ABSTRACT
Green space could influence adult cognition and childhood neurodevelopment , and is hypothesized to be partly driven by epigenetic modifications. However, it remains unknown whether some of these associations are already evident during foetal development. Similar biological signals shape the developmental processes in the foetal brain and placenta.Therefore, we hypothesize that green space can modify epigenetic processes of cognition-related pathways in placental tissue, such as DNA-methylation of the serotonin receptor HTR2A. HTR2A-methylation was determined within 327 placentas from the ENVIRONAGE (ENVIRonmental influence ON early AGEing) birth cohort using bisulphite-PCR-pyrosequencing. Total green space exposure was calculated using high-resolution land cover data derived from the Green Map of Flanders in seven buffers (50 m-3 km) and stratified into low (<3 m) and high (≥3 m) vegetation. Residential nature was calculated using the Land use Map of Flanders. We performed multivariate regression models adjusted for several a priori chosen covariables. For an IQR increment in total green space within a 1,000 m, 2,000 m and 3,000 m buffer the methylation of HTR2A increased with 1.47% (95%CI:0.17;2.78), 1.52% (95%CI:0.21;2.83) and 1.42% (95%CI:0.15;2.69), respectively. Additionally,, we found 3.00% (95%CI:1.09;4.91) and 1.98% (95%CI:0.28;3.68) higher HTR2A-methylation when comparing residences with and without the presence of nature in a 50 m and 100 m buffer, respectively. The methylation status of HTR2A in placental tissue is positively associated with maternal green space exposure. Future research is needed to understand better how these epigenetic changes are related to functional modifications in the placenta and the consequent implications for foetal development.
Introduction
Exposure to green spaces has been linked to various improved health outcomes across all age groups. Of particular interest are the psychological-related outcomes, such as mental wellbeing, neurobehavior and cognitive function. For example, children living in an environment with more surrounding green were better at memorizing, were more attentive and had an increased intelligence quotient [Citation1–3]. However, as described by the hypothesis of Developmental Origins of Health and Diseases” (DoHaD), some of these observed associations could already originate during foetal development [Citation4–7], which is considered an important phase in which the brain develops and neurocognitive maturation takes place [Citation8,Citation9].
A proposed way through which green space could influence child (neuro)development is by affecting epigenetic mechanisms [Citation10,Citation11], such as DNA methylation, thereby shaping early-life conditions and development [Citation12–14]. For example, a recent study by Lee et al. (2021) provided evidence for an association between residential green space and cognitive ability-related DNA methylation in children [Citation10]. Given this knowledge it is particularly interesting to investigate the epigenetics that take place within the placenta, as they can be readily influenced by the maternal environment [Citation15–17]. The placenta is a key mediator during intrauterine development. This organ is important in exchanging and transferring nutrients and waste products, as well as the secretion of hormones necessary in foetal growth and development [Citation18]. In particular, the placenta’s role in serotonergic signalling is essential, because the foetus relies on maternal and placental derived serotonin during early foetal brain development [Citation19–22]. Several functional elements of this pathway are present within this organ, including the HTR2A gene. The HTR2A gene encodes the G-protein coupled, 5-hydroxytryptamine (serotonin) receptor 2A and functions as a mitogen, affecting placental implantation and mitogenesis [Citation23,Citation24]. The serotonergic pathway is closely involved in foetal neurodevelopment [Citation20,Citation25,Citation26], and placental methylation changes in this serotonin receptor have been associated with infant neurobehavioral outcomes [Citation27]. Considering green space is similarly closely involved in neural-related health outcomes and has been associated with cognitive ability-associated DNA methylation changes [Citation10], we hypothesize that green space exposure is capable of modifying the methylation status of an important target for cognitive development at an earlier time as measurable within placental tissue. To our knowledge, we are the first to investigate the association between green space and the placental methylation and hope to motivate future research into this field.
Methods
Study design and population
The ongoing ENVIRONAGE (ENVIRonmental Influence ON AGEing in early life) birth cohort recruits mother-newborn pairs at birth at the East-Limburg Hospital in Genk (Belgium). Only singletons, mothers without a planned caesarean section and able to fill out a questionnaire in Dutch are eligible for the cohort. Written informed consent was obtained from all participating mothers and the procedures were approved by the Ethical Committee of Hasselt University and East- Limburg Hospital. The overall participation rate of eligible mothers was 61%. Questionnaires with detailed information about demographic and lifestyle characteristics were collected, and perinatal parameters were obtained by birth records as described elsewhere [Citation28].
In the present study, 400 bio-banked placental tissue samples were randomly selected from 502 mother–newborn pairs recruited between February 2010 and May 2013. After exclusion of samples with missing data of green space exposure (n = 1) or lifestyle characteristics (n = 2) and those not meeting the pyrosequencing quality control criteria (3 failed runs of 20 samples, n = 60), statistical analyses were carried out for 327 mother-newborn pairs.
Placenta collection
Within 10 minutes after the delivery, whole placentas were stored at −20°C. For further processing, the placentas were thawed and placental biopsies were taken at four standardized sites across the middle region of the foetal side about 1 to 1.5 cm below the chorion-amniotic membrane to avoid membrane contamination and approximately 4 cm away from the umbilical cord [Citation28]. One biopsy was used in our analysis, which was taken to the right of the main artery. The biopsies were 1–2 cm3 and were collected one in each quadrant of the foetal side across the middle region of the placenta and one at the maternal side opposite to the first foetal biopsy. Each biopsy was washed in a Petri dish with phosphate buffered saline to remove blood, snap-frozen in liquid nitrogen and stored at – 80°C until DNA methylation measurements were performed, as published previously [Citation16].
DNA methylation
After DNA isolation with the QIAamp DNA mini Kit (Qiagen, Hilden, Germany) the samples were treated with sodium bisulphite to convert unmethylated cytosines into uracil using the EZ DNA methylation-GoldTM Kit (ZYMO RESEARCH CORPORATION, Irvine, CA, USA.). The bisulphite-treated DNA was amplified via polymerase chain reaction (PCR) in accordance with the manufacturer’s instructions (Pyromark PCR kit, QIAgen, Hilen, Germany) and PCR amplification success was visualized via post-PCR gel electrophoresis (Bio-Rad, Hercules, CA, USA).
Percentage DNA methylation pattern was quantified via pyrosequencing using PyroMark Q48 Autoprep and PyroMark Q48 Advanced Reagents (Qiagen, Hilden, Germany). We investigated two CpG sites in the promoter region (chr13:47,471,407–47,471,554) of the HTR2A gene, because of their potential effect on neurodevelopment function [Citation27]. CpG sites were selected based on the region analysed by Falkenberg et al. 2011 and Paquette et al. 2013, the latter describing an association with infant neurobehavioral outcomes [Citation27,Citation29], furthermore GRCh37/hg19 UCSC genome browser 2009 was used to explore the selected region for specific features of the sequence such as the presence of SNPs (Supplemental Figure 1). The PyroMark Assay Design 2.0 was used to develop appropriate PCR- and sequence primers (forward primer: 5´-TAGGTTGAAGGGTGAAGAGAG-3´; biotinylated reverse primer: 5´-CCACCCTAAACCTATATA ACCAATATC-3´; and sequencing primer: 5´-ATAAGGTTAGAAAATAGT ATGTT-3´). In both CpG sites there is the possibility of single nucleotide polymorphisms (SNPs) occurring. Based on a European population, the rs6306 SNP on the first CpG site has a prevalence of Ref allele G = 0.92 and Alt allele A = 0.08, and the rs6311 SNP on the second CpG site has a prevalence of Ref allele T = 0.41 and Alt allele C = 0.59 [Citation30]. Considering, the SNP in the second CpG site has a rather high prevalence and since this would lead to the loss of the CG methylation site in a substantial proportion of the population, we decided to exclusively proceed with the first CpG site in all further analyses.
Green space exposure
ArcGIS 10 software (Esri, Redlands, CA) was used to calculate residential green space (total green, low vegetation, high vegetation, and nature). The first three variables were based on land cover data from the Green Map of Flanders 2012 (Agency for Geographic Information Flanders, AGIV). The Green Map of Flanders contains high-resolution (1x1m) information derived from a segment-based classification using aerial ortho-photographs of 2012. The total green area, including all non-agricultural vegetation, was further divided into low-growing green (less than 3 m in height) and high-growing green (i.e., all vegetation more than 3 m in height), further referred to as low vegetation and high vegetation, respectively. In addition, nature was calculated using the Land use Map of Flanders 2012 (Flanders Department of Environment and Spatial Development). The land use data contains information about the actual use of the land according to 22 land use classes. Nature was defined as the sum of the following 10 land use classes: thickets and bushes; poplars stands; deciduous, coniferous and alluvial forests, semi-natural grassland, heath and swamp, coastal dune, and bay mud (Supplemental Table 1). We calculated the area percentage of green space (total green space, low vegetation, high vegetation, and nature) surrounding the residence in seven geographic buffers (50 m, 100 m, 300 m, 500 m, 1,000 m, 2,000 m and 3,000 m). ENVIRONAGE is a cohort study including a range of rural to urban households, that includes participants from the region of Limburg, Belgium. This region has one of the highest percentages of green space in Belgium [Citation31] and therefor our study population has on average a high percentage of green space compared to cohort studies located in metropolitan areas and large cities.
Covariables
Information on maternal pre-pregnancy body mass index (BMI) and newborn characteristics including gestational age and sex was derived from medical records. In addition, lifestyle characteristics, such as maternal education, job and smoking behaviour was obtained using a questionnaire. Maternal pre-pregnancy BMI was measured to the nearest gram and cm at the first antenatal visit and expressed as kg/m2 and gestational age was estimated based on the last menstrual cycle and first foetal ultrasound examination and expressed in days. Maternal education was defined as low (no diploma or primary school), middle (high school), or high (college or university degree). Maternal occupational levels (low, middle, and high) were assessed using the International Standard Classification of Occupation described in detail in supplement. Information about smoking behaviour was divided in non-smokers (those who never smoked), past-smoker (those who quit smoking before pregnancy), and current-smokers (those who continue smoking during pregnancy). A spatiotemporal interpolation approach [Citation32] was used to obtain daily exposure levels of PM2.5 (particulate matter with an aerodynamic diameter ≤ 2.5 µm, µg/m3) at the residential address. This method takes both land cover data and pollution data from fixed monitoring stations into account in combination with a dispersion model [Citation33,Citation34]. A leave-one-out cross-validation was carried out to measure overall model performance, which included 34 monitoring stations. The validation statistics of the interpolation tool explained > 80% of the spatial-temporal variability in the Flemish Region of Belgium for PM2.5 [Citation35]. The model was additionally validated by associating the modelled residential PM2.5 and BC levels with the biomarkers of internal exposure to nanosized black carbon particles in urine [Citation16] and placental tissue [Citation36]. These daily values were then averaged over the whole pregnancy to obtain an estimation on the mother’s chronic ambient PM2.5 exposure. In addition, we assessed neighbourhood income based on median annual household income of the statistical sector based on the residential address as described in detail in supplement.
Statistical analysis
For the statistical processing we used the R environment version 3.10 for statistical computing [Citation37]. To examine the associations between the green space exposure and methylation of the HTR2A gene we used a generalized linear model. We adjusted our model for a priori chosen covariates including maternal demographic characteristics: maternal age, pre-pregnancy BMI, maternal education, and maternal smoking behaviour, as well as newborn characteristics: gestational age and sex. Additionally, we adjusted for date of delivery and maternal exposure to ambient PM2.5-levels. To investigate sex-specific effects, we assessed interaction terms between green space and sex, which were not found to be significant. Consequently, we did not stratify the analysis for newborn sex.
The results are presented as an absolute percentage change in placental HTR2A methylation for an interquartile range (IQR) increment in green space exposure. The exposure to nature in a 50 m and 100 m buffer however was stratified into a discrete variable of none versus some presence of nature, to better deal with the low number of maternal residences with surrounding nature. Statistical significance was defined as p-value < 0.05.
In a sensitivity analysis, we examined the associations between placental HTR2A methylation and green space exposure while excluding mothers with gestational diabetes, gestational hypertension, or preeclampsia. In addition, to investigate seasonal variability, we additionally adjusted our model for season at delivery. Lastly, because there exist a strong association between green space and socio-economic status (SES) we investigated two other SES variables, i.e., maternal job and neighbourhood income. First, we adjusted our main model for maternal job instead of maternal education and second, we additionally adjusted our main model with neighbourhood income.
Results
Demographic and lifestyle characteristics of the mother-newborn pairs of the ENVIRONAGE birth cohort are presented in . The mothers were median (p25-p75) 29.0 (26.0–32.0) years old and had a pre-pregnancy BMI of 23.4 kg/m2 (21.3–26.8 kg/m2). Half of the mothers completed a high education programme and the majority (66.1%) never smoked cigarettes. For the newborn population, approximately half (52%) were boys and the median gestation duration was 279.0 (273.0–284.0) days.
Table 1. Characteristics of the mother-newborn pairs with available HTR2A methylation information (n = 327)
An overview of the correlation between the green space variables is provided in . We observed that the correlation between low vegetation and total green was strongest in the smallest buffer (Spearman r = 0.69 in 50 m) and gradually decreased with expanding buffer sizes. In contrast, the correlation between high vegetation and total green increased with expanding buffer sizes, ranging from r = 0.58 (50 m) to r = 0.92 (1,000 m). Moreover, considering the nature variable correlated strongly with high vegetation, we observed a similar increase in correlation between nature and total green with expanding buffer sizes.
Figure 1. Overview of the Spearman correlation matrix between the green space variables (total green, low vegetation, high vegetation, and nature) in the seven buffer sizes (50 m, 100 m, 300 m, 500 m and 1,000 m, 2,000 m and 3,000 m).
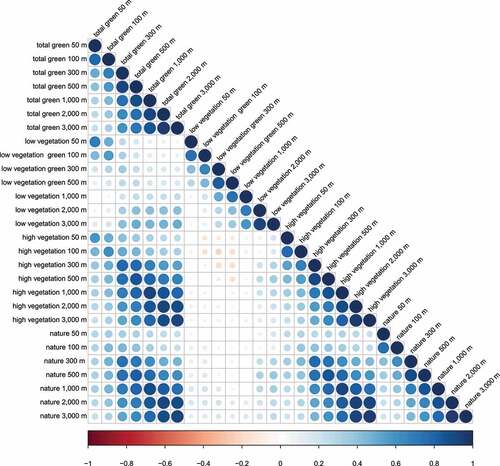
Overall, maternal residences had a higher percentage of low vegetation than high vegetation in their surroundings, in all included buffers (). Furthermore, we observed that the number of maternal residences with surrounding nature was rather low within a 50 m and 100 m radius. Accordingly, we stratified these green space variables into a discrete variable of none versus some presence of nature (n = 85 and n = 166, respectively).
Table 2. Maternal gestational green space exposure
After adjustment to a priori chosen covariables, placental HTR2A methylation was increasingly positively associated with residential green space, reaching significance in larger buffers surrounding the residential address. More specifically, for an IQR increment in residential total green exposure within a 1,000 m, 2,000 m and 3,000 m buffer the methylation of HTR2A increased with 1.47% (95% CI: 0.17; 2.78), 1.52% (95% CI: 0.21; 2.83) and 1.42% (95% CI: 0.15; 2.69), respectively ( and Supplemental Table 2). When examining whether this positive association was attributed to low or high vegetation, we observed an overall positive trend in both green space variables, reaching statistical significance in residential high vegetation in a 3,000 m buffer surrounding the household, where we found a 1.38% (95% CI: 0.08;2.68) higher HTR2A methylation. Regarding exposure to nature surrounding the residence, we observed a significant positive association of HTR2A methylation with nearby nature, i.e., 50 m and 100 m buffer surrounding the residence. Here, we found 3.00% (95% CI: 1.09; 4.91) and 1.98% (95% CI: 0.28; 3.68) higher HTR2A methylation in maternal residences surrounded with residential nature compared to residences without the presence of residential nature in a 50 m and 100 m buffer respectively ( and Supplemental Table 2).
Figure 2. Estimates (with 95% CI) of 327 placental HTR2A DNA-methylation in association with land cover green space variables (total green, low vegetation, and high vegetation) for seven buffers surrounding the residence (50 m, 100 m, 300 m, 500 m, 1,000 m, 2,000 m and 3,000 m). The model was adjusted for newborn sex, maternal age, maternal education, maternal smoking status, gestational age, pre-pregnancy BMI, date of delivery and ambient airborne PM2.5 concentration. Buffer-specific IQRs are given in the x-axis.
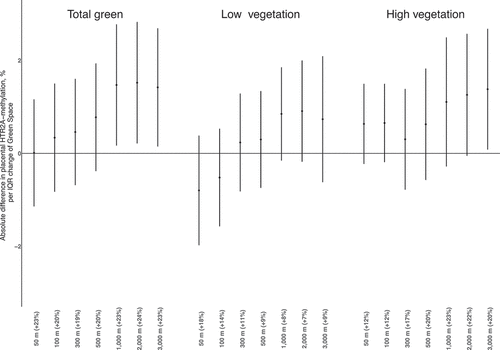
Figure 3. Estimates (with 95% CI) of 327 placental HTR2A DNA-methylation in association with the land use variable nature for seven buffers surrounding the residence (50 m, 100 m, 300 m, 500 m, 1,000 m, 2,000 m and 3,000 m). Variables with low amount of green within the buffers are recalculated as discrete categorical variables (50 m and 100 m). Results are expressed as estimates [95% confidence interval] for an IQR increment in residential green space unit change [95% confidence interval] compared to the corresponding reference level for the discrete variables (50 m and 100 m nature). The model was adjusted for newborn sex, maternal age, maternal education, maternal smoking status, gestational age, pre-pregnancy BMI, date of delivery and ambient airborne PM2.5 concentration. Buffer-specific IQRs are given in the x-axis.
![Figure 3. Estimates (with 95% CI) of 327 placental HTR2A DNA-methylation in association with the land use variable nature for seven buffers surrounding the residence (50 m, 100 m, 300 m, 500 m, 1,000 m, 2,000 m and 3,000 m). Variables with low amount of green within the buffers are recalculated as discrete categorical variables (50 m and 100 m). Results are expressed as estimates [95% confidence interval] for an IQR increment in residential green space unit change [95% confidence interval] compared to the corresponding reference level for the discrete variables (50 m and 100 m nature). The model was adjusted for newborn sex, maternal age, maternal education, maternal smoking status, gestational age, pre-pregnancy BMI, date of delivery and ambient airborne PM2.5 concentration. Buffer-specific IQRs are given in the x-axis.](/cms/asset/7613e6ef-aafa-42d8-9266-97d2e27356a3/kepi_a_2088464_f0003_b.gif)
In a sensitivity analysis, we examined the associations between placental HTR2A methylation and green space exposure while excluding mothers with gestational diabetes, gestational hypertension or preeclampsia and found no substantial differences to our previously reported results (Supplemental Table 3). In addition, we adjusted the main analysis for season of delivery to account for season variability in PM2.5 and found no changes to the observed associations (Supplemental Table 4). In addition, we investigate maternal job instead of maternal education as a covariate in our model (Supplemental Table 5) and in another analysis we additionally adjusted our main model for neighbourhood income (Supplemental Table 6) and found no major changes to the observed associations.
Discussion
In this study, we provide evidence for an association between maternal green space exposure and placental methylation of an important component of the serotonergic pathway related to foetal growth and neurodevelopment. More specifically, our results show that early-life green space exposure, represented by total green, high vegetation and nature, is associated with an increase in the methylation status of a key CpG site of the HTR2A gene.
Previous findings support the relationship between chronic exposure to green space and cognitive functioning and development in children. [Citation1–3] A proposed mechanism explaining this relationship is through DNA-methylation of IQ-associated genes, as seen in a recent study by Lee et al. (2021) which included, among others, genes involved in dopaminergic and serotonergic pathways [Citation10]. We assume that these observed associations find their origin earlier during foetal cognitive development, where maternal green space exposure could shape the placental environment through epigenetic changes. This intrauterine reprogramming occurs during a vulnerable window of brain development [Citation8,Citation9], during which environmental factors are highly efficient in modulating the placental epigenome, thereby resulting in long-term consequences. [Citation15–17,Citation38] Accordingly, epigenetic changes in genes involved in serotonin signalling could result in changes to foetal neurodevelopment [Citation39,Citation40]. In the present study, we investigated the placental HTR2A serotonin receptor, which is expressed in placental villous cytotrophoblast and syncytiotrophoblast layers, comprising the barrier between maternal and foetal blood compartments [Citation41]. This receptor has mitogenic properties, and its activation has been linked to the JAK2/STAT pathway and is consequently involved in trophoblast growth and placental implantation [Citation23,Citation24]. Moreover, this particular receptor in placental tissue, has been implicated in infant cognitive outcomes. In a study by Paquette et al. (2013), neurodevelopmental outcomes, as defined by the NICU Network Neurobehavioral Scales were assessed in newborn infants. Here, they found placental HTR2A-methylation to be negatively associated with quality of movement and positively associated with infant attention scores [Citation27]. However, more research is required to better understand the physiological and psychological long-term health outcomes related to this placental HTR2A methylation.
Interestingly, we observed a difference in the associations between the two types of green space measures, i.e., derived from land cover (total green, low vegetation and high vegetation) or land use (nature variable) data, with regard to scale. Whereas the land cover green space variables were statistically associated with HTR2A-methylation in large-scale buffers (1,000 to 3,000 m) surrounding the household, the observed associations for the nature variable were evident in buffers close to the residence (50 m and 100 m). This difference might be explained by the differences in the type of data used to construct these green space variables. Land use variables, such as residential nature, only consider land suitable for a specific use, referring to larger areas of connected qualitative green. In contrast, land cover variables are quantified at high-resolution and by taking all elements of green space into account. This different approach is most noticeable in buffers closer to the residence. Here, we observe a lower correlation between the land cover and land use variables, due to the higher contribution of non-qualitative vegetation in the land cover variables that are not included in the nature variable (). Overall, these different associations suggest, a stronger effect of qualitative green in closer buffers, whereas in larger buffers, all elements of green space might be contributing to the observed association [Citation42,Citation43].
Although it has been hypothesized that epigenetic modulation is a regulatory pathway through which green space could be associated with foetal development, the specific pathways are still unclear. Green space could exert its effect via biophysical-related processes such as reducing urban-related environmental hazards, including noise and heat [Citation44–47], which are directly and indirectly associated with placental DNA methylation [Citation48,Citation49]. Additionally, the presence of green space promotes physical activity [Citation50,Citation51] and improves cognitive restoration [Citation52], both associated with functional and epigenetic changes of placental tissue [Citation25,Citation53–56] However, the underlying mechanisms of these pathways are still being investigated. A proposed way green space could result in alterations in DNA methylation, might be through their capability to reduce oxidative stress [Citation57–60], which is known to impact epigenetic mechanisms [Citation16,Citation61]. Moreover, oxidative stress is hypothesized to be associated with neurodevelopment, supporting the oxidative-stress pathway as a link between green space exposure and methylation changes in neural-related components, such as within the serotonergic pathway [Citation10,Citation62–64].
This study has several strengths. First of all, we included a large number of mother-newborn pairs (n = 327), which were representative for the gestational segment of population in Flanders, as described earlier [Citation28]. Additionally, we used bisulphite-PCR-pyrosequencing to asses DNA methylation, which is a highly standardized technique with an excellent detection limit to obtain accurate results [Citation65,Citation66]. A limitation however is that we did not measure the occurrence of SNPs in our population, but the prevalence of the rs6306 SNP is rather low, limiting potential implications. A second important limitation is the that we only investigated one CpG site, which will not reflect the full promoter region of the HTR2A gene, making our observed associations difficult to translate to important health changes. However this selected site has been implicated in important infant neurobehavioral outcomes, highlighting the health relevance of this investigation. A third limitation is the fact that the placenta is a heterogeneous tissue, resulting in compositional differences potentially contributing to the observed methylation patterns. However, a standardized protocol was used for the placental sampling, as stated before [Citation16]. Moreover, we used high-resolution green exposure data to obtain detailed information on the type of green space, which might be an important contributing factor to unravel the underlying mechanisms between green space exposure and health outcomes [Citation67,Citation68]. This research is an important first step to investigate the relationship between prenatal green space exposure and foetal cognitive development.
Conclusion
Overall, we found a significant positive association between the amount of green space surrounding the residential address of the participating women and the methylation of the placental serotonin receptor, coded by the HTR2A gene. However, future research is needed to understand better how these epigenetic changes are related to functional modifications in the placenta and the consequent implications for foetal development. Overall, these results are a first step to establish a potential association between green space exposure and epigenetic changes within placental tissue, that might give further support to the importance of green space, in particular, in early developmental processes.
Supplemental Material
Download MS Word (3.1 MB)Acknowledgments
The ENVIRONAGE birth cohort was established by the EU research council “project ENVIRONAGE” (ERC-2012-StG 310,890) and received support for this study from the Flemish Scientific Fund (G073315N/G048420N), from the Belgian Science Policy Office BELSPO (grant nr. BR/154/A1/RespirIT) and Kom op Tegen Kanker. Further, Esmée M Bijnens holds a fellowship from the Marguerite-Marie Delacroix foundation.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. https://www.uhasselt.be/nl/aparte-sites-uhasselt/limburgs-geboortecohort
Disclosure statement
No potential conflict of interest was reported by the author(s).
supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15592294.2022.2088464
Additional information
Funding
References
- Dadvand P, Tischer C, Estarlich M, et al. Lifelong residential exposure to green space and attention: a population-based prospective study. Environ Health Perspect. 2017;125(9):097016.
- Dockx Y, Bijnens EM, Luyten L, et al. Early life exposure to residential green space impacts cognitive functioning in children aged 4 to 6 years. Vol. 161. ; 2022. p. 107094.
- Bijnens EM, Derom C, Thiery E, et al. Residential green space and child intelligence and behavior across urban, suburban, and rural areas in Belgium: a longitudinal birth cohort study of twins. PLoS Med. 2020;17(8):e1003213.
- Barker DJ. Developmental origins of adult health and disease. J Epidemiol Community Health. 2004;58(2):114–115.
- Barker D, Thornburg K. Placental programming of chronic diseases, cancer and lifespan: a review. Placenta. 2013;34(10):841–845.
- O’Connor TG, Miller RK, Salafia C. Placental studies for child development. Child Dev Perspect. 2019;13(3):193–198
- O’Donnell KJ, Glover V, Lahti J, et al. Maternal prenatal anxiety and child COMT genotype predict working memory and symptoms of ADHD. PloS one. 2017;12(6):e0177506. PloS one
- Gilmore JH, Knickmeyer RC, Gao W. Imaging structural and functional brain development in early childhood. Nat Rev Neurosci. 2018;19(3):123–137.
- Nelson CA, Gabard-Durnam LJ. Early adversity and critical periods: neurodevelopmental consequences of violating the expectable environment. Trends Neurosci. 2020;43(3):133–143.
- Lee K-S, Choi Y-J, Cho J-W, et al. Children’s greenness exposure and IQ-associated DNA Methylation: a prospective cohort study. Int J Environ Res Public Health. 2021;18(14):7429.
- Xu R, Li S, Li S, et al. Residential surrounding greenness and DNA methylation: an epigenome-wide association study. Vol. 154. ; 2021. p. 106556.
- Dwi Putra SE, Reichetzeder C, Hasan AA, et al. Being born large for gestational age is associated with increased global placental DNA methylation. Sci Rep. 2020;10(1):927.
- Li Y, et al. Differential placental methylation in preeclampsia, preterm and term pregnancies. Vol. 93. ; 2020. p. 56–63.
- Bahado-Singh RO, Vishweswaraiah S, Aydas B, et al. Placental DNA methylation changes and the early prediction of autism in full-term newborns. PLOS ONE. 2021;16(7):e0253340.
- Everson TM, Vives-Usano M, Seyve E, et al. Placental DNA methylation signatures of maternal smoking during pregnancy and potential impacts on fetal growth. Nat Commun. 2021;12(1):5095.
- Saenen ND, Vrijens K, Janssen BG, et al. Lower placental leptin promoter methylation in association with fine particulate matter air pollution during pregnancy and placental nitrosative stress at birth in the ENVIR ON AGE cohort. Environ Health Perspect. 2017;125(2):262–268.
- Tsamou M, Vrijens K, Madhloum N, et al. Air pollution-induced placental epigenetic alterations in early life: a candidate miRNA approach. Epigenetics. 2018;13(2):135–146.
- Griffiths SK, Campbell JP. Placental structure, function and drug transfer. Continuing Educ Anaesth Crit Care Pain. 2014;15(2):84–89.
- Bonnin A, Goeden N, Chen K, et al. A transient placental source of serotonin for the fetal forebrain. Nature. 2011;472(7343):347–350.
- Bonnin A, Levitt P. Fetal, maternal, and placental sources of serotonin and new implications for developmental programming of the brain. Vol. 197. 2011.1–7
- Rosenfeld CS. Placental serotonin signaling, pregnancy outcomes, and regulation of fetal brain development†. Biol Reprod. 2020;102(3):532–538.
- Kliman HJ, Quaratella SB, Setaro AC, et al. Pathway of maternal serotonin to the human embryo and fetus. Endocrinology. 2018;159(4):1609–1629.
- Oufkir T, Vaillancourt C. Phosphorylation of JAK2 by serotonin 5-HT (2A) receptor activates both STAT3 and ERK1/2 pathways and increases growth of JEG-3 human placental choriocarcinoma cell. Placenta. 2011;32(12):1033–1040.
- Sonier B, Lavigne C, Arseneault M, et al. Expression of the 5-HT2A serotoninergic receptor in human placenta and choriocarcinoma cells: mitogenic implications of serotonin. Placenta. 2005;26(6):484–490.
- Reynolds RM, Pesonen A-K, O’Reilly JR, et al. Maternal depressive symptoms throughout pregnancy are associated with increased placental glucocorticoid sensitivity. Psychol Med. 2015;45(10):2023–2030.
- Ranzil S, Walker D, Borg A, et al. The relationship between the placental serotonin pathway and fetal growth restriction. Vol. 161. ; 2019. p. 80–87.
- Paquette AG, Lesseur C, Armstrong DA, et al. Placental HTR2A methylation is associated with infant neurobehavioral outcomes. Epigenetics. 2013;8(8):796–801.
- Janssen BG, Madhloum N, Gyselaers W, et al. Cohort profile: the ENVIRonmental influence ON early AGEing (ENVIRONAGE): a birth cohort study. Int J Epidemiol. 2017;46(5):1386–1387.
- Falkenberg VR, Gurbaxani BM, Unger ER, et al. Functional genomics of serotonin receptor 2A (HTR2A): interaction of polymorphism, methylation, expression and disease association. Neuromolecular Med. 2011;13(1):66–76.
- Phan L, Jin Y, Zhang H, et al. ALFA: Allele Frequency Aggregator. 2020 2020 Oct Oct. 2020 Oct. 2020 Oct Oct 10]; Available from: www.ncbi.nlm.nih.gov/snp/docs/gsr/alfa/.
- Een cijfermatig zicht op het gebruik van de ruimte in Limburg - A numerical view on the use of space in Limburg. ; 2020.
- Janssen S, Dumont G, Fierens F, et al. Spatial interpolation of air pollution measurements using CORINE land cover data. Atmos Environ. 2008;42(20):4884–4903.
- Lefebvre W, Vercauteren J, Schrooten L, et al. Validation of the MIMOSA-Aurora-IFDM model chain for policy support: modeling concentrations of elemental carbon in Flanders. Atmos Environ. 2011;45(37):6705–6713.
- Lefebvre W, Degrawe B, Beckx C, et al. Presentation and evaluation of an integrated model chain to respond to traffic-and health-related policy questions. Environ Modell Software. 2013;40:160–170.
- Maiheu B, et al. Identifying the best available large-scale concentration maps for air quality in Belgium. Mechelen Belgium: Flemish Institute for Technological Research (VITO; 2013.
- Bové H, Bongaerts E, Slenders E, et al. Ambient black carbon particles reach the fetal side of human placenta. Nat Commun. 2019;10(1):3866.
- R Development Core Team. R: a langugage and environment for statistical computing. Vienna Austria: R Foundation for Statistical Computing; 2019.
- Lester BM, Marsit CJ. Epigenetic mechanisms in the placenta related to infant neurodevelopment. Epigenomics. 2018;10(3):321–333.
- Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci. 2003;4(12):1002–1012.
- Hadden C, Fahmi T, Cooper A, et al. Serotonin transporter protects the placental cells against apoptosis in caspase 3-independent pathway. J Cell Physiol. 2017;232(12):3520–3529.
- Viau M, Lafond J, Vaillancourt C. Expression of placental serotonin transporter and 5-HT 2A receptor in normal and gestational diabetes mellitus pregnancies. Reprod Biomed Online. 2009;19(2):207–215.
- Browning M, Lee K. Within what distance does “Greenness” best predict physical health? A systematic review of articles with GIS buffer analyses across the lifespan. Int J Environ Res Public Health. 2017;14(7):675.
- Su JG, Dadvand P, Nieuwenhuijsen M, et al. Associations of green space metrics with health and behavior outcomes at different buffer sizes and remote sensing sensor resolutions. Vol. 126. ; 2019. p. 162–170.
- Nieuwenhuijsen MJ. Green Infrastructure and Health. Annu Rev Public Health. 2021;42(1):317–328.
- Markevych I, Schoierer J, Hartig T, et al. Exploring pathways linking greenspace to health: theoretical and methodological guidance. Vol. 158. ; 2017. p. 301–317.
- Dzhambov AM, Markevych I, Tilov B, et al. Lower noise annoyance associated with GIS-derived greenspace: pathways through perceived greenspace and residential noise. Int J Environ Res Public Health. 2018;15(7):1533.
- Schwaab J, Meier R, Mussetti G, et al. The role of urban trees in reducing land surface temperatures in European cities. Nat Commun. 2021;12(1):6763.
- Abraham E, Rousseaux S, Agier L, et al. Pregnancy exposure to atmospheric pollution and meteorological conditions and placental DNA methylation. Environ Int. 2018;118:334–347.
- Kingsley SL, Eliot MN, Whitsel EA, et al. Maternal residential proximity to major roadways, birth weight, and placental DNA methylation. Environ Int. 2016;92-93:43–49.
- Almanza E, Jerrett M, Dunton G, et al. A study of community design, greenness, and physical activity in children using satellite, GPS and accelerometer data. Health Place. 2012;18(1):46–54.
- Ward JS, Duncan JS, Jarden A, et al. The impact of children’s exposure to greenspace on physical activity, cognitive development, emotional wellbeing, and ability to appraise risk. Health Place. 2016;40:44–50.
- Kondo MC, Fluehr J, McKeon T, et al. Urban green space and its impact on human health. Int J Environ Res Public Health. 2018;15(3):445.
- Monk C, Feng T, Lee S, et al. Distress during pregnancy: epigenetic regulation of placenta glucocorticoid-related genes and fetal neurobehavior. Am J Psychiatry. 2016;173(7):705–713.
- Rasmussen L, Knorr S, Antoniussen CS, et al. The impact of lifestyle, diet and physical activity on epigenetic changes in the offspring—A systematic review. Nutrients. 2021;13(8):2821.
- Kertes DA, Kamin HS, Hughes DA, et al. Prenatal maternal stress predicts methylation of genes regulating the hypothalamic-Pituitary-Adrenocortical system in mothers and newborns in the democratic Republic of Congo. Child Dev. 2016;87(1):61–72.
- Janssen AB, Kertes DA, McNamara GI, et al. A role for the placenta in programming maternal mood and childhood behavioural disorders. J Neuroendocrinol. 2016; 28: 28.
- Yeager R, Riggs DW, DeJarnett N, et al. Association between residential greenness and cardiovascular disease risk. J Am Heart Assoc. 2018;7(24):e009117.
- De Petris S, Squillacioti G, Bono R, et al. Geomatics and epidemiology: associating oxidative stress and greenness in urban areas. Environ Res. 2021;197:110999.
- Squillacioti G, Carsin AE, Borgogno-Mondino E, et al. Greenness and physical activity as possible oxidative stress modulators in children. Eur J Public Health. 2020;30(Supplement_5). 10.1093/eurpub/ckaa165.090.
- Squillacioti GBV, V B, F G, et al. Greenness effect on oxidative stress and respiratory flows in children. Environ Epidemiol. 2019; 3: 35–36.
- Scarpato R, Testi S, Colosimo V, et al. Role of oxidative stress, genome damage and DNA methylation as determinants of pathological conditions in the newborn: an overview from conception to early neonatal stage. Mutat Res/Rev Mutat Res. 2019;783:108295.
- Salim S. Oxidative stress and the central nervous system. J Pharmacol Exp Ther. 2017;360(1):201–205.
- Herman F, Westfall S, Brathwaite J, et al. Suppression of presymptomatic oxidative stress and inflammation in neurodegeneration by grape-derived polyphenols. Front Pharmacol. 2018;9:867.
- Ponsonby AL, Symeonides C, Saffery R, et al. Prenatal phthalate exposure, oxidative stress-related genetic vulnerability and early life neurodevelopment: a birth cohort study. Vol. 80. ; 2020. p. 20–28.
- Dejeux E, El abdalaoui H, Gut IG, et al. Identification and quantification of differentially methylated loci by the pyrosequencing technology. Methods Mol Biol. 2009;507:189–205.
- Tost J, Gut IG. Analysis of gene-specific DNA methylation patterns by pyrosequencing technology. Methods Mol Biol. 2007;373:89–102.
- Astell-Burt T, Feng X. Association of urban green space with mental health and general health among adults in Australia. JAMA Network Open. 2019;2(7):e198209–e198209.
- Nguyen PY, Astell-Burt T, Rahimi-Ardabili H, et al. Green space quality and health: a systematic review. Int J Environ Res Public Health. 2021;18(21):11028.
