between these two. Some chimpanzee personality dimensions have a heritable component, indicating that part of the variation is attributable to genetic similarity of individuals in the population [Citation5,Citation6]. In particular, the dimensions that reflect affection (Extraversion and Dominance) generally show higher levels of heritability than the other dimensions [Citation5]. The remaining variation in personality is shaped by environmental factors, like maternal effects or early social rearing experiences [Citation5,Citation7]. For example, gmother-reared chimpanzees scored higher on Agreeableness than individuals reared by humans in a nursery setting [Citation5]. Nursery-reared chimpanzees, on the other hand, scored higher on Intellect, a dimension highly resembling chimpanzee Openness [Citation1,Citation5]. Finally, the development of personality is also influenced by the interaction of early social background with the genetic predisposition of the individual, leading to varying degrees of heritability for certain dimensions depending on rearing background. Extraversion, for example, showed the highest overall levels of heritability (h2 = 0.381), but was significantly more highly expressed in individuals that were mother-reared (h2 = 0.916) versus nursery-reared (h2 = 0.369). Similarly, Dominance had a significant heritable component (h2 = 0.195) but the heritability of this trait was again higher among mother-reared individuals (h2 = 0.469), than nursery-reared (h2 = 0.247) [Citation5]. In nursery-reared individuals, environmental factors thus potentially play a bigger role in explaining variation in personality scores than in mother-reared individuals. This shows that early-life experiences in chimpanzees are crucial for the development of personality in later life and that maternal care, or the lack thereof, might play an important role in modifying the genetic architecture of complex social personality traits.
Gene-environment interaction dynamics that result in long-lasting behavioural effects are often due to epigenetic mechanisms, for example CpG methylation [Citation8]. CpG methylation, or DNA methylation (‘methylation’ hereafter), comprises the addition of a methyl chemical group to cytosine DNA bases within the context of CpG sites (CpGs), or cytosines next to guanine bases. Methylation can reflect and maintain a gene’s transcriptional status by altering transcription factor activity and chromatin structure [Citation9]. CpG methylation levels change across development, but can also change through the influence of environmental factors like nutrition and stress, especially during critical stages of early development [Citation10]. Methylation levels at CpGs then often become fixed and can last throughout an individual’s lifetime. A well-known example of this is found in rats, where the stable alteration of DNA methylation levels in the hippocampus at the glucocorticoid receptor (NR3C1) depends on whether they received high versus low levels of maternal grooming during the first postnatal week [Citation11]. Studies in humans have found similar relationships between methylation levels at CpGs of candidate genes and various long-lasting behavioural phenotypes, for example of NR3C1 and stress responses [Citation12] or the serotonin transporter gene (SLC6A4) and aggression [Citation13].
To date, variation in chimpanzee personality has been linked mainly to variation in DNA sequence variation in genes implicated in behaviour. The main candidate genes in the search for mechanisms underlying personality differences in great apes include the genes coding for the receptors for androgens (AR), oestrogens (ERα and ERβ), vasopressin (AVPR1A), oxytocin (OXTR), serotonin (HTR1A) and dopamine (DRD4) [Citation14–23]. While some of these studies have repeatedly found links between genetic variants and personality, effect sizes are modest and explain only part of the variation [Citation24]. Epigenetic modifications of these genes are likely to contribute to additional variance in personality, but this has not been studied yet in chimpanzees and other nonhuman primates [for a review see Citation24]. Results from primate studies are nonetheless required to determine to what extent the biological mechanisms that lie at the basis of personality development in humans are unique or rather generalizable across species. Several studies have suggested that neuroanatomical and brain gene expression differences in cortical dopaminergic systems between humans and other primate species have functional and evolutionary implications [Citation25–28]. But it remains unclear to what extent these changes result in differential dopaminergic regulation of personality across species.
Therefore, in this study, we investigated the link between CpG methylation of a key behaviour-related candidate gene, dopamine receptor gene D2 (DRD2), and personality in captive chimpanzees. Dopamine is a neurotransmitter with a known role in reward-regulated processes. In humans, dopamine signalling is typically associated with personality traits related to exploration behaviour, like Novelty Seeking, Openness and Extraversion [Citation29–34]. It has been suggested that the release of dopamine in the brain increases the individual’s motivation to explore and facilitates cognitive and behavioural processes that are useful in exploration [Citation30]. Dopamine is linked with Extraversion, for example, a personality dimension from human literature, with item loadings for sociable, dominance, activity, and excitement seeking [Citation30,Citation35,Citation36]. Links between dopamine and Extraversion are easily explained through the social facet of the dimension, as one of the most potent human rewards can be found from social interactions such as friendships, but also from leadership interactions and gaining status [Citation30]. Although Extraversion is often viewed as a social trait, it also has item loadings like activity and positive emotionality, which are not necessarily associated with social situations but are relevant in the framework of exploratory behaviour, which is in turn linked to the dopaminergic system. We also chose the dopaminergic system to investigate the role of epigenetic modification on chimpanzee personality because the two personality dimensions with the highest levels of heritability (and thus perhaps the largest genetic component) in our sample of chimpanzees are Extraversion and Dominance [Citation5]. Both dimensions have item loadings that reflect facets of the Extraversion dimension in humans, with chimpanzee Dominance showing high item loadings for dominant, bold, and aggressive behaviour, whereas chimpanzee Extraversion reflects high levels of activity, play, sexual, and affiliative behaviours [Citation1,Citation5].
The molecular bases of the dopaminergic system are coded for by a number of genes. Gene-personality studies have mostly focused on the genes coding for the receptors for dopamine like DRD2 and dopamine receptor gene D4 (DRD4) [Citation31–33,Citation37]. Other studies focused on genes associated with dopamine metabolism like the catechol-O-methyltransferase gene (COMT) [Citation38], or the gene coding for its transporter (SLC6A3) [Citation39]. Here, we focus on epigenetic modification of DRD2, as this receptor subtype is most widespread in the central nervous system, whereas DRD4, for example, is primarily expressed in the prefrontal cortex [Citation40]. We expect that epigenetic modification of DRD2 is a reasonable candidate model to explain part of the variation on Extraversion and Dominance in our population of chimpanzees. To test this, we first determined the level of DRD2 CpG methylation using peripheral blood samples, and then we investigated whether such variation might be associated with aspects of personality, using chimpanzee personality trait rating data that were collected in a previous study Citation1. When such an effect was found, we used repeat methylation measures that are available for a subset of individuals to test for the stability of methylation patterns across the individuals’ lifespan. Finally, we included rearing background as a factor to investigate the role of early-life social experiences on methylation patterns and their potential associations with personality dimensions. Again, we expected to find associations between rearing background, epigenetic background, and the personality dimensions Extraversion and Dominance, as in a previous study these two dimensions showed the biggest difference in heritability between mother-reared and nursery-reared individuals [Citation5].
Materials and method
Subjects
All chimpanzees were housed at the National Center for Chimpanzee Care, which is part of the Michale E. Keeling Center for Comparative Medicine and Research [KCCMR), UT MD Anderson Cancer Center, Bastrop, TX. The guidelines from the American Psychological Associations for the ethical treatment of animals in research were followed throughout this project.
Personality
We obtained the measures of chimpanzee personality published by 1,for 51 out of 99 rated chimpanzees for which we have blood samples available (29 males and 22 females, range of age at time of personality sampling: 12–51 years, mean age: 25 years]. These measures were collected using a chimpanzee-specific personality questionnaire that has undergone extensive validation Citation1]. The questionnaire consists of 41 personality trait adjectives alongside definitions. For example, the adjective ‘anxious’ is defined as ‘hesitant, indecisive, tentative, and jittery.’ Each trait is rated on a Likert scale ranging from 1 (least descriptive of chimpanzee) to 7 (most descriptive of chimpanzee). Observers who were familiar with each individual completed the questionnaires to characterize overall impressions of the behaviours for each chimpanzee. Observers were 17 staff members who had worked with the chimpanzees for a minimum period of 6 months and up until 21 years, including 13 keepers, the behavioural research coordinator, a trainer, the colony manager, and an enrichment technician. Questionnaires were only completed for those individuals for which the observers felt they had enough experience to produce an accurate result, resulting in ratings per observer for on average 72 chimpanzees out of the whole population of 99 chimpanzees living in the colony in the period of 2006–2008 (range: 9–99). Interrater-reliabilities were high for all but one adjective (predictable), which was excluded from subsequent analysis [for details see Citation1]. Principal component analysis (PCA) was then conducted on the means of the remaining 40 rating adjectives, combined with expert evaluations of the factor solution. This resulted in 6 personality dimensions: Reactivity/Undependability, Dominance, Extraversion, Openness, Agreeableness, and Methodical (). See supplementary Table S1 for factor adjective scores. The first five dimensions show strong evidence for convergent and predictive validity, meaning they are consistent among studies, among raters, across time, and show correlations with independently collected quantitative behavioural observations that are in line with expectations [Citation1,Citation2–4]. For the sixth dimension, Methodical, evidence is mixed and loadings vary among studies Citation1. Given that there is little proof for construct validity of the sixth dimension, it was not included in further analysis of methylation effects on personality.
Table 1. Adjectives loading onto varimax-rotated chimpanzee personality traits.
DNA extraction and methylation
DNA was extracted from blood samples available for 51 chimpanzees with known rearing histories and personality ratings. Blood samples were collected on average within 2.11 years (SD = 3.96) from the personality rating date and between the ages of 12 and 42 (supplementary Table S2). Genomic DNA was extracted from 200 μl of whole blood using the QIAampDNA Mini Kit automated on a QiaCube (Qiagen). DNA concentrations were quantified using a Nanodrop 2000 (Thermo-Fisher Scientific) spectrophotometer. To analyse variation in methylation, extracted DNA samples were brought to a concentration of ~70 ng/μL. These samples then underwent bisulphite conversion prior to being run on the Illumina Infinium Methylation EPIC array at the Yale Center for Genome Analysis. Because this array was designed to assay methylation levels at over 850,000 CpG sites in the human genome, analyses were limited to CpG sites expected to also be successfully assayed in chimpanzees [Citation41]. These CpG sites mapped to the chimpanzee genome (panTro2.1.4) with one or zero mismatches [Citation42]. Raw intensity data were additionally filtered to remove probes with spectral intensities not significantly different from background levels using the illumina GenomeStudio software.
Blood versus brain methylation
As the genome-wide methylation levels in our study are measured from peripheral whole blood samples, we also investigated whether these can serve as a proxy for methylation levels of the primary tissue of relevance, in this case the brain. While this approach has been validated in several other studies [for a review see Citation43], caution in extending these conclusions is nevertheless warranted. Therefore, for eight individuals (5 females and 3 males, range of age at time of sampling: 20–50 years, mean age: 36.5 years) in which post-mortem brain tissue was available, we determined methylation levels at each DRD2-associated CpG from the dorsolateral prefrontal cortex and the lateral hemisphere of the cerebellum [as described in Citation44]. Given that blood and brain tissue samples were not available for the same individuals, we compared mean methylation levels at each of the CpGs in these two brain regions to mean blood CpG methylation levels.
Statistical analysis
Statistical analyses were performed using the statistical software program R (www.r-project.org, version 3.3.2). To test for DRD2 methylation effects on personality, we ran linear models using the lm function in the lme4 package in R [Citation45]. Each personality factor was tested as an outcome variable in a separate model. Sex, methylation scores, and two-way interactions were included as fixed effects. The age at which blood was collected, and relatedness coefficient calculated from the pedigree were entered as covariates. To reduce the number of predictor variables and thus model complexity, we performed a PCA on all CpGs associated with the DRD2 gene (N = 16) to look for underlying correlations. This PCA was done using a varimax rotation with Kaiser normalization. The number of dimensions to extract was determined using a visual analysis of the scree plot and parallel analysis [Citation46] and the resulting components were entered as predictor variables in the analysis. Coefficients of correlation for methylation scores >|0.5| were considered high and therefore salient and kept for further analysis. We corrected for the degree of relatedness of each individual to all other individuals in the colony, by including relatedness coefficients in the model. We used the kinship2 package in R (https://cran.r-project.org/package=kinship2) to extract relatedness coefficients. Model selection was based on the Akaike Information Criterion (AIC). We examined diagnostic plots (residuals vs. leverage, QQ plots, etc.), undertook Shapiro-Wilk tests, and calculated variance inflation factors to confirm the assumptions of linear models. A p-value correction was done using a false discovery rate (FDR) correction [Citation47] to account for multiple testing. Finally, we investigated if methylation scores were influenced by rearing background (mother- versus nursery-reared) for those composite CpG scores that showed a significant association with personality dimensions. These models were run separately from the models above, given that we excluded three wild-born individuals from this analysis. This was done because a sample size of three does not allow for making a meaningful statistical comparison to the other two rearing categories, and because often the background of wild-born individuals is complex as they were partially mother-and human-reared, depending on how old they were when they entered into captivity. For the remaining 13 nursery-reared and 35 mother-reared chimpanzees in our sample, we treated each composite methylation score as an outcome variable and rearing background, personality dimension score, and the interaction of the two were entered as fixed effects while a correction for age, sex and relatedness remains in the model, similar as described for the linear models above. Both mother- and nursery-reared chimpanzees had access to outdoor enclosures.
Results
DRD2 epigenetic modification
Sixteen DRD2-specific CpG sites were identified, of which 4 were in the promoter region, 1 was in the exonic region, and 11 were in the 5’ regulatory region. CpG IDs and their respective coordinates in the human and chimpanzee genome are shown in . Fifteen of the CpGs were also present after quality filtering in the brain data. Mean peripheral blood CpG methylation levels at these sites correlated highly with mean levels of CpG methylation in brain regions, prefrontal cortex (R = 0.86, p < 0.001) and cerebellum (R = 0.59, p = 0.02) (). For methylation patterns at individual CpG sites in all three tissue types, see supplementary Figure S1. The proportion of methylated probes decreased with position across the gene, with the highest proportions found in the gene body and the lowest in the promoter region (supplementary Figure S2).
Figure 1. Correlation between peripheral blood CpG methylation levels and methylation at same sites in tissue from (a] the prefrontal cortex, and (b) the cerebellum.
![Figure 1. Correlation between peripheral blood CpG methylation levels and methylation at same sites in tissue from (a] the prefrontal cortex, and (b) the cerebellum.](/cms/asset/24935032-6c6c-431e-9cb5-689b0fc8ea52/kepi_a_2058224_f0001_oc.jpg)
Table 2. 16 Chimpanzee DRD2 CpG sites identified and included in this study.
Composite CpG measures
All 16 CpGs were then entered into a PCA, resulting in four components (). All CpG sites had salient loadings exceeding l0.5 l on at least one of the four factors. Sampling adequacy was high (KMO = 0.808) and inter-variable correlations were sufficiently high (Bartlett’s test of sphericity χ2 = 592.00, df = 120, p < 0.001). The first component, PC1, explained a majority of the epigenetic variation (43%) and 8 out of 16 CpG sites clustered together, 5 with positive loadings (cg14809166, cg03608783, cg21141217, cg02198192, cg16823554) and three with negative loadings (cg23881278, cg12758687, cg18248586). PC2 explained 13% of the variation and combined 3 CpG sites, one with a positive loading (cg11323042) and two with negative loadings (cg12176709, cg20629239). PC3 and PC4 each explained <10% of the variation and clustered three (cg00525373, cg22404572, cg02332042) and two (cg20016411, cg19804476) CpGs, respectively. One individual with an outlier on PC2 was removed from the dataset for further analysis as its PC2 score was more than 4 standard deviations above the mean.
Table 3. CpG factor item loadings on varimax-rotated factors.
DRD2 methylation and personality
Three significant associations were found between variation in the personality trait Extraversion and CpG composite measure scores (PC1: t = 2.650, df = 43, padj = 0.020; PC3: t = −2.429, df = 43, padj = 0.019; PC4: t = −2.781, df = 43, padj = 0.020; see ). Higher scores on PC1 were associated with higher Extraversion (est = 0.169; ), while higher scores on PC3 and PC4 were associated with lower Extraversion (PC3: est = −0.159; , PC4: est = −0.159; ). One significant association was found between personality trait Openness and CpG composite measure scores for PC3 (t = −2.771, df = 43, padj = 0.008; see ). For the remaining personality traits, none of the associations were significant after FDR correction (). Visual inspection of the variation in individual CpG methylation levels, rather than composite measure scores, showed a similar pattern for Extraversion, with higher methylation scores at cg14809166, cg03608783, cg21141217, cg02198192 and cg16823554 being associated with higher Extraversion scores. Higher methylation scores at cg23881278, cg12758687, cg18248586, the CpG sites loading on the negative end of PC1, were associated with lower Extraversion scores (). Similarly, methylation levels at the individual CpGs of PC3 and PC4 showed clear associations with Extraversion and PC3 also with Openness (supplementary Figure S3). Finally, we also analysed associations between age and sex with personality dimensions. Although both variables were included as fixed effects in the models, we sought to characterize the distribution of personality data with respect to these covariates. We found that most personality dimensions did not differ by age and sex in our sample, with the exception of Dominance (age: p = 0.03; sex: p < 0.001).
Figure 2. Association between personality scores and composite DRD2 CpG composite measure scores. Higher scores are associated with (a) higher Extraversion for PC1; (b) lower Extraversion for PC3 (c) lower Extraversion for PC4 (d) lower Openness for PC3.
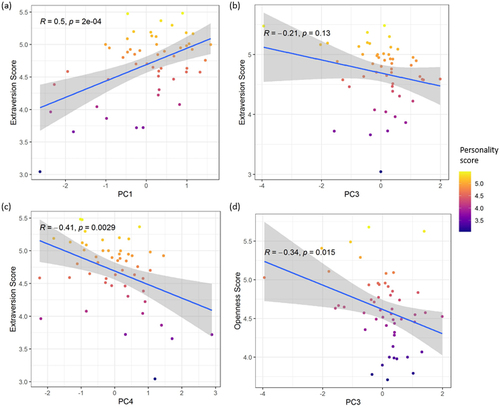
Figure 3. Methylation levels for individual DRD2 CpG sites loading onto composite measures PC1 and PC4. Each point represents methylation values for each individual at each CpG site and is coloured by that individual’s Extraversion score. Lines are loess smoothed methylation values for each quartile for Extraversion score. TSS 1500 = within 1500 bp of a transcription start site and 5’ UTR = within the 5’ untranslated region of DRD2. Chromosomal coordinates are in base pairs and refer to human genome build hg19.
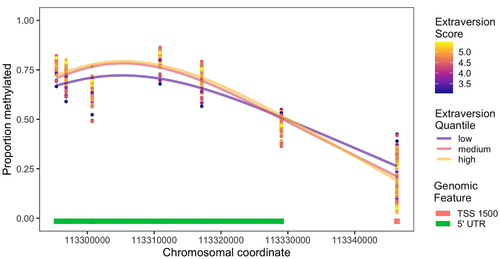
Table 4. Model statistics for DRD2 CpG composite measure scores and their association with personality dimensions in chimpanzees.
DRD2 methylation and rearing background
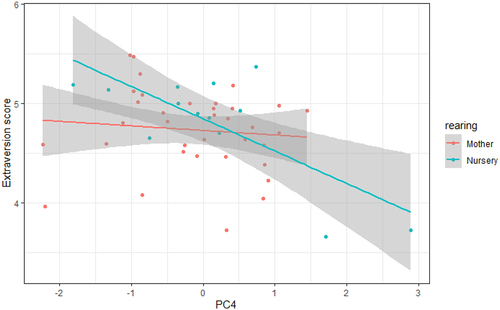
DRD2 methylation levels were significantly influenced by rearing background for PC4 (t(42) = −2.259, SE = 0.623, est = −1.407, p = 0.029). Individuals that were nursery-reared showed a negative association between Extraversion and PC4 scores whereas this association was not present in mother-reared individuals (). No such rearing interaction effects with Extraversion were present for PC1 (t(42) = −0.300, SE = 0.572, est = −0.172, p = 0.766) or PC3 (t(42) = 1.337, SE = 0.664, est = 0.888, p = 0.1885) or for rearing and Openness for PC3 (t(42) = 0.103, SE = 0.721, est = 0.075, p = 0.918).
Discussion
We investigated the role of CpG methylation at the dopamine receptor DRD2 gene in explaining variation in personality dimensions in a sample of 51 captive chimpanzees. In line with our predictions, we found that epigenetic modification of DRD2 showed the strongest association with Extraversion, a personality dimension reflecting a more social, active, and affiliative personality [1].
To investigate the effects of the epigenetic modification of chimpanzee DRD2, we first used principal component analysis as a data reduction method and found a four-component structure on which all 16 CpG sites loaded. The first component represented a majority of the variation in DRD2 methylation as it contained loadings for half of the sites found. The other three components each explained relatively small proportions of the remaining variation in methylation data. We found three significant associations between Extraversion, for PC1, PC3, and PC4. Individual scores on PC1 were positively associated with Extraversion, while scores on PC3 and PC4 were negatively associated with Extraversion. Given that these component scores reflect composite measures of methylation scores at different CpG sites, we also investigated methylation patterns at individual CpGs to better understand the directionality of the methylation pattern. The location of the CpGs appeared to determine the directionality of the methylation effect on personality (). The CpGs that loaded negatively on PC1, indicating a negative association with Extraversion, were all present in or near the promoter region of the gene. For PC4, the pattern was similar, with lower levels of methylation being associated with higher levels of Extraversion for the CpG site closest to the promoter region (cg20016411), which loaded positively on PC4. It thus appears that in general, higher levels of methylation around the promoter region of DRD2 are associated with lower Extraversion, likely due to higher DRD2 gene expression. Hypermethylation of promoter CpG sites located in the promoter region have been associated with an antagonizing effect of antipsychotic drugs on DRD2 receptor function in human neuroblastoma cells, indicating the important role of these CpGs in suppressing receptor function and expression when they are methylated [Citation48].
In line with our prediction, we found the strongest association between epigenetic modification of DRD2 and chimpanzee Extraversion, as three out of four CpG composite measures were significantly linked with this personality trait. For Openness, only one component was significant, PC3. In humans, many studies have reported associations between components of the dopaminergic system and approach-related personality traits, like Extraversion and Novelty Seeking [Citation29,Citation31,Citation32,Citation49–51]. Both dimensions originate from different questionnaire methods but are correlated [Citation52]. Extraversion is typically measured using either the Neuroticism Extraversion Openness Personality Inventory (NEO PI-R) [Citation35] or the Eysenck Personality Questionnaire (EPQ) [Citation36] and reflects gregariousness, sensation seeking, and high levels of activity. Novelty seeking is measured using the Cloninger tridimensional theory of personality and temperament [Citation53], and reflects a tendency to pursue new experiences with intense emotional sensations [Citation54]. It includes items such as thrill seeking, novelty preference, risk taking, harm avoidance, and reward dependence [Citation54]. Finally, Novelty Seeking also correlates with Openness, a personality dimension measured using the same instruments as Extraversion [Citation52]. High scores on this dimension reflect individuals that are open to new experiences and have a preference for variety over routine [Citation35]. The common core of all these dimensions is that they reflect a tendency to actively explore or engage with novelty [Citation30].
Chimpanzee Extraversion in our study is characterized by high item loadings of active, playful, sexual, and affiliative and low item loadings for solitary and depressed [1]. Chimpanzee Openness has item loadings for human oriented, curious/inquisitive, inventive, intelligent, affectionate/friendly, and persistent. Both dimensions thus reflect largely the same tendency to actively explore or engage with novelty as described for the human personality dimensions Extraversion, Novelty Seeking, and Openness. Given that Openness was not found to be heritable in chimpanzees [Citation5], we did not expect to find a direct association between DRD2 methylation and Openness. Indeed, the link with Openness was less strong in our study, with only one significant association (PC3), but the association was nonetheless in the same direction as the link between Extraversion and PC3. It is likely that this significant finding originates from the correlation of the two personality dimensions (r = 0.61, p < 0.001, Figure S4), and that DRD2 methylation thus primarily influences behavioural facets of the Extraversion dimension. We did not find a significant association between DRD2 methylation and Dominance. While the human Extraversion dimension typically has item loadings related to status (dominant, assertive), these items load onto a separate dimension in chimpanzees, Dominance. It thus appears that DRD2 methylation is rather linked with exploration-related traits than items linked to dominance or status in our study. Chimpanzee Extraversion is also directly associated with observed behaviours like play and sexual activity [1]. These behaviours are typically experienced as positive and rewarding, and are regulated by common mechanisms across animal species. For example, increases in dopamine receptor activity are associated with increased play and sexual behaviour in rats [Citation55,Citation56]. Our study shows, that in chimpanzees, similar mechanisms likely underlie the regulation of these same behaviours.
Finally, we also investigated the potential impact of social rearing background on DRD2 methylation levels and personality. Previous research had shown that rearing background significantly influenced the heritability of Extraversion, with mother-reared individuals having much higher heritability scores than nursery-reared individuals. The results in our study reveal that this result might partly be attributable to epigenetic mechanisms, and more specifically through epigenetic modification of DRD2. We find that methylation levels at PC4 are closely related with Extraversion scores in nursery-reared chimpanzees, while in mother-reared individuals, no such association was present. This shows that chimpanzees who were reared by their mothers in age-graded social groups will have more stable Extraversion scores throughout life independent of DRD2 methylation, whereas individuals that were reared by humans in a nursery setting have Extraversion scores that are more susceptible to DRD2 methylation changes. It is possible that the reward chimpanzees receive from behaviours such as exploration, sex, and play is therefore different between individuals that were mother-reared versus nursery-reared and that their motivation to pursue these behaviours is not the same as a result. In line with this, mother-reared chimpanzees have been shown to be more successful at initiating play than chimpanzees with an atypical rearing background [but see Citation57, Citation58]. A link between DRD2 and early life adversity has also been documented in other species. For example, in rats, differences were found in DRD1 and DRD2 expression between individuals that were raised with or without their mothers, especially on glutamatergic neurons that project from the prefrontal cortex to the nucleus accumbens [Citation59]. In rhesus macaques, atypical rearing conditions were associated with a decrease in promoter methylation of dopamine receptor 3 (DRD3) in prefrontal cortex tissue [Citation60]. While the exact impact of DRD2 methylation at all CpGs in our study remains unknown in chimpanzees, it would be interesting to investigate DRD2 brain expression patterns in mother- and nursery-reared individuals to investigate if such a link is present.
One limitation in our study was that personality data were not collected at the same date of blood sample collection. It is thus possible that methylation levels do not fully represent the methylome profile associated with the personality profile at the time of sampling. However, we do not expect this to have a major impact on the results as Extraversion and Openness are two personality traits with good stability across time in chimpanzees [Citation61], and the mean difference between the dates of personality rating and blood sample collection was relatively low (Mean = 2.11 years, SD = 3.96) for a long-lived species like chimpanzees. Our study sample was also limited in age range for properly estimating methylation effects of dopamine on personality across the chimpanzee lifespan. While we did not find an age effect on levels of methylation, our study sample did not include individuals younger than 12 years of age. Previous research has shown that epigenetic modifications of brain tissue are most pronounced in the immediate postnatal state, as environmental factors have a larger impact on developing brain circuits at a young age due to higher plasticity of the brain [Citation62,Citation63]. It is thus possible that age effects would have been more pronounced if data for juveniles and subadults had been available and included. Another limitation is that the methylation results were determined using peripheral blood samples. It is unclear to what extent methylation patterns from blood are similar to methylation patterns in relevant brain tissue for these same individuals. To investigate this further, we determined DRD2 methylation patterns in brain tissue from the cerebellum and prefrontal cortex to determine how well they correlate with patterns from blood samples for eight chimpanzees. Unfortunately, none of these chimpanzees were included in the personality study given that they were already deceased of natural causes at the time. The data did show a significant correlation between blood and brain tissue samples, with the highest correlation found for the prefrontal cortex. This is notable given that DRD2 expression is lower in the cerebellum than in the prefrontal cortex [Citation64]. Furthermore, this is in line with other studies that have reported similar associations between blood and brain methylation patterns [for a review see Citation43].
On a final note, many studies have reported differences in forebrain dopaminergic systems between humans and other primate species, which are suggested to have functional and evolutionary implications [Citation25–28]. Humans differ from other primates, for example, in their patterns of cortical and basal ganglia dopaminergic innervation [Citation25,Citation27] and have unique dopamine biosynthesis gene expression in the striatum [Citation28]. While further research is needed to clarify the implications of variation in the dopaminergic system among primates on cognitive function, our study shows that the broad regulatory effects of dopamine on personality are largely similar in chimpanzees compared to humans. Chimpanzees, together with bonobos, show the highest level of genetic similarity to humans due to their close phylogenetic relatedness [Citation65]. They therefore show the largest overlap in brain structure and functioning with humans and offer much better models to study the uniqueness of the biological foundation and evolutionary origin of human personality development than common animal models, including rodents and nonhuman primates such as macaques and marmosets [Citation66]. If proximate mechanisms are shared in both species, as is the case here, they were most likely already present in the last common ancestor to Pan and Homo [Citation67]. As the overall personality structure is also fundamentally different in chimpanzees than humans, with the Extraversion domain only containing aspects associated with exploratory behaviour in chimpanzees while in humans items associated with both status and exploration are present [1], our study indicates an evolutionarily conserved role of dopamine on exploratory behaviour rather than status.
In conclusion, our results highlight the role of dopaminergic signalling in chimpanzee personality, and indicate that environmental factors such as early social rearing background, can have long-lasting behavioural effects, potentially through modification of the epigenome. These findings add to the growing field of evidence demonstrating the importance of the experience-dependent methylome for the development of complex social traits.
Supplemental Material
Download Zip (262.2 KB)Acknowledgments
We thank all members of the GW Primate Genomics Lab, the GW Laboratory for Evolutionary Neuroscience, the Antwerp Primate Ethology and Evolution Lab, and specifically Dr Philippe Helsen from the Antwerp Zoo Centre for Research and Conservation for helpful feedback. This work was supported by the National Science Foundation under Grants BCS-1733896, BSC-2127961, EF-2021785, and INSPIRE Grant SMA-1542848; the James S. McDonnell Foundation under Grant 220020293; The Leakey Foundation; The Yale MacMillan Center for International Studies; The Yale Institute for Biospheric Studies; The George Washington University; and the Research Foundation Flanders.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the supplementary materials https://doi.org/10.1080/15592294.2022.2058224.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed here
Additional information
Funding
References
- Freeman HD, Brosnan SF, Hopper, LM et al. Developing a comprehensive and comparative questionnaire for measuring personality in chimpanzees using a simultaneous top-down/bottom-up design. Am J Primatol. 2013;75(10):1042–1053.
- Dutton DM. Subjective assessment of chimpanzee (Pan troglodytes) personality: reliability and stability of trait ratings. Primates. 2008;49(4):253–259.
- King JE, Figueredo AJ. The Five-Factor Model plus Dominance in Chimpanzee Personality. J Res Personality. 1997;31(2):257–271.
- Weiss A, Inoue-Murayama M, Hong KW, et al. Assessing chimpanzee personality and subjective well-being in Japan. Am J Primatol. 2009;71(4):283–292.
- Latzman RD, Hopkins WD, Keebaugh AC, et al. Personality in chimpanzees (Pan troglodytes): exploring the hierarchical structure and associations with the vasopressin V1A receptor gene. PLoS ONE. 2014;9(4):e95741.
- Weiss A, King JE, Figueredo AJ. The heritability of personality factors in chimpanzees (Pan troglodytes). Behav Genet. 2000;30(3):213–221.
- Murray LE. The effects of group structure and rearing strategy on personality in Chimpanzees Pan troglodytes at Chester, London ZSL and Twycross Zoos. Int Zoo Yearbook. 1998;36(1):97–108.
- Weaver I, Cervoni N, Champagne F, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7(8):847–854.
- Field AE, Robertson NA, Wang T, et al. Review DNA methylation clocks in aging : categories, causes, and consequences. Mol Cell. 2018;71(6):882–895.
- Faulk C, Dolinoy DC. Timing is everything. The when and how of environmentally induced changes in the epigenome of animals. Epigenetics. 2011;6(7):791–797.
- Mcgowan PO, Suderman M, Sasaki A, et al. Broad epigenetic signature of maternal care in the brain of adult rats. PLoS ONE. 2011;6(2):e14739.
- Rooij SRD, Costello PM, Veenendaal MVE, et al. Associations between DNA methylation of a glucocorticoid receptor promoter and acute stress responses in a large healthy adult population are largely explained by lifestyle and educational differences. Psychoneuroendocrinology. 2012;37(6):782–788.
- Wang D, Szyf M, Benkelfat C, et al. Peripheral SLC6A4 DNA methylation is associated with in vivo measures of human brain serotonin synthesis and childhood physical aggression. PLoS ONE. 2012;7(6):e39501.
- Anestis SF, Webster TH, Kamilar JM, et al. AVPR1A variation in chimpanzees (Pan troglodytes): population differences and association with behavioral style. Int J Primatol. 2014;35(1):305–324.
- Garai C, Furuichi T, Kawamoto Y, et al. Androgen receptor and monoamine oxidase polymorphism in wild bonobos. Meta Gene. 2014;2:831–843.
- Hong KW, Hibino E, Takenaka O, et al. Comparison of androgen receptor cag and ggn repeat length polymorphism in humans and apes. Primates. 2006;47(3):248–254.
- Hong KW, Iwatsuki H, Takenaka O, et al. Comparative analysis of estrogen receptor gene polymorphisms in apes. Primates. 2007;48(2):151–155.
- Hopkins WD, Donaldson ZR, Young LJ. A polymorphic indel containing the RS3 microsatellite in the 5’ flanking region of the vasopressin V1a receptor gene is associated with chimpanzee (Pan troglodytes) personality. Genes Brain Behav. 2012;11(5):552–558.
- Inoue-Murayama M, Adachi S, Mishima N, et al. Variation of variable number of tandem repeat sequences in the 3’-untranslated region of primate dopamine transporter genes that affects reporter gene expression. Neurosci Lett. 2002;334(3):206–210.
- Shimada MK, Inoue-Murayama M, Ueda Y, et al. Polymorphism in the second intron of dopamine receptor D4 gene in humans and apes. Biochem Biophys Res Commun. 2004;316(4):1186–1190.
- Staes N, Koski SE, Helsen P, et al. Chimpanzee sociability is associated with vasopressin (Avpr1a) but not oxytocin receptor gene (OXTR) variation. Horm Behav. 2015;75:84–90.
- Staes N, Sherwood CC, Freeman H, et al. Serotonin receptor 1A variation is associated with anxiety and agonistic behavior in chimpanzees. Mol Biol Evol. 2019;36(7):1418–1429.
- Staes N, Weiss A, Helsen P, et al. Bonobo personality traits are heritable and associated with vasopressin receptor gene 1a variation. Sci Rep. 2016;6(1):38193.
- Rogers J. The behavioral genetics of nonhuman primates : status and prospects. Am J Phys Anthropol. 2018;165:23–36.
- Hirter K, Miller E, Stimpson C, et al. The nucleus accumbens and ventral pallidum exhibit greater dopaminergic innervation in humans compared to other primates. Brain Struct Funct. 2021;226(6):1909–1923.
- Raghanti MA, Edler MK, Stephenson AR, et al. A neurochemical hypothesis for the origin of hominids. Pnas. 2018;115(6):E1108–E1116.
- Raghanti MA, Stimpson CD, Marcinkiewicz JL, et al. Cortical dopaminergic innervation among humans, chimpanzees, and macaque monkeys: a comparative study. Neuroscience. 2008;155(1):203–220.
- Sousa AMM, Zhu Y, Raghanti MA, et al. Molecular and cellular reorganization of neural circuits in the human lineage. Science. 2017;358(6366):1027–1032.
- Chmielowiec J, Chmielowiec K, Suchanecka A, et al. Associations between the dopamine D4 receptor and DAT1 dopamine transporter genes polymorphisms and personality traits in addicted patients. Int J Environ Res Public Health. 2018;15(10):2076.
- Deyoung CG. The neuromodulator of exploration : a unifying theory of the role of dopamine in personality. Front Hum Neurosci. 2013;7:762.
- Fischer R, Lee A, Verzijden MN. Dopamine genes are linked to Extraversion and Neuroticism personality traits, but only in demanding climates. Sci Rep. 2018;8(1):1733.
- Munafò MR, Yalcin B, Willis-Owen SA, et al. Association of the dopamine D4 receptor (DRD4) gene and approach-related personality traits: meta-analysis and new data. Biol Psychiatry. 2008;63(2):197–206.
- Noble EP, Ozkaragoz TZ, Ritchie TL, et al. D 2 and D 4 dopamine receptor polymorphisms and personality. Am J Med Genet A. 1998;81(3):257–267.
- Oak JN, Oldenhof J, Van Tol HHM. The dopamine D4 receptor: one decade of research. Eur J Pharmacol. 2000;405(1–3):303–327.
- Costa P, McCrae R. Normal personality assessment in clinical practice: the NEO personality inventory. Psychol Assess. 1992;4(1):5–13.
- Eysenck HJ, Eysenck SBG. Manual of the Eysenck Personality Questionnaire. London: Hodder & Stoughton; 1975.
- Lee HJ, Lee HS, Kim YK, et al. D2 and D4 dopamine receptor gene polymorphisms and personality traits in a young Korean population. Am J Med Genet B Neuropsychiatr Genet. 2003;121B(1):44–49.
- Reuter M, Schmitz A, Corr P, et al. Molecular genetics support Gray’s personality theory: the interaction of COMT and DRD2 polymorphisms predicts the behavioural approach system. Int J Neuropsychopharmacol. 2006;9:155–166.
- Kazantseva AV, Gaĭsina DA, Malykh SB, et al. Role of dopamine transporter gene (DAT1) polymorphisms in personality traits variation. Russian J Genet. 2009;45(8):974–980.
- Mulcrone J, Kerwin RW. The regional pattern of D4 gene expression in human brain. Neurosci Lett. 1997;234(2–3):147–150.
- Guevara EE, Lawler RR, Staes N, et al. Age-associated epigenetic change in chimpanzees and humans. Philos Trans Royal Soc B. 2020;375(1811):20190616.
- Needhamsen M, Ewing E, Lund H, et al. Usability of human Infinium Methylation EPIC Bead Chip for mouse DNA methylation studies. BMC Bioinformatics. 2017;18(1):486.
- Tylee DS, Kawaguchi DM, Glatt SJ. On the outside, looking in: a review and evaluation of the comparability of blood and brain “-omes.” American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2013;162(7):595–603.
- Guevara EE, Hopkins WD, Hof PR, et al. Comparative analysis reveals distinctive epigenetic features of the human cerebellum. PLoS Genet. 2021;17(5):e1009506.
- Bates D, Machler M, Bolker B, et al. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67(1):1–48.
- O’Connor BP. SPSS and SAS programs for determining the number of components using parallel analysis and velicer’s MAP test. Behav Res Methods Instrum Comput. 2000;32(3):396–402.
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B (Statistical Methodology). 1995;57(1):289–300.
- Murata Y, Nishioka M, Bundo M, et al. Comprehensive DNA methylation analysis of human neuroblastoma cells treated with blonanserin. Neurosci Lett. 2014;563:123–128.
- Golimbet VE, Alfimova MV, Gritsenko IK, et al. Relationship between dopamine system genes and Extraversion and Novelty Seeking. Neurosci Behav Physiol. 2007;37(6):601–606.
- Smillie LD, Cooper AJ, Proitsi P, et al. Variation in DRD2 dopamine gene predicts Extraverted personality. Neurosci Lett. 2010;468(3):234–237.
- Wacker J, Smillie LD. Trait Extraversion and dopamine function. Soc Personal Psychol Compass. 2015;96(10):225–238.
- Goclowska AM, Ritter SM, Elliot AJ, et al. Novelty seeking is linked to openness and extraversion, and can lead to greater creative performance. J Pers. 2019;87(2):252–266.
- Cloninger CR. A unified biosocial theory of personality and its role in the development of anxiety states. Psychiatr Dev. 1986;4(3):167–226.
- Arenas MC, Manzanedo C. Novelty Seeking. In: Zeigler-Hill V, Shackelford T, editors. Encyclopedia of Personality and Individual Differences. Cham: Springer; 2016;5. doi:10.1007/978-3-319-28099-8_1095-1.
- Guadarrama-bazante IL, Canseco-alba A, Rodríguez-manzo G. Dopamine receptors play distinct roles in sexual behavior expression of rats with a different sexual motivational tone. Behav Pharmacol. 2014;25(7):684–694.
- Trezza V, Vanderschuren LJMJ. Divergent effects of anandamide transporter inhibitors with different target selectivity on social play behavior in adolescent rats. J Pharmacol Exp Ther. 2009;328(1):343–350.
- Clay AW, Bard KA, Mollie A. Effects of sex and early rearing condition on adult behavior, health, and well-being in captive chimpanzees (Pan troglodytes). Behav Processes. 2018;156:58–76.
- Martin JE. The effects of rearing conditions on grooming and play behaviour in captive chimpanzees. Animal Welfare. 2005;14:125–133.
- Brenhouse HC, Lukkes JL, Andersen SL. Early life adversity alters the developmental profiles of addiction-related prefrontal cortex circuitry. Brain Sci. 2013;3(4):143–158.
- Massart R, Suderman M, Provencal N, et al. Hydroxymethylation and DNA methylation profiles in the prefrontal cortex of the non-human primate rhesus macaque and the impact of maternal deprivation on hydroxymethylation. Neuroscience. 2014;268:139–148.
- Rawlings B, Flynn E, Freeman H, et al. Sex differences in longitudinal personality stability in chimpanzees. Evol Human Sci. 2020;2(e46):1–17.
- Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6(11):877–888.
- Kolb B, Harker A, Gibb R. 2017. Principles of plasticity in the developing brain. Developmental Medicine and Child Neurology. Vol. 59. 12 1218–1223. doi:10.1111/dmcn.13546.
- Uhlén M, Fagerberg L, Hallström B, et al. Tissue-based map of the human proteome. Science. 2015;347(6220):1260419.
- Prüfer K, Munch K, Hellmann I, et al. The bonobo genome compared with the chimpanzee and human genomes. Nature. 2012;486(7404):1–5.
- Preuss TM. Taking the measure of diversity: comparative alternatives to the model-animal paradigm in cortical neuroscience. Brain Behav Evol. 2000;55(6):287–299.
- Staes N, Guevara EE, Helsen P, et al. The Pan social brain: an evolutionary history of neurochemical receptor genes and their potential impact on sociocognitive differences. J Hum Evol. 2021;152:102949.