ABSTRACT
Foetuses exposed to maternal gestational diabetes (GDM) and type 2 diabetes (T2D) have an increased risk of adverse perinatal outcomes. Epigenetic mechanisms, including DNA methylation and histone modifications, may act as mediators of persistent metabolic memory in endothelial cells (ECs) exposed to hyperglycaemia, even after glucose normalization. Therefore, we investigated alterations in global DNA methylation and epigenetic modifier expression (DNMT1, DNMT3a, DNMT3b, HDAC1, and HDAC2) in human umbilical vein ECs (HUVECs) from the umbilical cords of mothers with GDM (n = 8) and T2D (n = 3) compared to that of healthy mothers (n = 6). Global DNA alteration was measured using a 5-methylation cytosine colorimetric assay, followed by quantitative real-time polymerase chain reaction to measure DNA methyltransferase and histone acetylase transcript expression. We revealed that DNA hypermethylation occurs in both GDM- and T2D-HUVECs compared to that in Control-HUVECs. Furthermore, there was a significant increase in HDAC2 mRNA levels in GDM-HUVECs and increase in DNMT3b mRNA levels in T2D-HUVECs. Overall, our results suggest that GDM and T2D are associated with global DNA hypermethylation in foetal endothelial cells under normoglycemic conditions and the aberrant mRNA expression of HDAC2 and DNMT3b could play a role in this dysregulation.
Key policy highlights
Global DNA hypermethylation was reported in foetal endothelial cells of both gestational and type 2 diabetes cultured under normal glucose condition
HDAC2 mRNA was significantly increased in gestational diabetes
DNMT3b mRNA was significantly increased in type 2 diabetes
Our data support the concept of epigenetic programming occurs in foetal endothelial cells exposed to diabetes which retain even after glucose normalization
Introduction
Type 2 diabetes (T2D) is a multifactorial disorder characterized by impaired carbohydrate metabolism and hyperglycaemia [Citation1]. Saudi Arabia has the second highest prevalence of diabetes among Middle Eastern countries and ranks seventh in the world [Citation2]. The prevalence of Saudi Arabian women with either pre-existing T2D or gestational diabetes mellitus (GDM) is approximately 40% [Citation3]. Such increased percentages suggest that further research should be conducted to understand the pathological effects of diabetes in both mothers and foetuses. T2D affects 90% of patients with diabetes mellitus and is caused by resistance to insulin, which is also accompanied by a deficiency in insulin secretion. GDM is defined as the first diagnosis of glucose intolerance during pregnancy. GDM is associated with long-term complications in the mother and foetus and is characterized by hyperglycaemia due to insulin insensitivity, which usually reverts to normal after delivery. However, some mothers with GDM develop T2D after delivery for unknown reasons. The prevalence of GDM in pregnant Saudi Arabian women is the highest in the world and can increase maternal and foetal morbidities and mortalities [Citation4]. Untreated GDM is associated with a wide-spectrum of foetal complications, starting with being overweight at birth, cardiac dysfunction, and malformations [Citation5]. Numerous studies have verified the association between intrauterine exposure to hyperglycaemia and an increased risk of obesity, diabetes, and cardio-metabolic disorders in offspring, suggesting that epigenetic modifications may be the primary cause of metabolic memory [Citation6–10]. El-Osta et al. [Citation11,Citation12] reported that transient exposure of cultured human and bovine aortic endothelial cells to elevated glucose concentration (30 mM) induced persistent epigenetic changes in the promoter region of the nuclear factor (NF)-κB p65 subunit and altered gene expression of molecules including vascular cell adhesion molecule 1 and monocyte chemotactic protein 1 during subsequent normoglycemia.
Epigenetic changes involve DNA methylation and histone modifications. Histone acetyltransferases mediate histone acetylation, which opens the DNA double helix and allows free access for gene transcription. Conversely, histone deacetylases cause hypoacetylation of histones, which condenses chromatin structure and represses gene transcription [Citation13]. A family of enzymes responsible for histone modification (e.g., acetylation or deacetylation) includes histone methyltransferases, histone acetyltransferases, and demethylases [Citation14]. Under hyperglycaemic conditions, specific histone methylation occurs in endothelial cells and is associated with a chronic inflammatory phenotype [Citation11,Citation15].
Few studies have addressed the importance of DNA methylation in the pathogenesis of diabetes and its associated complications [Citation16–18]. DNA methylation is regulated by three DNA methyltransferase (DNMTs) enzymes, DNMT1, DNMT2, and DNMT3 (subunits a and b). Both specific and global DNA methylation are believed to contribute to endothelial dysfunction caused by complications of diabetes [Citation19,Citation20]. Both hypermethylation and hypomethylation have been reported in diabetes, suggesting their possible involvement in disease onset and progression by regulation of gene expression related to diabetes. Interestingly, insulin gene expression is also regulated by DNA methylation [Citation21]. In addition, increased global DNA methylation has been reported in the placenta of patients with GDM [Citation22]. Zhu et al. [Citation23] identified 361 hypomethylation-high and 541 hypermethylation-low genes in GDM. Moreover, DNA hypomethylation at the Long Interspersed Nuclear Element 1 (LINE-1) sequences, may contribute to cardiovascular and metabolic complications in T2D [Citation24]. However, it remains to be fully elucidated whether alterations in global DNA methylation and epigenetic enzymes occur in the foetal endothelium in GDM or T2D under normal glucose conditions. Therefore, this study aimed to determine whether GDM and T2D are associated with global DNA methylation changes and alterations in the transcript expression of five epigenetic modifiers (DNMT1, DNMT3a, DNMT3b, HDAC1, and HDAC2) in foetal endothelial cells under normoglycemic conditions.
Materials and methods
All reagents were purchased from UFC biotechnology (Buffalo, NY, USA) unless otherwise stated.
Participants and HUVECs isolation
Umbilical cords were collected from women after full-term pregnancy at the East Jeddah General Hospital, Saudi Arabia. All participants were notified of the aims of the study, and written informed consent was obtained before the collection of umbilical cords. Approval for the study was obtained from the Ethics Committee at the Research and Studies Department, Directorate of Health Affairs, Jeddah (IRB registration number H-02-J-002; approved on 29 January 2020). Samples were collected on the day of delivery. Control subjects who were on medication, or had a family history of diabetes, high blood pressure, and/or smoking were excluded from the study. Participants with a fasting plasma glucose concentration of >7 mmol/L or HbA1c > 7% were considered to have T2D according to the American Diabetes Association [Citation25]. Subjects with GDM had a positive oral glucose tolerance test according to the WHO criteria [Citation26]. All patients with GDM were on a diet whereas patients with T2D were on insulin therapy. None of the patients experienced any other obstetric complications such as pre-eclampsia. HUVECs were isolated from participants with GDM (n = 8), T2D (n = 3), and healthy mothers (n = 6) using the collagenase method [Citation27]. Subsequently, HUVECs were cultured in M199 medium supplemented with 20% foetal bovine serum, 100 U penicillin/100 mg/mL streptomycin, 2.5 μg/mL fungizone, and 2 mM L-glutamine at 37°C under 95% air and 5% CO2 humidified conditions. The cells were used for up to four passages. Characterization of cultured HUVECs with CD31 was performed as described previously [Citation28].
DNA extraction
DNA extraction was performed using the QIAamp DNA Mini Kit (Qiagen, Manchester, UK) following the manufacturer’s protocols. DNA concentration was quantified using a Nanodrop 2000 spectrophotometer (Agilent, Edinburgh, UK).
Global DNA methylation
Global methylated DNA quantification (colorimetric) 5-methylation cytosine (5-mC) analysis was performed using a 5-mc colorimetric assay (Abcam, Cambridge, UK) following the manufacturer’s instructions. Briefly, 100 ng of relatively pure DNA with a 260/280 ratio of 1.8 were treated with the binding solution and incubated at 37°C for 60 min. The plate was washed with a buffer. DNA samples were incubated with anti-5-mC monoclonal and detection antibodies. After the addition of the enhancer and developer solutions, the absorbance was read at 450 nm using a microplate spectrophotometer system.
RNA extraction and cDNA conversion
Total RNA was extracted from HUVECs using an RNeasy Mini Kit (Qiagen). RNA quantity was checked using a spectrophotometer and quality was assessed using an Agilent 2100 Bioanalyzer (Agilent). DNase I digestion was performed during RNA isolation to exclude contamination with genomic DNA. cDNA was synthesized from 1 µg of total RNA using the ImProm-II™ Reverse Transcription System (Promega, Madison, WI, USA), according to the manufacturer’s protocols.
Quantitative real-time polymerase chain reaction (Qrt-PCR)
Primers were designed using Primer3web version 4.1.0 and the sequences are listed in . Beta-actin was used as the endogenous reference gene to normalize the results. Amplifications were performed using the QuantiTect SYBR Green PCR Kit (Qiagen) in the iCycleriQ RT-PCR System (Applied Biosystems, Cheshire, UK) following the manufacturer’s instructions. Relative gene expression was quantified using Rest 2009 software version 2.0.13 [Citation29].
Table 1. The primers used in RT-PCR.
Statistical analysis
Normality distribution was checked using the Shapiro-Wilk test. Data are expressed as the mean ± standard error of mean. Student’s t-tests were performed to compare the differences between the groups using GraphPad Prism (version 8.0.0; GraphPad Software Inc., San Diego, CA, USA). P < 0.05 was considered to indicate statistically-significant differences.
Results
The clinical parameters of the donors recruited in this study are summarized in . Random plasma glucose (RPG) levels were significantly higher in the GDM group than in the control group (P = 0.01). However, the T2D group had higher haemoglobin A1c (HbA1c)% levels than the healthy control group (P = 0.004). There were no significant differences in other parameters between the two groups.
Table 2. List of Mean characteristics of subjects participating in the study.
Results of DNA methylation
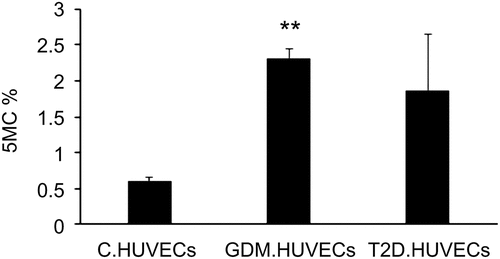
In the present study, DNA isolated from GDM-HUVECs exhibited a significant increase in the 5-mC% status (P = 0.002) compared to that in the Control-HUVECs, as shown in . Additionally, there was a near significant (P = 0.05) increase in global DNA methylation in T2D-HUVECs compared to that in Control-HUVECs.
Gene expression analysis using Qrt-PCR
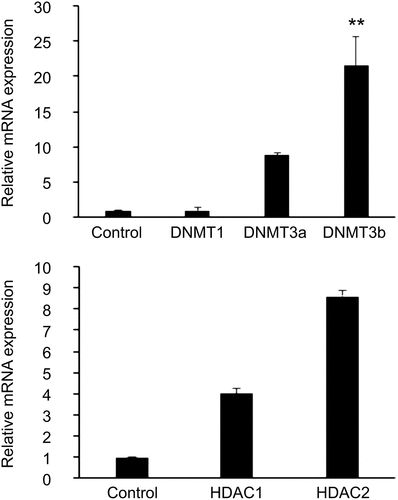
An approximately 431-fold increase in the mRNA level of HDAC2 was observed in GDM-HUVECs compared to in Control-HUVECs (P = 0.019), as shown in . Although we noticed an increase in DNMT3b expression in GDM-HUVECs when compared to that in Control-HUVECs, it was not statistically significant (P > 0.05). In addition, there was an increase in the mRNA levels of DNMT3b in T2D-HUVECs compared to that in Control-HUVECs (P = 0.002, ).
Figure 2. Expression levels of the epigenetic markers (a) DNMTs and (b) HDACs in GDM-HUVECs (n = 8) and Control-HUVECs (n = 6) were measured using Qrt-PCR. Gene expression was normalized to the mRNA level of the housekeeping gene β-actin in each sample. Results are expressed as the mean ± SEM. **P < 0.5 versus controls.
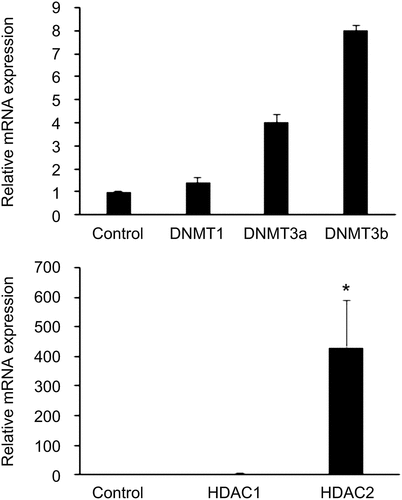
Figure 3. Expression levels of the epigenetic markers (a) DNMTs and (b) HDACs in T2D-HUVECs (n = 3) and Control-HUVECs (n = 6), were measured using Qrt-PCR. Gene expression was normalized to the mRNA level of the housekeeping gene β-actin in each sample. Results are expressed as the mean ± SEM. **P < 0.01 versus controls.
Discussion
In the present study, an in vitro model was employed to provide some evidence that both GDM and T2D are associated with global changes in DNA methylation in HUVECs cultured under normal glucose conditions. In addition, both GDM and T2D were found to affect epigenetic enzyme modifiers, as higher mRNA levels of HDAC2 and DNMT2 were observed in GDM-HUVECs and T2D-HUVECs, respectively. These findings support the concept of glycaemic memory, wherein the damaging effect of hyperglycaemia can induce epigenetic changes even after the normalization of glucose levels. Previous studies have verified the relationship between diabetes and epigenetic changes in HUVECs [Citation30–32]. Moreover, several studies have noted DNA methylation as the main cause of glycaemic memory that leads to diabetic end-organ complications, such as diabetic nephropathy and retinopathy [Citation33–40]. Epigenetic changes include DNA methylation, histone modifications, and microRNA gene dysregulation. The most common epigenetic modification occurs because of methylation of cytosine at enriched gene promoters and 5’ regions of CpG dinucleotide sites [Citation41]. Accordingly, hypomethylation of promoters generally facilitates gene transcription, whereas hypermethylation is associated with gene silencing [Citation42].
Consistent with our present findings, a previous study showed that overall hypermethylation increased the expression of genes related to glycolipid metabolism and related signalling pathways, including Agap2, in the pancreas of offspring of GDM mice [Citation43]. Another study showed that progressive epigenetic silencing via DNA methylation of the Pdx1 promoter – a transcription factor that affects beta cell differentiation – promotes the development of diabetes [Citation44]. Interestingly, Pepin et al. [Citation45] found that transient exposure to a high concentration of glucose (75, 175, and 200 mg/dL) for 72 h induces epigenetic changes and DNA methylation in genes involved in endothelial dysfunction, including vascular endothelial growth factor, and nitric oxide production in aortic endothelial cells. In contrast to our model, Howe et al. [Citation46] found that maternal GDM is associated with DNA hypomethylation in cord blood. This may be explained by the fact that different tissues display different forms of methylation.
DNMT3a and DNMT3b are members of a family of DNA methyltransferases that can methylate hemimethylated and un-methylated CpG at the same rate [Citation47,Citation48]. The architecture of DNMT3 is similar to that of DNMT1, with a regulatory region attached to the catalytic domain. Interestingly, overexpression of DNMT3b plays an essential role in the development of endothelial cell dysfunction through increased DNA hypermethylation and subsequent suppression of the anti-atherosclerosis cellular repressor of E1A-stimulated genes [Citation49]. The mechanism underlying increased DNMT3b expression in T2D-HUVECs is currently unknown; however, we speculate it to be induced by oxidative stress. Hyperglycaemia increases an inflammatory marker nitric oxide synthase that induces increased oxidative stress and endothelial dysfunction in mothers as well as their foetuses [Citation50]. Increased oxidative stress has been previously reported in HUVECs isolated from patients with GDM [Citation51] and T2D [Citation52]. Interestingly, oxidative stress has been identified as the main cause of epigenetic changes in methylation enzymes in diabetes [Citation53]. This suggests that increased oxidative stress in T2D could facilitate activation of DNMT3b, which could eventually lead to the observed hypermethylation. Consistently, previous studies showed that overexpression of DNMT induces CpG hypermethylation in cultured cell lines [Citation54,Citation55].
HDAC1 and HDAC2 promote the regulation of the chromatin structure. HDAC1 is involved in endothelial dysfunction by reducing nitric oxide levels in endothelial cells [Citation56,Citation57]. Our study provides quantitative data on the increase in mRNA expression of the epigenetic modifier enzyme HDAC2 in GDM-HUVECs. This is supported by a study that showed that exposure to elevated glucose (25 mM) for 24 h enhanced the protein expression of the epigenetic marker HDAC2 in cardiomyocytes [Citation58]. Moreover, two genome-wide association studies have shown a linkage between the chromosomal region (6q21) of HDAC2 and type 1 and T2D, indicating its possible pathogenic role [Citation59,Citation60]. Furthermore, several studies have highlighted the effects of HDAC2 on endothelial cell dysfunction in offspring of cardiovascular diseases [Citation61]. Hyperglycaemia induces HDAC2 to promote endothelial dysfunction [Citation62,Citation63]. As shown in , increased glucose concentrations were observed in participants with GDM and T2D, indicating the pivotal role of glucose in the observed changes in isolated HUVECs. Although, we noted increased body mass index in the GDM group; however, this was not significant when compared to the healthy controls, and hence its effect was ruled out ().
The main limitation of the present study is the small sample size; however, the significant values obtained strongly confirm the validity of the findings. Large-scale studies are required to further verify the findings of this study. In addition, colorimetric assays do not provide information with regards which genes are hypomethylated or hypermethylated. Although we did not measure protein levels of epigenetic enzymes, a previous study using a different model showed that the mRNA expression of epigenetic enzymes correlated with their protein levels [Citation64].
We recommend extending this study to test various enzymes related to metabolic syndrome, endothelial dysfunction, and oxidative stress. It would also be interesting to assess hydroxy-methylation and TET enzyme levels in the future. Such a study would provide broader insight into epigenetic foetal programming caused by diabetes.
Overall, our results suggest that the aberrant expression of epigenetic modifiers HDAC2 and DNMT3b in foetal endothelial cells exposed to GDM and T2D, respectively could be involved in the observed dysregulation of global DNA methylation under normoglycemic condition. These findings support the growing notion that foetal exposure to GDM and T2D may induce persistent epigenetic changes in endothelial cells.
Acknowledgments
We are grateful to all the participants of the study and the nursing staff who helped in the sample collection.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
No large sets of sequencing or proteomic data were generated. All the data that support the findings of this study are included in the manuscript.
Additional information
Funding
References
- American Diabetes, Association. Standards of medical care in diabetes. Diabetes Care. 2014;37(Supplement 1):S14–9. doi: 10.2337/dc14-S014.
- Robert A, Asirvatham MAAD, Braham R, et al. Type 2 diabetes mellitus in Saudi Arabia: major challenges and possible solutions. Curr Diabetes Rev. 2017;13(1):59–64.
- Al-Rubeaan K, Al-Manaa HA, Khoja TA, et al. A community-based survey for different abnormal glucose metabolism among pregnant women in a random household study (Saudi-Dm). BMJ Open. 2014;4(8):e005906.
- Wahabi H, Fayed A, Esmaeil S, et al. Prevalence and complications of pregestational and gestational diabetes in Saudi Women: analysis from Riyadh mother and baby cohort study (rahma). BioMed Res Int. 2017;2017:6878263.
- Carpenter MW. Gestational diabetes, pregnancy hypertension, and late vascular disease. Diabetes Care. 2007;30(2):S246–50.
- Dabelea D, Knowler WC, Pettitt DJ. Effect of diabetes in pregnancy on offspring: follow-up research in the pima Indians. J Matern-Fetal Neonatal Med. 2000;9(1):83–88.
- Franks PW, Hanson RL, Knowler WC, et al. Childhood obesity, other cardiovascular risk factors, and premature death. N Engl J Med. 2010;362(6):485–493.
- Clausen TD, Mathiesen ER, Hansen T, et al. High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: the role of intrauterine hyperglycemia. Diabetes Care. 2008;31(2):340–346.
- Shiau S, Wang L, Liu H, et al. PrenataL gestational diabetes mellitus exposure and accelerated offspring DNA methylation age in early childhood. Epigenetics. 2021;16(2):186–195.
- Dalfrà MG, Burlina S, Giovanna Del Vescovo G, et al. Genetics and epigenetics: new insight on gestational diabetes mellitus. Front Endocrinol. 2020;11:602477.
- El-Osta A, Brasacchio D, Yao D, et al. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med. 2008;205(10):2409.
- Brasacchio D, Okabe J, Tikellis C, et al. Hyperglycemia induces a dynamic cooperativity of histone methylase and demethylase enzymes associated with gene-activating epigenetic marks that coexist on the lysine tail. Diabetes. 2009;58(5):1229–1236.
- Kouzarides T. Chromatin modifications and their function. Cell. 2007;128(4):693–705.
- Reddy MA, Natarajan R. Epigenetic mechanisms in diabetic vascular complications. Cardiovasc Res. 2011;90(3):cvr024.
- Martin-Nunez M, Rubio-Martín ERM, Cabrera-Mulero R, et al. Type 2 diabetes mellitus in relation to global line-1 DNA methylation in peripheral blood: a cohort study. Epigenetics. 2014;9(10):1322–1328.
- Hong X, Wu Z, Cao W, et al. Longitudinal association of DNA methylation with type 2 diabetes and glycemic traits: a 5-year cross-lagged twin study. Diabetes. 2022;71(12):2804–2817.
- Napoli C, Benincasa G, Schiano C, et al. Differential epigenetic factors in the prediction of cardiovascular risk in diabetic patients. Eur Heart J Cardiovasc Pharmacother. 2020;6(4):239–247.
- Benincasa G, Franzese M, Schiano C, et al. DNA methylation profiling of Cd04(+)/Cd08(+) T cells reveals pathogenic mechanisms in increasing hyperglycemia: piramide pilot study. Ann Med Surg (Lond). 2020;60:218–226.
- Prattichizzo F, Giuliani A, Ceka A, et al. Epigenetic mechanisms of endothelial dysfunction in type 2 diabetes. Clin Epigenetics. 2015;7(1):1–12.
- Jiang YZ, Manduchi E, Stoeckert CJ, et al. Arterial endothelial methylome: differential DNA methylation in athero-susceptible disturbed flow regions in vivo. BMC Genomics. 2015;16(1):1–15.
- Kuroda A, Rauch TA, Todorov I, et al. Insulin gene expression is regulated by DNA methylation. PLoS ONE. 2009;4(9):e6953.
- Reichetzeder C, Putra SD, Pfab T, et al. Increased global placental DNA methylation levels are associated with gestational diabetes. Clin Epigenetics. 2016;8(1):82.
- Zhu W, Shen Y, Liu J, et al. Epigenetic alternations of microRNAs and DNA methylation contribute to gestational diabetes mellitus. J Cell Mol Med. 2020;24(23):13899–13912.
- Martín-Núñez M, Gracia ERM, Cabrera-Mulero R, et al. Type 2 diabetes mellitus in relation to global line-1 DNA methylation in peripheral blood: a cohort study. Epigenetics. 2014;9(10):1322–1328.
- Handelsman Y, Mechanick J, Blonde L, et al. American association of clinical endocrinologists medical guidelines for clinical practice for developing a diabetes mellitus comprehensive care plan. Endocr Pract. 2011;17(2):287–302.
- Weinert LS. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy: comment to the International Association of Diabetes and Pregnancy Study Groups Consensus Panel. Diabetes Care. 2010;33(7):e97–e.
- Eccles KA, Sowden H, Porter KE, et al. Simvastatin alters human endothelial cell adhesion molecule expression and inhibits leukocyte adhesion under flow. Atherosclerosis. 2008;200(1):69–79.
- Sultan S. Aberrant expression of proatherogenic cytokines and growth factors in human umbilical vein endothelial cells from newborns of type 2 diabetic women. SAGE Open Med. 2021;9:20503121211026832.
- Pfaffl MW. Relative expression software tool (Rest(c)) for group-wise comparison and statistical analysis of relative expression results in real-time Pcr. Nucleic Acids Res. 2002;30(9):36e.
- Peng HY, Hua-Ping L, Ming-Qing L. High glucose induces dysfunction of human umbilical vein endothelial cells by upregulating Mir-137 in gestational diabetes mellitus. Microvascular Res. 2018;118:118.
- Blue EK, DiGiuseppe R, Derr-Yellin E, et al. Gestational diabetes induces alterations in the function of neonatal endothelial colony-forming cells. Pediat Res. 2014;75(2):266–272.
- Ambra R, Manca S, Palumbo MC, et al. Transcriptome analysis of human primary endothelial cells (HUVEC) from umbilical cords of gestational diabetic mothers reveals candidate sites for an epigenetic modulation of specific gene expression. Genomics. 2014;103(5–6):337–348.
- Sommese L, Zullo A, Paolo Mancini F, et al. Clinical relevance of epigenetics in the onset and management of type 2 diabetes mellitus. Epigenetics. 2017;12(6):401–415.
- Perrone L, Matrone C, Singh LP. Epigenetic modifications and potential new treatment targets in diabetic retinopathy. J Ophthalmol. 2014;2014:1–10.
- Gautier JF, Porcher R, Abi Khalil C, et al. Kidney dysfunction in adult offspring exposed in utero to type 1 diabetes is associated with alterations in genome-wide DNA methylation. PLoS ONE. 2015;10(8):e0134654.
- Alam F, Islam A, Hua Gan S, et al. DNA methylation: an epigenetic insight into type 2 diabetes mellitus. Curr Pharm Des. 2016;22(28):4398–4419.
- Simmons RA. Programming of DNA methylation in type 2 diabetes. Diabetologia. 2013;56(5):947–948.
- Nilsson E, Anders Jansson P, Perfilyev A, et al. Altered DNA methylation and differential expression of genes influencing metabolism and inflammation in adipose tissue from subjects with type 2 diabetes. Diabetes. 2014;63(9):2962–2976.
- Maghbooli Z, Larijani B, Emamgholipour S, et al. Aberrant DNA methylation patterns in diabetic nephropathy. J Diabetes Metab Disord. 2014;13:1–8.
- Chan Y, Fish JE, D’Abreo C, et al. The cell-specific expression of endothelial nitric-oxide synthase: a role for DNA methylation. J Biol Chem. 2004;279(33):35087–35100.
- Chen ZX, Riggs AD. DNA methylation and demethylation in mammals. J Biol Chem. 2011;286(21):18347–18353.
- Suzuki MM, Bird A. DNA methylation landscapes: provocative insights from epigenomics. Nat Rev Genet. 2008;9(6):465–476.
- Zhu Z, Chen X, Xiao Y, et al. GestationaL diabetes mellitus alters DNA methylation profiles in pancreas of the offspring mice. J Diabetes Complications. 2019;33(1):15–22.
- Park JH, Stoffers DA, Nicholls RD, et al. Development Of type 2 diabetes following intrauterine growth retardation in rats is associated with progressive epigenetic silencing of Pdx1. J Clin Investig. 2008;118(6):2316–2324.
- Pepin ME, Schiano C, Miceli M, et al. The human aortic endothelium undergoes dose-dependent DNA methylation in response to transient hyperglycemia. Exp Cell Res. 2021;400(2):112485.
- Howe CG, Cox B, Fore R, et al. Maternal gestational diabetes mellitus and newborn DNA methylation: findings from the pregnancy and childhood epigenetics consortium. Diabetes Care. 2020;43(1):98–105.
- Smith SS, Kaplan BE, Sowers LC, et al. Mechanism of human methyl-directed DNA methyltransferase and the fidelity of cytosine methylation. 1992;89(10):4744–4748.
- Kar S, Deb M, Sengupta D, et al. An insight into the various regulatory mechanisms modulating human DNA methyltransferase 1 stability and function. Epigenetics. 2012;7(9):994–1007.
- Liu Y, Tian X, Liu S, et al. DNA hypermethylation: a novel mechanism of creg gene suppression and atherosclerogenic endothelial dysfunction. Redox Biol. 2020;32:101444.
- Mordwinkin NM, Ouzounian JG, Yedigarova L, et al. Alteration oF endothelial function markers in women with gestational diabetes and their fetuses. J Matern-Fetal Neonatal Med. 2013;26(5):507–512.
- Sultan SA, Liu W, Peng Y, et al. The role of maternal gestational diabetes in inducing fetal endothelial dysfunction. J Cell Physiol. 2015;230(11):2695–2705.
- Sultan S. The effect of maternal type 2 diabetes on fetal endothelial gene expression and function. Acta Diabetol. 2018;56(1):1–13.
- Pinzón-Cortés JA, Perna-Chaux A, Steven Rojas-Villamizar N, et al. Effect of diabetes status and hyperglycemia on global DNA methylation and hydroxymethylation. Endocr Connect. 2017;6(8):708–725.
- Vertino PM, Yen RW, Gao J, et al. De Novo methylation of cpg island sequences in human fibroblasts overexpressing DNA (Cytosine-5-)-methyltransferase. Mol Cell Biol. 1996;16(8):4555–4565.
- Khalil H, Tazi M, Caution K, et al. Aging is associated with hypermethylation of autophagy genes in macrophages. Epigenetics. 2016;11(5):381–388.
- Lagger G, O’Carroll D, Rembold M, et al. Essential function of histone deacetylase 1 in proliferation control and cdk inhibitor repression. Embo J. 2002;21(11):2672–2681.
- Hyndman KA, Ho DH, Sega MF, et al. Histone deacetylase 1 reduces no production in endothelial cells via lysine deacetylation of no synthase 3. Am J Physiol Heart Circ Physiol. 2014;307(5):H803–9.
- Thakur V, Alcoreza N, Cazares J, et al. Changes in stress-mediated markers in a human cardiomyocyte cell line under hyperglycemia. Int J Mol Sci. 2021;22(19). DOI:10.3390/ijms221910802
- Studies ECFIG. A genomewide scan for type 1–diabetes susceptibility in Scandinavian families: identification of new loci with evidence of interactions. Am J Hum Genet. 2001;69(6):1301–1313.
- Xiang K, Wang Y, Zheng T, et al. Genome-wide search for type 2 diabetes/impaired glucose homeostasis susceptibility genes in the Chinese: significant linkage to chromosome 6q21-Q23 and chromosome 1q21-Q24. Diabetes. 2004;53(1):228–234.
- Yoon S, Hyeon Eom G. HDAC and HDAC inhibitor: from cancer to cardiovascular diseases. Chonnam Med J. 2016;52(1):1.
- Hou Q, Hu K, Liu X, et al. HADC regulates the diabetic vascular endothelial dysfunction by targetting MnSOD. Biosci Rep. 2018;38(5). DOI:10.1042/BSR20181042
- Reddy MA, Tak Park J, Natarajan R. Epigenetic modifications in the pathogenesis of diabetic nephropathy. Semin Nephrol. 2013;33(4):341–353.
- Yan W, Strawn E, Basir Z, et al. Aberrant expression of deoxyribonucleic acid methyltransferases Dnmt1, Dnmt3a, and Dnmt3b in women with endometriosis. Fertil Sterility. 2007;87(1):24–32.