ABSTRACT
Intramuscular fat development is regulated by a series of complicated processes, with non-coding RNA (ncRNA) such as microRNA (miRNA) having a critical role during intramuscular preadipocyte proliferation and differentiation in pigs. In the present study, the miRNA expression profiles of intramuscular preadipocytes from the longissimus dorsi muscle of Chinese Guizhou Congjiang Xiang pigs were detected by RNA-seq during various differentiation stages, namely, day 0 (D0), day 4 (D4), and day 8 (D8). A total of 67, 95, and 16 differentially expressed (DE) miRNAs were detected between D4 and D0, D8 and D0, and D8 and D4, respectively. According to gene ontology and Kyoto Encyclopedia of Genes analysis, target genes of DE miRNAs were enriched in categories and pathways related to lipid metabolic process, lipid biosynthetic process, as well as the PI3K-Akt, AMPK, and MAPK signalling pathways. Notably, miR-148a-3p was differentially expressed, with highest expressed abundance in D0, D4, and D8. Overexpression of miR-148a-3p in intramuscular preadipocytes increased cell proliferation and differentiation, and decreased apoptosis, in comparison to the knockdown of miR-148a-3p in intramuscular preadipocytes. Luciferase activity assays, quantitative polymerase-chain reaction, and western blot analysis confirmed that miR-148a-3p regulated adipogenesis by repressing PPARGC1A expression. Accordingly, the effect of miR-148a-3p mimic was attenuated by overexpression of PPARGC1A intramuscular preadipocytes. Furthermore, miR-148a-3p promoted intramuscular preadipocyte differentiation by inhibiting the AMPK/ACC/CPT1C signalling pathway. Taken together, we identified expression profiles of miRNAs in intramuscular preadipocytes and determined that miR-148a-3p acted as a promoter of adipogenesis.
Introduction
Intramuscular fat (IMF) content has a key role in various quality traits, such as flavour, tenderness, and juiciness [Citation1]. Isolation and in vitro culture of primary intramuscular preadipocytes facilitate understanding mechanisms of IMF deposition [Citation2]. Intramuscular fat content is positively correlated with the number and size of fat cells [Citation3]. Therefore, understanding proliferation and differentiation of swine intramuscular preadipocytes may provide valuable information regarding body development and improvement of meat quality [Citation4].
Based on genome-wide analyses of mammalian transcriptomes, >50% of transcripts are not translated into proteins but act as transcriptional noise or functional RNAs, including non-coding RNA (ncRNA) [Citation5]. Generally, ncRNAs are divided into small or short ncRNA and long ncRNAs (lncRNAs) [Citation6]. MicroRNAs (miRNA) are 18–23 nucleotides small non-coding RNAs that direct post-transcriptional repression of mRNA targets in mammals [Citation6]. In recent years, numerous miRNAs have been identified in eukaryotic organisms from nematodes to humans [Citation7–9]. Moreover, miRNAs have been regarded as key regulators because they have critical roles in various biological processes, including animal development, cell proliferation, and differentiation, transcriptional regulation, and homoeostasis [Citation10]. Numerous studies have demonstrated that miRNAs regulate intramuscular preadipocytes, including adipogenesis and differentiation through versatile gene-regulatory mechanisms [Citation11]. Wei et al. [Citation12] studied the expression pattern of miRNAs with longissimus dorsi muscles from the higher and lower longissimus dorsi content of pigs by RNA-Seq and reported that 73 differentially expressed miRNAs were potential candidates influencing the longissimus dorsi trait by their target genes. Several researchers reported that miR-199a-3p [Citation13], miR-21 [Citation14], miRNA-143a-3p [Citation15], miR-425 [Citation16] influenced fat deposition by their target genes, which were involved in cell proliferation and differentiation. Therefore, identifying pivotal miRNA controllers of adipogenesis is important for understanding the metabolic regulation network of adipocytes. However, there is limited research on expression profiles and the function of miRNAs in intramuscular preadipocytes during various differentiation stages, especially during differentiation of livestock intramuscular preadipocytes.
Chinese Guizhou Congjiang Xiang pigs, a domestic pig breed, are famous for their excellent meat qualities. However, miRNA expression profiles of these pigs during intramuscular preadipocyte differentiation have not yet been reported. In the present study, expression characteristics of miRNAs were detected using RNA sequencing (RNA-Seq) during various differentiation stages (day 0 (D0), day 4 (D4), day 8 (D8)) of intramuscular preadipocyte differentiation in Chinese Guizhou Congjiang Xiang pigs. Objectives were to identify differentially expressed miRNAs and their targets and verify their functions.
Materials and methods
Assurance of regulatory compliance and animals
Chinese Guizhou Congjiang Xiang piglets (n = 3), 3-d old, were obtained from a local livestock farm. All animal care and experimental procedures were approved by the Guizhou University Animal Care and Use Committee, Guizhou, China. The ethics approval document number was EAE-GZU-2021-P004.
Intramuscular preadipocyte culture and differentiation
The three piglets were euthanized by intravenous administration of sodium pentobarbital (30 mg/kg of body weight). The skin was sterilized with 75% ethanol and then incised to obtain samples of the longissimus dorsi muscle. After tissue samples were excised, they were washed three times with phosphate-buffered saline (PBS) and cut into 1–2 cm pieces that were digested with 2 mg/mL collagenase type II at 37°C for 65 min and shaken well every 10 min. The digested tissue was diluted with an equal volume of DMEM/F12 growth medium and filtered with gauze to remove undigested tissues. The filtered solution was then strained using 200 and 400 μm cell strainers and centrifuged at 1500 r/min for 10 min to collect progenitor cells. These cells were cultured in DMEM/F12 growth medium containing 10% foetal bovine serum (FBS) and 1% penicillin-streptomycin at 37°C in an atmosphere of 5% CO2. Because preadipocytes attach much earlier than myoblasts, after culturing for 1 h, cultured cells were washed with PBS three times to remove nonadherent cells and insoluble myofibrillar proteins [Citation4]. After preadipocytes reached confluence (D0), the growth medium was substituted with induction medium (growth medium supplemented with 5 mM IBMX, 1 μM DEX, and 5 μg/mL insulin). After 2 d, cells were cultured in maintenance medium (growth medium supplemented with 5 μg/mL insulin) for an additional 2 d (D4), and the medium was changed every 2 d until D8. Three samples were collected on D0, D4, and D8 for sequencing.
RNA extraction, library preparation, clustering, and sequencing
Total RNA was isolated on D0, D4, and D8 using Trizol reagent (Takara, Dalian, China). First, RNA integrity was assessed using an RNA Nano 6000 Assay Kit in a Bioanalyzer 2100 system (Agilent Technologies, CA, USA) and 1% agarose gel electrophoresis. For all samples, RNA integrity numbers (RINs) were >9.8. Then, RNA concentration was measured using a Qubit® RNA Assay Kit in a Qubit® 2.0 Fluorometer (Life Technologies, CA, USA). A total of 3 μg total RNA per sample were used as input material for the small RNA library. Sequencing libraries were generated using NEBNext® Multiplex Small RNA Library Prep Set for Illumina® (NB, USA) following manufacturer’s recommendations and index codes were added to attribute sequences to each sample. Library quality was assessed on the Agilent Bioanalyzer 2100 system using DNA High Sensitivity Chips. Clustering of the index-coded samples was performed on a cBot Cluster Generation System using TruSeq PE Cluster Kit v3-cBot-HS (Illumina) according to the manufacturer’s instructions. After cluster generation, library preparations were sequenced on an Illumina HiSeq 2500/2000 platform and 50 bp single-end reads were generated.
Data analyses
Data were analysed as follows: First, raw data (raw reads) of fastq format were processed through custom perl and python scripts. Second, low-quality reads, including reads containing ploy-N, with 5’adapter contaminants, without 3’adapter or the insert tag, containing ploy A or T or G or C, were removed from raw data, and Q20, Q30, and GC-content of the raw data were calculated. Third, the remaining clean reads were mapped to the porcine reference genome sequence (Sus scrofa 11.1) by Bowtie [Citation17] without mismatch. Known porcine miRNA was searched using modified software mirdeep2 [Citation18] and srna-tools-cli. Fourth, the hairpin structures of miRNA precursor were used to predict novel miRNA through the software miREvo [Citation19] and mirdeep2 [Citation18].
Differentially expressed (DE) miRNAs and pathway analyses
The miRNA expression levels were estimated by TPM (transcript per million), using the following criteria (Zhou et al., 2010). The differential expression of miRNAs was performed using the DESeq R package (1.8.3) [Citation20]. The corrected P-value ≤0.05 was set as the threshold for significantly differential expression by default. The function of DE miRNAs was predicted by the GO analysis of their target mRNAs. Gene Ontology (GO) enrichment analysis was performed for DE miRNAs using GOseq [Citation21]. KOBAS [Citation22] software was used to test statistical enrichment of the target gene in KEGG (Kyoto Encyclopedia of Genes and Genomes) pathway enrichment.
cDNA synthesis and quantitative real-time RT-PCR
The RNA was reversed to cDNA with RevertAid First Strand cDNA Synthesis Kit (Thermo, Waltham, MA, USA). Stem-loop primers were used for reverse-transcribed cDNA of miRNA (Table S1). Meanwhile, U6 (for miRNA) and GAPDH (for mRNA) were used as reference genes, and gene expression was calculated using the 2−ΔΔCt method and qRT-PCR primers were listed in Table S2.
Cells transfection
The mature sequence of miR-148a-3p was obtained from miRbase [Citation23]. The sense sequence of mimic was the same as the mature sequence of miR-148a-3p, and the antisense sequence of mimic was an incomplete complementary pairing to sense in order to increase the stability of mimic. The mimic NC sequence was a double-stranded nonsense sequence similar to Opitz et al. [Citation24]. The inhibitor sequence was completely complementary to miR-148a-3p mature sequence. The inhibitor NC sequence was meaningless single strand similar to Wu et al. [Citation25]. Mimic (Sequence: 5’-UCAGUGCACUACAGAACUUUGU-3’, 5’-AAAGUUCUGUAGUGCACUGAUU-3’), mimic NC (Sequence: 5’-UUCUCCGAACGUGUCACGUTT-3’, 5’-ACGUGACACGUUCGGAGAATT-3’), inhibitor (Sequence: 5’-ACAAAGUUCUGUAGUGCACUGA-3’), and inhibitor NC (Sequence: 5’-CAGUACUUUUGUGUAGUACAA-3’) oligonucleotides of miRNA-148a-3p were synthesized by GenePharma (Shanghai, China). For proliferation and apoptosis, intramuscular preadipocyte was transfected with the miR-148a-3p mimic (60 nM), mimic NC (60 nM), inhibitor (60 nM), and inhibitor NC (60 nM) using the FuGENE®HD Transfection Reagent (Promega Corporation, Madison, WI, USA) according to the manufacturer’s introductions, when cell confluence reached 40%. For differentiation, when cell confluence reached 80%, miR-148a-3p mimic, mimic NC, inhibitor, and inhibitor NC were similarly transfected into cells. After 6 h, the medium was replaced by growth medium. The growth medium was replaced 2 d after transfection with the adipogenic induction medium, which contained 5 mM IBMX, 1 μM DEX, and 5 μg/mL insulin. After 2 d, the cells were cultured in maintenance medium which contained 5 μg/mL insulin for an additional 2 d, and the medium was changed every 2 d until D8. Thereafter, cells were collected on D8 to analyse the effects of miR-148a-3p on differentiation of intramuscular preadipocytes.
Cell proliferation assay
Porcine intramuscular preadipocytes were plated on 96-well culture plates. Each treatment had eight independent replicates. Cell proliferation was measured at 0, 24, 48, 72, 96, 120, 144, 168, and 192 h after transfection with miR-148a-3p mimic, mimic NC, inhibitor, inhibitor NC according to the manufacturer’s protocol using cell counting kit-8 (CCK-8) assay (APExBIO, Houston, TX, USA). Furthermore, after transfection for 48 h, EdU was used to assess cell proliferation, following manufacturer’s instructions (APExBIO).
Flow cytometry for cell cycle assays
To analyse the effects of miR-148a-3p in various periods of the cell cycle, we analysed the cell cycle with miR-148a-3p mimic, mimic NC, inhibitor, and inhibitor NC groups using a cell cycle testing kit (BD, NJ, USA). Cells cultivated in six-well plates were collected and then centrifuged at 800 g for 5 min. The supernatant was discarded, cells were washed with PBS and fixed with 75% alcohol for 24 h. The fixed cells were centrifuged at 1000 g for 10 min, washed with PBS to remove alcohol, stained with PI/RNase for 15 min in the dark at room temperature, and then detected for flow cytometry (FACS Canto II plus, BD BioSciences, USA).
Flow cytometry for apoptosis assays
To analyse the effects of miR-148a-3p on cell apoptosis, the Annexin V-FITC/PI staining assay (BD, NJ, USA) was used. After transfection, cells from various treatment groups (three independent replicates per treatment) were washed three times with PBS buffer (pH = 7.4), collected by trypsinization, washed again with PBS, and then resuspended in 1 ml 1× binding buffer (BD, NJ, USA). Thereafter, cells were incubated for 15 min in the dark at room temperature in the presence of Annexin V-FITC (5 μl) and propidium iodide (5 μl) (BD, New Jersey, USA). Afterwards, cells were analysed using flow cytometry (FACS Canto II plus, BD BioSciences, USA).
Hoechst 33342 and PI dual staining assays
Hoechst 33342 and PI double staining (Solarbio, Beijing, China) were used to detect apoptosis. In brief, after transfection with miR-148a-3p for 48 h, cells were washed three times with PBS and then incubated with Hoechst 33342 and PI for 30 min at 4°C. Thereafter, cells were washed with PBS and the fluorescence signal was assessed using a fluorescence microscope (Nikon 80i, Tokyo, Japan).
Luciferase activity assay
The wild-type and mutant-type 3’UTR of PPARGC1A was cloned into a commercial pmir-GLO vector (TsingKe Biotech, Chongqing, China). The miR-148a-3p mimic, mimic NC, PmirGLO-3’UTR-WT, PmirGLO-3’UTR-MUT were co-transfected into 293 T cells, when cell confluence reached ~80%. At 24 h after transfection, cells were washed with PBS and lysed with 500 μL passive lysis buffer (PLB). Dual-luciferase activity was measured with the Dual-Luciferase® Reporter Assay System (Promega Corporation) according to manufacturer’s instructions.
Oil red O staining and triglyceride analysis
Cultured cells were washed thrice with PBS, fixed in 4% paraformaldehyde for 30 min, and stained with Oil Red O for 30 min. Stained cells were washed three times with PBS, observed under an inverted microscope, and photographed. Stained Oil Red O cells were eluted with isopropanol (v/v) for 30 min and quantified by measuring optical absorbance at 510 nm. Triglyceride concentrations were measured using a commercial assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Western blotting
Proteins were extracted from various treatments using RIPA Lysis Buffer (Solarbio, Beijing, China), according to the manufacturer’s instructions. Protein concentrations were determined using a BCA assay kit (Solarbio, Beijing, China) and then separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and subsequently transferred to a PVDF membrane and blocked with 5% skim milk powder solution for 3 h at 37°C. Membranes were incubated overnight with primary antibodies; then, membranes washed three times with TBST (8 min each time) and incubated for 2 h with horseradish peroxidase-conjugated secondary antibodies (Abcam, Cambridge, England). Anti-PPARγ and anti-CEBPα were purchased from Abcam (Abcam, Cambridge, England). Anti-CDK4, anti-Phospho-AMPK, anti-AMPK, anti-ACC, and anti-LPL were purchased from Thermo Fisher Scientific (Thermo, Waltham, MA, USA). Anti-cyclinD1 was purchased from Aviva Systems Biology (Aviva Systems Biology, CA, USA). Anti-Phospho-ACC was purchased from Biorbyt Company (Biorbyt, Cambridge, England). Anti-GAPDH, anti-PCNA, anti-PPARGC1A, anti-CPT1C, and anti-HSL were purchased from Proteintech Group (Proteintech, Wuhan, China). Protein bands were detected after treatment with BeyoECL Moon (Beyotime, Shanghai, China).
Statistical analyses
Data were analysed using one-way ANOVA, with SPSS Version 18.0 software. Differences were considered significant at P< 0.05.
Results
Characters of RNA-seq results and quality control
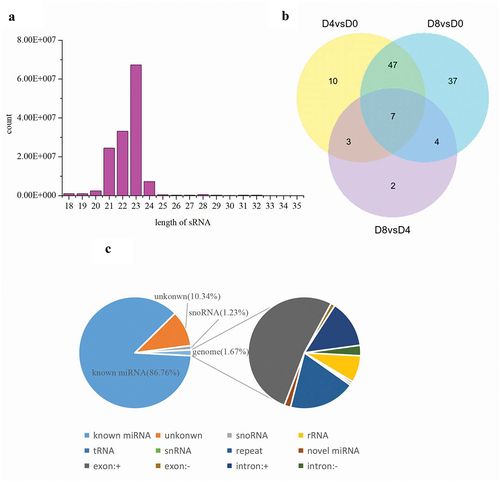
We constructed nine small RNA libraries (d0_1, d0_2, d0_3, d4_1, d4_2, d4_3, d8_1, d8_2, d8_3) using porcine intramuscular preadipocytes at various stages of differentiation. A total of 16050754, 20667858, 18521184, 15356301, 14585607, 16028024, 14393598, 15354839, and 13928901 high-quality clean reads were obtained from d0_1, d0_2, d0_3, d4_1, d4_2, d4_3, d8_1, d8_2, and d8_3, respectively (Table S3). Clean reads were mapped to the pig reference genome, with a ratio ranging from 98.71% to 99.10% (Table S4). Therefore, our data quality and mapping ratio were satisfactory for further analyses. The size distribution of mappable reads was similar in nine libraries, and the majority of reads were distributed to 23 nt in length ()). Numbers and percentage of the reads mapping to certain known classes of RNA sequences, including miRNA, rRNA, tRNA, snRNA, snoRNA, exon, and intron are shown in ).
Differentially expressed (DE) of miRNAs during intramuscular preadipocyte differentiation
The TPM (tag per million) was used to calculate and normalize the relative expression level of miRNA. Three comparison groups (D4 vs D0, D8 vs D0, D8 vs D4) were set to analyse the DE miRNAs during intramuscular preadipocyte differentiation. A total of 471 miRNAs were obtained from three differentiation stages (Table S5). Of these, 37 ()), 48 ()), and 8 ()) miRNAs were significantly upregulated, whereas 30 ()), 47 ()), and 8 ()) miRNAs were significantly downregulated in group D4 vs D0, D8 vs D0, and D8 vs D4, respectively. There were seven miRNAs that were commonly differentially expressed genes during the entire differentiation process ()).
Prediction and function analysis of genes targeted by the identified miRNAs
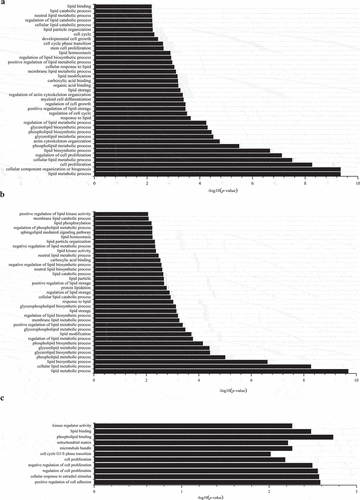
To explore the potential function during intramuscular preadipocyte differentiation, the target genes of DE miRNAs were predicted. A total of 130,041 target genes were predicted in our research. Based on differentially expressed miRNAs with target genes, significant GO terms were mainly involved in the following: (1) lipid metabolic process (GO:0006629), cellular component organization or biogenesis (GO:0071840), and cell proliferation (GO:0008283) between D4 and D0 ()); (2) lipid metabolic process (GO:0006629), cellular lipid metabolic process (GO:0044255), and lipid biosynthetic process (GO:0008610) between D8 and D0 ()); (3) kinase regulator activity (GO:0019207), lipid binding (GO:0008289), phospholipid binding (GO:0005543), mitochondrial matrix (GO:0005759), microtubule bundle (GO:0097427), and cell cycle G1/S phase transition (GO:0044843) between D8 and D4 ()).
Figure 3. Gene Ontology (GO) functional enrichment analysis of differentially expressed miRNA with their target genes in group D4 vs D0 (a), D8 vs D0 (b), and D8 vs D4 (c).
Based on differentially expressed miRNAs with target genes, significant KEGG enrichment was mainly involved in (1) sphingolipid signalling pathway, regulation of actin cytoskeleton, PI3K-Akt signalling pathway, pathway in cancer, oxytocin signalling pathway, metabolic pathway, and MAPK signalling pathway between D4 and D0 ()); (2) TNF signalling pathway, sphingolipid signalling pathway, regulation of actin cytoskeleton, ras signalling pathway, rap1 signalling pathway, proteoglycans in cancer, PI3K-Akt signalling pathway, Pathways in cancer, NF-kappa B signalling, and metabolic pathways between D8 and D0 ()); (3) viral myocarditis, type I diabetes mellitus, transcriptional misregulation in cancer, toxoplasmosis, T cell receptor signalling pathway, staphylococcus aureus infection, rheumatoid arthritis, proteoglycans in cancer, and PI3K-Akt signalling pathway between D8 and D4 ()).
Validation of RNA-Seq by qRT-PCR
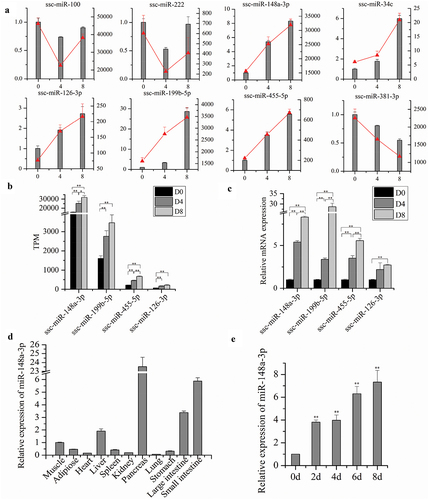
To validate the RNA-Seq data, qRT-PCR was conducted. We randomly selected eight miRNAs including miR-34c, miR-100, miR-126-3p, miR-148a-3p, miR-199b-5p, miR-222, miR-381-3p, and miR-455-5p to validate. Nine cell samples used in RNA-Seq were also used for qRT-PCR validation. Relative gene expression levels were calculated based on the mean value from nine cell samples using the comparative Ct method with U6 as a reference gene. After comparison to RNA-Seq data, similar expression trends for qRT-PCR were discovered, suggesting that the DE genes identified via RNA-seq were reliable ()). Interestingly, we noticed that the expression of ssc-miR-148a-3p and ssc-miR-455-5p were significantly upregulated for both RNA-Seq ()) and qRT-PCR ()) data in group D4 vs D0, D8 vs D0, and D8 vs D4, respectively. Also, the expression of ssc-miR-148a-3p was much higher than ssc-miR-455-5p, suggesting that ssc-miR-148a-3p may be an important regulator of IM fat deposition in Chinese Guizhou Congjiang Xiang pigs.
Figure 5. Validation of miRNAs involved in various differentiation stages of lipid metabolism using qRT-PCR. Data from qRT-PCR are shown as column and Y-axis on the left, whereas data from RNA-Seq are shown as line and Y-axis on the right (a). Expression of miRNAs related to lipid metabolism (ssc-miR-148a-3p, ssc-miR-199b-5p, ssc-miR-455-5p, ssc-miR-126-3p) derived from the RNA-seq data (b). Expression of these miRNA levels using qRT-PCR (c). The relative expression level of miR-148a-3p in Chinese Guizhou Congjiang Xiang pig tissues (d) and various differentiation stages of intramuscular preadipocyte (e).
Expression pattern analysis of miR-148a-3p
QRT-PCR was used to explore expression characteristics of miR-148a-3p in Chinese Guizhou Congjiang Xiang pig tissues and various differentiation stages of intramuscular preadipocytes. MiR-148a-3p was most highly expressed in the pancreas, followed by the small intestine, large intestine, liver, muscle, adipose, spleen, stomach, kidney, heart, and lung ()). Moreover, expression of ssc-miR-148a-3p was analysed during porcine intramuscular preadipocyte differentiation, and a continuous increase was detected ()).
Effects of miR-148a-3p on porcine intramuscular preadipocyte proliferation
In this study, synthetic miR-148a-3p mimic, mimic NC, inhibitor, and inhibitor NC were transfected into porcine intramuscular preadipocyte, respectively ()). As expected, miR-148a-3p mimic significantly increased the expression of miR-148a-3p by 13-fold compared to mimic NC in porcine intramuscular preadipocyte. Meanwhile, after transfection with the miR-148a-3p inhibitor in porcine intramuscular preadipocyte, expression of miR-148a-3p was 42.1% lower ()).
Figure 6. MiR-148a-3p promotes proliferation of porcine intramuscular preadipocytes. (a) Relative expression of miR-148a-3p in porcine intramuscular preadipocyte transfected with miR-148a-3p mimic, mimic NC, inhibitor, and inhibitor NC. (b) Cell proliferation was detected using cell counting kit 8 (CCK-8) assay. (c) Porcine intramuscular preadipocytes were transfected with miR-148a-3p mimic, mimic NC, inhibitor, inhibitor NC, and cell phases were analysed using flow cytometry. (d) 5-ethynyl-2’-deoxyuridine (EdU) was used to determine the cell proliferation. (e) The mRNA expression of proliferation marker genes (CDK2, CDK3, CDK4, cyclinB, cyclinG1, cyclinD1) were detected using qRT-PCR. (f–g) The protein level of cyclinD1, CDK4, and PCNA were detected by western blotting. Data are shown as means ± SEM. *P< 0.05, **P< 0.01.
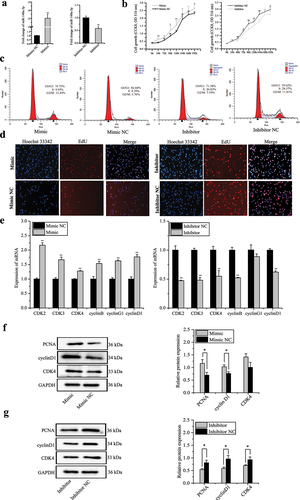
To elucidate the potential effects of miR-148a-3p on porcine intramuscular proliferation, cell counting kit-8 (CCK-8), cell cycle assay, 5-Ethynyl-2’-deoxyuridine (EdU), qRT-PCR, and western blotting assays were used. Based on the CCK-8 assay, transfection miR-148a-3p mimic significantly increased the total number of preadipocytes compared to the mimic NC group. However, the total number of preadipocytes was significantly decreased by transfection with miR-148a-3p inhibitor compared to inhibitor NC group ()). A cell phase assay revealed that miR-148a-3p mimic decreased the proportion of cells in G0/G1 phase and increased the number of preadipocytes in S and G2 phases, whereas the proportion of cells in the G0/G1 phase significantly increased and decreased the number of preadipocytes in S and G2 phases by transfection of miR-148a-3p inhibitor compared to inhibitor NC group ()). EdU assay also indicated that miR-148a-3p overexpression promoted cell proliferation ()). We also determined the effects of miR-148a-3p on the mRNA expression of cell proliferation-related genes (CyclinB, CyclinG1, CyclinD1), and cyclin-dependent-kinase 2, 3, 4 (CDK2, CDK3, CDK4). MiR-148a-3p significantly increased transcription of these genes at mRNA levels ()). Protein expression of proliferating cell nuclear antigen (PCNA), CyclinD1, and CDK4 were analysed; miR-148a-3p significantly increased expression of these genes at protein levels ().
Effects of miR-148a-3p on porcine intramuscular preadipocyte apoptosis
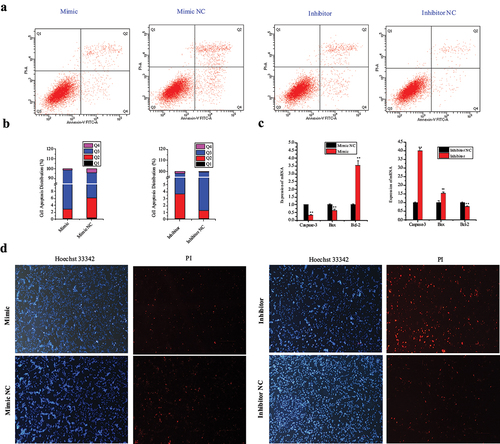
Hoechst 33342 and propidium iodide (PI), Annexin V-fluorescein isothiocyanate (FITC)/PI dual staining, and qRT-PCR assays were used to elucidate the potential effects of miR-148a-3p in porcine intramuscular apoptosis. Based on the Annexin V-FITC/PI staining assay, miR-148a-3p overexpression inhibited apoptosis in porcine intramuscular preadipocytes ()). Overexpression of miR-148a-3p decreased expression levels of Bax and Caspase-3 and promoted expression of the anti-apoptotic protein Bcl-2 ()). Based on Hoechst 33342 and propidium iodide (PI) dual staining assays, miR-148a-3p significantly decreased numbers of dead cells ()).
Figure 7. MiR-148a-3p inhibits apoptosis of porcine intramuscular preadipocytes. (a–b) Porcine intramuscular preadipocytes apoptosis was analysed by Annexin V-FITC and PI binding using flow cytometry and counted. (c) The mRNA expression of apoptosis marker genes (Caspase-3, Bax, Bcl-2) were detected using qRT-PCR. (d) Cell apoptosis was detected by Hoechst 33342 and PI dual staining assays. Data are shown as means ± SEM. *P< 0.05, **P< 0.01.
Effects of miR-148a-3p on porcine intramuscular preadipocyte differentiation
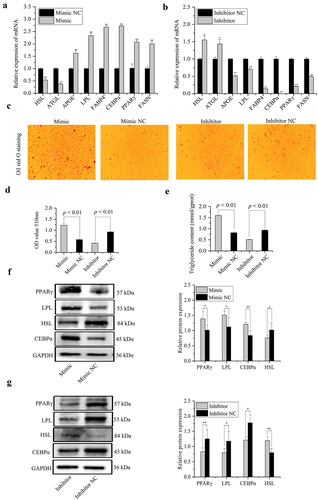
To assess effects of miR-148a-3p on porcine intramuscular preadipocyte differentiation, expression of adipose metabolism-related marker genes (hormone-sensitive lipase (HSL), adipose triglyceride lipase (ATGL), apolipoprotein E (APOE), lipoprotein lipase (LPL), fatty acid-binding protein 4 (FABP4), CCAAT/enhancer-binding protein-α (CEBPα), peroxisome proliferator-activated receptor γ (PPARγ), and fatty acid synthase (FASN)) were detected in porcine intramuscular preadipocyte transfected with miR-148a-3p mimic, mimic NC, inhibitor, or inhibitor NC on differentiated 8 d. The mRNA expression of adipose differentiation-related genes, including APOE, LPL, FABP4, CEBPα, PPARγ, and FASN were significantly increased by transfection with miR-148a-3p mimic ()), whereas miR-148a-3p inhibitor remarkably decreased their mRNA expression level ()). Furthermore, lipolysis marker genes, e.g., hormone-sensitive lipase (HSL) and adipose triglyceride lipase (ATGL), were significantly decreased after being treated with miR-148a-3p mimic ()). Overexpression of miR-148a-3p significantly increased the Oil red O staining signal and triglyceride content on preadipocyte differentiated 8 d ()). Protein levels of LPL, CEBPα, and PPARγ were significantly increased in the miR-148a-3p mimic group ()), whereas the HSL protein level was significantly decreased ()). Meanwhile, miR-148a-3p inhibitor remarkably transformed their protein expression level ()).
Figure 8. MiR-148a-3p promotes differentiation of porcine intramuscular preadipocytes. (a,b) The mRNA expression of differentiation marker genes were detected using qRT-PCR. (c) The lipid accumulation of porcine differentiated intramuscular adipocytes were detected by Oil Red O staining. (d,e) Relative absorbance of Oil Red O and TG content. (f,g) Protein expression level of PPARγ, LPL, HSL, and CEBPα were examined by western blotting. Results were presented as means ± SEM, *P< 0.05, **P< 0.01.
MiR-148a-3p promote porcine intramuscular preadipocyte differentiation by directly targeting PPARGC1A
To explore the regulatory mechanism by which miR-148a-3p promotes porcine intramuscular preadipocyte differentiation, we speculated and screened PPARGC1A as a potential target gene of miR-148a-3p by bioinformatics software TargetScan 7.2. The 3’-UTR region of PPARGC1A mRNA had a binding site for the miR-148a-3p seed sequence ()). To confirm this prediction, dual-luciferase reporter assays were performed. Transfection of miR-148a-3p mimic in 293 T cells directly repressed the luciferase activities of the wild-type PPARGC1A 3’-UTR reporter when compared to the control group, whereas mutation of the miR-148a-3p binding site in porcine PPARGC1A 3’-UTR completely stopped this response ()). On day 8 of adipogenic induction, the miR-148a-3p mimic decreased PPARGC1A protein and mRNA expression ().
Figure 9. MiR-148a-3p promotes porcine intramuscular preadipocyte differentiation by targeting PPARGC1A. (a) RNAhybrid and TargetScan predicted miR-148a-3p binding sites in the PPARGC1A 3’UTR. (b) 293 T cells were co-transfected with miR-148a-3p mimic, PmirGLO-PPARGC1A-3’UTR-MUT, and PmirGLO-PPARGC1A-3’UTR-WT, and Renilla and firefly luciferase activity were analysed. (c) The mRNA expression of PPARGC1A was detected using qRT-PCR. (d,e) The protein expression of PPARGC1A was detected by western blotting. (f–h) Upon cotransfection with pEX-3_PPARGC1A construct, the levels of differentiation marker genes were detected on day 8 of differentiation by qRT-PCR and western blotting. (f–h) Upon cotransfection with pEX-3_PPARGC1A plasmid, the levels of differentiation marker genes were detected on differentiated 8 d by qRT-PCR and western blotting.
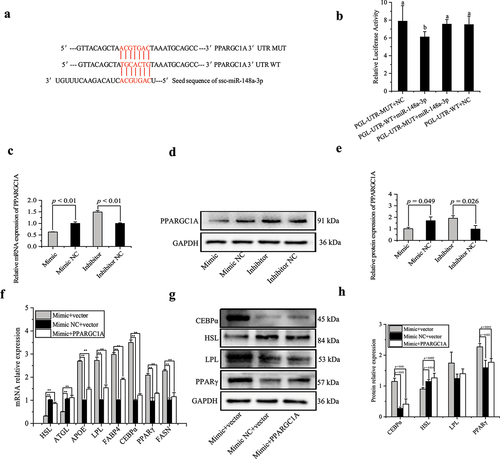
To further confirm the relationship between miR-148a-3p and PPARGC1A in adipocyte differentiation, intramuscular preadipocytes were transfected with miR-148a-3p mimic or mimic NC. After 24 h, the pEX-3_PPARGC1A plasmid was used to transfect intramuscular preadipocytes. On day 8 of induced differentiation, PPARGC1A overexpression attenuated the function of the miR-148a-3p mimic, as it decreased mRNA expression of APOE, LPL, FABP4, CEBPα, PPARγ, and FASN, but increased mRNA expression of HSL and ATGL ()). Meanwhile, the PPARGC1A overexpression decreased protein expression of CEBPα and PPARγ, and increased protein expression of HSL ().
MiR-148a-3p promote porcine intramuscular preadipocyte differentiation by inhibiting AMPK/ACC/CPT-1 signalling pathway
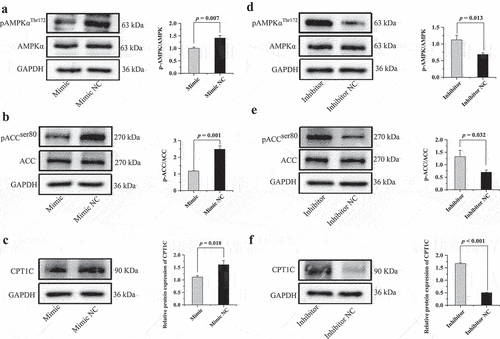
To further explore the underlying mechanism of how miR-148a-3p promotes porcine intramuscular preadipocyte differentiation, the signal transducer and the activator of transcription of the AMPK/ACC/CPT-1 signalling pathway were detected on day 8 of adipogenic differentiation. Levels of p-AMPK/AMPK, p-ACC/ACC, and carnitine palmitoyltransferase 1C (CPT1C) were significantly decreased when miR-148a-3p was over-expressed (). In contrast, knockdown of miR-148a-3p expression significantly increased the protein level of p-AMPK/AMPK, p-ACC/ACC, and CPT1C ().
Figure 10. MiR-148a-3p promotes porcine intramuscular preadipocyte differentiation through inhibiting AMPK/ACC/CPT-1 signalling pathway. (a,b) Protein levels of phosphorylated and total AMPK and ACC treated with miR-148a-3p mimic. (c) The protein level of CPT1C treated with miR-148a-3p mimic. (d,e) Protein levels of phosphorylated and total AMPK and ACC treated with miR-148a-3p inhibitor. (f) The protein level of CPT1C was treated with miR-148a-3p inhibitor. All cells were measure on differentiated 8 d.
Discussion
Meat quality can be evaluated by multiple indicators, including intramuscular fat content (IMF), muscle tenderness, meat colour, and water-holding capacity [Citation26]. Certainly, IMF is an important economic trait, a major determinant of meat quality, and IMF content is positively correlated with meat marble, taste, flavour, colour, and other characteristics of meat [Citation27]. Due to the differences in breeds and genetic backgrounds and breeds, there are considerable differences between native Chinese pigs and Western pigs in IMF content. The Chinese Guizhou Congjiang Xiang pig, a heritage breed that is largely restricted to the Guizhou Province, is known for high-quality, fragrant, and tender meat, due to its high IMF content (>4.5%) [Citation28]. Therefore, in view of the importance of IMF to livestock production economics, it is of great significance to clarify molecular mechanisms of IMF deposition.
Most research examining molecular mechanisms in porcine intramuscular preadipocyte development has focused on protein-coding genes; therefore, studies using high-throughput RNA-seq analysis usually detected mRNAs [Citation29]. Hou et al. reported that 43, 92, and 145 mRNAs were differentially expressed in group Landrace vs. Tongcheng pigs, Tongcheng vs. Wuzhishan pigs, and Landrace vs. Wuzhishan pigs, respectively. However, the potential function of miRNAs in porcine intramuscular preadipocyte development remains largely unknown. The present study provided an overview of the types and relative abundances of miRNAs in porcine intramuscular preadipocytes at various differentiation stages (D0, D4, and D8). A total of 16050754, 20667858, 18521184, 15356301, 14585607, 16028024, 14393598, 15354839, and 13928901 clean reads from d0_1, d0_2, d0_3, d4_1, d4_2, d4_3, d8_1, d8_2, and d8_3, respectively, were obtained through RNA-seq, which was sufficient for quantitative analysis of miRNA expression. In this study, we identified 471 miRNAs (TPM ≥ 1) as reliably expressed genes during intramuscular preadipocyte development. Nevertheless, >178 miRNAs were differentially expressed in porcine intramuscular preadipocytes in various differentiation stages, which revealed that they may have a developmental stage-specific role in porcine intramuscular preadipocytes. In addition, we detected seven miRNAs that were shared as common differentially expressed genes during the entire differentiation process. The length of predicted novel miRNA was largely aggregated in 22 nt, whereas the length of known miRNA was mainly clustered in 23 nt differed from other reports [Citation30]. The different characteristics of miRNA in our present research may be due to the different animal species-specific and cell-specific situations [Citation31], suggesting the classes and expression abundant of miRNA may display spatial and temporal characteristics [Citation32]. Thus, our data may provide new insights to understand transcriptional regulation in intramuscular preadipocytes of Guizhou Congjiang Xiang pigs.
To explore potential regulatory functions affecting intramuscular preadipocytes, target genes of DE miRNAs were predicted. Thereafter, GO and KEGG pathway analyses were performed with DE miRNA-regulated target genes. As expected, many classical functional categories such as cellular component, single-organism process, metabolic process, organic substance metabolic process, cytoplasm, cytoplasmic part, and protein binding were detected. These functional groups may have critical roles in intramuscular preadipocyte differentiation [Citation33]. Furthermore, the PI3K-Akt signalling pathway was commonly enriched in group D4 vs. D0, D8 vs. D0, and D8 vs. D4. The PI3K-Akt signalling pathway has a pivotal role in lipid metabolism, glucose metabolism, and cell development, regulated by insulin receptor substrate [Citation34]. The signalling pathway, including regulation of actin cytoskeleton, metabolic pathway, MAPK signalling pathway, and AMPK signalling pathway, were significantly enriched during intramuscular preadipocyte differentiation. This result was similar to previous research [Citation32,Citation35,Citation36] and may be related to the development of intramuscular preadipocytes [Citation37]. Unexpectedly, the related-disease signalling pathway, including proteoglycans in cancer, pathway in cancer, and HTLV-I infection, were enriched, which may give us a new view to comprehensively understand mechanisms of miRNA in diseases [Citation38,Citation39].
Based on our observation of several miRNAs being differentially expressed across intramuscular preadipocyte differentiation, we propose that studying miRNAs may lead to the discovery of a large number of regulatory miRNAs involved in porcine intramuscular preadipocyte proliferation and differentiation. In the present study, we exemplify the involvement of one miRNA (miR-148a-3p) in regulating intramuscular preadipocyte proliferation, differentiation, and apoptosis. In this study, miR-148a-3p expression level was increased during intramuscular preadipocyte differentiation, indicating that miR-148a-3p may regulate intramuscular preadipocyte adipogenesis.
Cell-cycle entry in the early G1 phase relies on the activation of CDK4 and CDK6 by combining with cyclin D [Citation40,Citation41]. Cyclin-dependent kinases 2 (CDK2) have been recognized as key regulators of cell growth and proliferation and are essential for G1 to S phase transition by binding of cyclin E [Citation42,Citation43]. Cyclin G can associate with CDK5 and serves as a negative regulator of p53 by activating Mdm2 through dephosphorylation to improve cell proliferation [Citation44]. Gain and loss-of-function studies in porcine intramuscular preadipocytes were applied to determine the function of miR-148a-3p in proliferation of porcine intramuscular preadipocytes. Based on the genes and protein expression level assay, overexpression of miR-148a-3p promoted expression of cell cycle-related genes, including CDK2, CDK3, CDK4, CCNB, CCNG1, and CCND1, and significantly accelerated the cell cycle progression by decreasing the proportion of cells in G0/G1 phase. Proliferation of bone marrow mesenchymal stem cell and non-small-cell lung cancer are promoted by miR-148a-3p mimic [Citation45,Citation46], consistent with our present research. However, overexpression of miR-148a-3p inhibited proliferation of gastric cancer cells [Citation47] and the diabetic retinopathy cell model [Citation48], respectively. Therefore, miR-148a-3p had inconsistent effects on proliferation of various cell types. Discrepancies in the function of miR-148a-3p in various cell lines may be due to miRNA-mRNA targeting and the interplay relationship that differs among tissues and cell types [Citation6,Citation10].
It is well known that miRNAs have a profound impact on many processes including cell proliferation, apoptosis, stress responses, maintenance of stem cell potency, and metabolism. Cell apoptosis can be impacted by several miRNAs, such as miR-34, miR-14-92, miR-372-373, and miR-155 [Citation49]. Caspases and the Bcl-2 family are the two most important groups of proteins that participate in all apoptotic pathways [Citation50]. Based on flow cytometry, PI dual staining and apoptosis-related genes expression level assay, miR-148a-3p inhibited apoptosis in porcine intramuscular preadipocytes. Similarly, in the previous research, miR-148a-3p decreased apoptosis in the human retinal microvascular endothelial cell by targeting TGFB2 and FGF2 [Citation48]. The similar anti-apoptosis function of miR-148a-3p in various cell types may give us a new insight to understand cell apoptosis [Citation51,Citation52].
Several studies have implicated miRNAs as having key roles in regulating adipogenesis [Citation4,Citation53]. For example, miR-425-5p, miR-540, miR-98, and miR-125a-5p inhibited adipogenic differentiation [Citation4,Citation33,Citation54,Citation55], whereas miR-146b, miR-223, and miR-145 promoted adipogenic differentiation [Citation56–58]. In the present study, we explored the physiological role of miR-148a-3p in porcine intramuscular preadipocyte differentiation and determined that miR-148a-3p not only promoted proliferation of porcine adipocytes but also their differentiation. Here, we obtained clear evidence that miR-148a-3p promoted differentiation of porcine adipocytes by increasing the expression of adipogenesis marker genes PPARγ, CEBPα, FASN, APOE, LPL, and FABP4. Moreover, HSL and ATGL expression were decreased by miR-148a-3p mimic transfection. Both HSL and ATGL are rate-limiting enzymes of lipolysis. Consistently, inhibiting miR-148a-3p expression declined intramuscular preadipocyte differentiation. All of these findings were consistent with how miR-148a-3p accelerates adipogenesis and increases intracellular lipid content in adipocytes.
It is well established that miRNA regulates many biological processes by negatively regulating target genes through interactions with 3’-untranslated regions (3’-UTR) of target mRNAs [Citation6]. To further explore the molecular mechanisms that miR-148a-3p promoted porcine intramuscular preadipocytes differentiation, we predicted target genes of miR-148a-3p using TargetScan 7.2. Among potential target genes, we observed that the 3’-UTR region of proliferator-activated receptor gamma coactivator 1-alpha (PPARGC1A) mRNA had a binding site for miR-148a-3p seed sequences. Luciferase reporter assays, quantitative polymerase chain reaction, and western blot experiment confirmed that miR-148a-3p was a direct target gene of miR-148a-3p during differentiation of porcine intramuscular preadipocyte. The PPARGC1A is a nuclear hormone receptor coactivator peroxisome that regulates gluconeogenesis, lipid metabolism, energy metabolism, and detoxification of reactive oxygen species produced by mitochondria [Citation59,Citation60]. In recent years, there are indications that PPARGC1A may promote insulin resistance [Citation61], regulate IRS1:IRS2 ratio [Citation59], and lipid oxidation [Citation62]. In support of this, in the present study, overexpression of PPARGC1A decreased the normal function of adipocytes transfected with miR-148a-3p mimic.
Specifically, PPARGC1A is a key fatty acid oxidation factor by regulating the fatty acid oxidation signalling pathway [Citation63]. Based on the present results, miR-148a-3p may promote adipocyte differentiation by specifically decreasing expression of PPARGC1A, which is associated with fatty acid oxidation [Citation63]. So, we further studied a mechanism for the promotion function of miR-148a-3p in porcine intramuscular preadipocyte adipogenesis through the fatty acid oxidation pathway. The 5’Adenosine monophosphate (AMP)-activated protein kinase (AMPK) is a highly adaptive complex that acts as a biological sensor and becomes active in response to the elevated AMP/ATP ratio via attachment to adenosine diphosphate and/or AMP [Citation64]. Furthermore, AMPK can suppress lipogenic gene expression and triglyceride synthesis and accumulation via phosphorylation of ACC and CPT1C pathways [Citation65]. Therefore, AMPK/ACC/CPT1C signalling pathway has an important role in regulation of fatty acid metabolism [Citation66]. In a diet-induced obese mice model, Hakka stir-fried tea effectively ameliorated obesity and related metabolic disorder by activation of the AMPK/ACC/CPT1 pathway in the liver [Citation67]. In an insulin resistance C57BL/6 J mouse model, Yin Zhi Huang associated decreased AMPK/SREBP-1 pathway-mediated de novo lipogenesis and increased AMPK/ACC/CPTI pathway-mediated mitochondrial fatty acid β oxidation to ameliorate insulin resistance and facilitate lipid metabolism [Citation68]. In the current study, miR-148a-3p inhibited AMPK to phosphorylate ACC and activating its ability to convert malonyl-CoA from acetyl-CoA consequently decreased CPT1C content to reduce mitochondrial fatty acid oxidation. These data were also parallel to the degree of increased adipose differentiation markers. Consistently, we determined that miR-148a-3p can positively regulate porcine intramuscular preadipocyte adipogenesis by decreasing the AMPK/ACC/CPTIC pathway-mediated mitochondrial fatty acid β oxidation.
Conclusions
In summary, our study provided a catalogue of miRNA expression during differentiation of porcine intramuscular preadipocytes. Many miRNAs were annotated, several of which present a highly different abundance among differentiation stages. We further characterized an abundant miRNA, miR-148a-3p, to promote proliferation and differentiation and suppress apoptosis during intramuscular adipogenesis in Chinese Guizhou Congjiang Xiang Pigs Breeds by targeting PPARGC1A. We anticipate that these results will be a stepping stone to identifying genetic mechanisms governing intramuscular adipogenesis formation and regeneration, which may enable improved meat quality.
Author contributions
Houqiang Xu provided the funding and conceived the study. Lulin Tan performed the research and wrote the manuscript. Zhaojun Chen and Yong Ruan participated in the statistical analyses of data. All authors have read and agreed to the submitted version of the manuscript.
Supplemental Material
Download Zip (56.3 KB)Acknowledgments
We thank the research assistants and laboratory technicians who contributed to this study. Especially, the authors are grateful for assistance with the manuscript provided by Professor John P. Kastelic.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Strains and plasmids are available upon request through [email protected] or [email protected]. The authors affirm that all data necessary for confirming the conclusions of the article are present within the article, figures, and tables. Supplementary material is available at Epigenetics online.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15592294.2022.2086675.
Additional information
Funding
References
- Hocquette JF, Gondret F, Baeza E, et al. Intramuscular fat content in meat-producing animals: development, genetic and nutritional control, and identification of putative markers. Animal. 2010;4:303–319.
- Dodson MV, Allen RE, Du M, et al. Evolution of meat animal growth research during the past 50 years: adipose and muscle stem cells. J Anim Sci. 2015;93:457–481.
- Sun WX, Dodson MV, Jiang ZH, et al. Myostatin inhibits porcine intramuscular preadipocyte differentiation in vitro. Domest Anim Endocrinol. 2016;55:25–31.
- Chen FF, Xiong Y, Peng Y, et al. MiR-425-5p inhibits differentiation and proliferation in porcine intramuscular preadipocytes. Int J Mol Sci. 2017;18:2101.
- Ambele MA, Dessels C, Durandt C, et al. Genome-wide analysis of gene expression during adipogenesis in human adipose-derived stromal cells reveals novel patterns of gene expression during adipocyte differentiation. Stem Cell Res. 2016;16:725–734.
- Bartel DP. Metazoan microRNAs. Cell. 2018;173:20–51.
- Liu J, Ning C, Li B, et al. Hepatic microRNAome reveals potential microRNA-mRNA pairs association with lipid metabolism in pigs. Asian-Australas J Anim Sci. 2019;32:1458–1468.
- Liu X, Wei H, Liao S, et al. MicroRNA transcriptome analysis of porcine vital organ responses to immunosuppressive porcine cytomegalovirus infection. Virol J. 2018;15:16.
- Seiri P, Abi A, Soukhtanloo M. PPAR‐γ: its ligand and its regulation by microRNAs. J Cell Biochem. 2019;120:10893–10908.
- Gebert LFR, MacRae IJ. Regulation of microRNA function in animals. Nat Rev Mol Cell Biol. 2019;20:21–37.
- Romao JM, Jin W, Dodson MV, et al. MicroRNA regulation in mammalian adipogenesis. Exp Biol Med (Maywood). 2011;236:997–1004.
- Wei W, Li B, Liu K, et al. Identification of key microRNAs affecting drip loss in porcine longissimus dorsi by RNA-Seq. Gene. 2018;647:276–282.
- Tan Z, Du J, Shen L, et al. miR-199a-3p affects adipocytes differentiation and fatty acid composition through targeting SCD. Biochem Biophys Res Commun. 2017;492:82–88.
- Wang W, Cheng M, Qiao S, et al. Gga-miR-21 inhibits chicken pre-adipocyte proliferation in part by down-regulating Kruppel-like factor 5. Poult Sci. 2017;96:200–210.
- Zhang P, Du J, Wang L, et al. MicroRNA-143a-3p modulates preadipocyte proliferation and differentiation by targeting MAPK7. Biomed Pharmacother. 2018;108:531–539.
- Qi R, Wang J, Wang Q, et al. MicroRNA-425 controls lipogenesis and lipolysis in adipocytes. Biochimica Et Biophysica Acta (BBA) - Mol Cell Biol Lipids. 2019;1864:744–755.
- Langmead B, Trapnell C, Pop M, et al. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R25.
- Friedlander MR, Mackowiak SD, Li N, et al. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res. 2012;40:37–52.
- Wen M, Shen Y, Shi S, et al. miREvo: an integrative microRNA evolutionary analysis platform for next-generation sequencing experiments. BMC Bioinformatics. 2012;13:140.
- Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106.
- Young MD, Wakefield MJ, Smyth GK, et al. Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biol. 2010;11:R14.
- Mao X, Cai T, Olyarchuk JG, et al. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics. 2005;21:3787–3793.
- Kozomara A, Birgaoanu M, Griffiths-Jones S. miRBase: from microRNA sequences to function. Nucleic Acids Res. 2019;47:D155–D162.
- Opitz B, Puschel A, Schmeck B, et al. Nucleotide-binding oligomerization domain proteins are innate immune receptors for internalized streptococcus pneumoniae. J Biol Chem. 2004;279:36426–36432.
- Wu Q, Luo X, Terp MG, et al. DDX56 modulates post-transcriptional Wnt signaling through miRNAs and is associated with early recurrence in squamous cell lung carcinoma. Mol Cancer. 2021;20:108.
- Belzunce MA, Henckel J, Di Laura A, et al. Intramuscular fat in gluteus maximus for different levels of physical activity. Sci Rep. 2021;11:21401.
- Cheng F, Liang J, Yang L, et al. Systematic identification and comparison of the expressed profiles of lncRNAs, miRNAs, circRNAs, and mRNAs with associated co-expression networks in pigs with low and high intramuscular fat. Animals (Basel). 2021;11. DOI:10.3390/ani11113212.
- Larzul C. How to improve meat quality and welfare in entire male pigs by genetics. Animals (Basel). 2021;11. DOI:10.3390/ani11030699
- Li B, Dong C, Li P, et al. Identification of candidate genes associated with porcine meat color traits by genome-wide transcriptome analysis. Sci Rep. 2016;6: 35224 .
- Bao H, Kommadath A, Liang G, et al. Genome-wide whole blood microRNAome and transcriptome analyses reveal miRNA-mRNA regulated host response to foodborne pathogen Salmonella infection in swine. Sci Rep. 2015;5. DOI:10.1038/srep12620.
- Goody D, Pfeifer A. MicroRNAs in brown and beige fat. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864:29–36.
- Li G, Li Y, Li X, et al. MicroRNA identity and abundance in developing swine adipose tissue as determined by solexa sequencing. J Cell Biochem. 2011;112:1318–1328.
- Du J, Xu Y, Zhang P, et al. MicroRNA-125a-5p affects adipocytes proliferation, differentiation and fatty acid composition of porcine intramuscular fat. Int J Mol Sci. 2018;19:501.
- Zhou YJ, Xu N, Zhang XC, et al. Chrysin improves glucose and lipid metabolism disorders by regulating the AMPK/PI3K/AKT signaling pathway in insulin-resistant HepG2 cells and HFD/STZ-induced C57BL/6J mice. J Agric Food Chem. 2021;69:5618–5627.
- Hao Y, Liu JR, Zhang Y, et al. The microRNA expression profile in porcine skeletal muscle is changed by constant heat stress. Anim Genet. 2016;47:365–369.
- Xi Y, Liu H, Zhao Y, et al. Comparative analyses of longissimus muscle miRNAomes reveal microRNAs associated with differential regulation of muscle fiber development between Tongcheng and Yorkshire pigs. PLoS One. 2018;13:e0200445.
- Oclon E, Latacz A, Zubel-Lojek J, et al. Hyperglycemia-induced changes in miRNA expression patterns in epicardial adipose tissue of piglets. J Endocrinol. 2016;229:259–266.
- Razavi ZS, Asgarpour K, Mahjoubin-Tehran M, et al. Angiogenesis-related non-coding RNAs and gastrointestinal cancer. Mol Ther Oncolytics. 2021;21:220–241.
- Xing Y, Ruan G, Ni H, et al. Tumor immune microenvironment and its related miRNAs in tumor progression. Front Immunol. 2021;12:624725.
- Pack LR, Daigh LH, Chung M, et al. Clinical CDK4/6 inhibitors induce selective and immediate dissociation of p21 from cyclin D-CDK4 to inhibit CDK2. Nat Commun. 2021;12:3356.
- Ekholm SV, Reed SI. Regulation of G1 cyclin-dependent kinases in the mammalian cell cycle. Curr Opin Cell Biol. 2000;12:676–684.
- Ohtsubo M, Theodoras AM, Schumacher J, et al. Human cyclin E, a nuclear protein essential for the G1-to-S phase transition. Mol Cell Biol. 1995;15:2612–2624.
- Vladar EK, Stratton MB, Saal ML, et al. Cyclin-dependent kinase control of motile ciliogenesis. Elife. 2018;7. DOI:10.7554/eLife.36375.
- Chen X. Cyclin G: a regulator of the p53-Mdm2 network. Dev Cell. 2002;2:518–519.
- Huang S, Li Y, Wu P, et al. microRNA-148a-3p in extracellular vesicles derived from bone marrow mesenchymal stem cells suppresses SMURF1 to prevent osteonecrosis of femoral head. J Cell Mol Med. 2020;24:11512–11523.
- Su H, Fan G, Huang J, et al. YBX1 regulated by Runx3-miR-148a-3p axis facilitates non-small-cell lung cancer progression. Cell Signal. 2021;85:110049.
- Bao C, Guo L. MicroRNA-148a-3p inhibits cancer progression and is a novel screening biomarker for gastric cancer. J Clin Lab Anal. 2020;34:e23454.
- Wang J, Yao Y, Wang K, et al. MicroRNA-148a-3p alleviates high glucose-induced diabetic retinopathy by targeting TGFB2 and FGF2. Acta Diabetol. 2020;57:1435–1443.
- He L, He X, Lowe SW, et al. MicroRNAs join the p53 network–another piece in the tumour-suppression puzzle. Nat Rev Cancer. 2007;7:819–822.
- Grilo AL, Mantalaris A. Apoptosis: a mammalian cell bioprocessing perspective. Biotechnol Adv. 2019;37:459–475.
- Pajvani UB, Trujillo ME, Combs TP, et al. Fat apoptosis through targeted activation of caspase 8: a new mouse model of inducible and reversible lipoatrophy. Nat Med. 2005;11:797–803.
- Wang SS, Wang C, Chen H. MicroRNAs are critical in regulating smooth muscle cell mineralization and apoptosis during vascular calcification. J Cell Mol Med. 2020;24:13564–13572.
- Kajimoto K, Naraba H, Iwai N. MicroRNA and 3T3-L1 pre-adipocyte differentiation. RNA. 2006;12:1626–1632.
- Chen L, Chen Y, Zhang S, et al. MiR-540 as a novel adipogenic inhibitor impairs adipogenesis via suppression of PPARγ. J Cell Biochem. 2015;116:969–976.
- Dai Y, Wu X, Dai D, et al. MicroRNA-98 regulates foam cell formation and lipid accumulation through repression of LOX-1. Redox Biol. 2018;16:255–262.
- Chen L, Dai Y-M, Ji C-B, et al. MiR-146b is a regulator of human visceral preadipocyte proliferation and differentiation and its expression is altered in human obesity. Mol Cell Endocrinol. 2014;393:65–74.
- Guan X, Gao Y, Zhou J, et al. MiR-223 regulates adipogenic and osteogenic differentiation of mesenchymal stem cells through a C/EBPs/miR-223/FGFR2 regulatory feedback loop. Stem Cells. 2015;33:1589–1600.
- Guo Y, Chen Y, Zhang Y, et al. Up-regulated miR-145 expression inhibits porcine preadipocytes differentiation by targeting IRS1. Int J Biol Sci. 2012;8:1408–1417.
- Besse-Patin A, Jeromson S, Levesque-Damphousse P, et al. PGC1A regulates the IRS1:IRS2 ratio during fasting to influence hepatic metabolism downstream of insulin. Proc Natl Acad Sci U S A. 2019;116:4285–4290.
- Puigserver P, Wu Z, Park CW, et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839.
- Koo SH, Satoh H, Herzig S, et al. PGC-1 promotes insulin resistance in liver through PPAR-alpha-dependent induction of TRB-3. Nat Med. 2004;10:530–534.
- Puigserver P. Tissue-specific regulation of metabolic pathways through the transcriptional coactivator PGC1-alpha. Int J Obes (Lond). 2005;29(Suppl 1):S5–9.
- Hou N, Huang Y, Cai SA, et al. Puerarin ameliorated pressure overload-induced cardiac hypertrophy in ovariectomized rats through activation of the PPARalpha/PGC-1 pathway. Acta Pharmacol Sin. 2021;42:55–67.
- Dehnavi S, Kiani A, Sadeghi M, et al. Targeting AMPK by statins: a potential therapeutic approach. Drugs. 2021;81:923–933.
- Pang Y, Xu X, Xiang X, et al. High fat activates o-glcNAcylation and affects AMPK/ACC pathway to regulate lipid metabolism. Nutrients. 2021;13:1740.
- Ho C, Gao Y, Zheng D, et al. Alisol a attenuates high-fat-diet-induced obesity and metabolic disorders via the AMPK/ACC/SREBP-1c pathway. J Cell Mol Med. 2019;23:5108–5118.
- Li Q, Lai X, Sun L, et al. Antiobesity and anti-inflammation effects of Hakka stir-fried tea of different storage years on high-fat diet-induced obese mice model via activating the AMPK/ACC/CPT1 pathway. Food Nutr Res. 2020;64. DOI:10.29219/fnr.v64.1681.
- Yao Q, Li S, Cheng X, et al. Yin Zhi Huang, a traditional Chinese herbal formula, ameliorates diet-induced obesity and hepatic steatosis by activating the AMPK/SREBP-1 and the AMPK/ACC/CPT1A pathways. Ann Transl Med. 2020;8:231.