Abstract
Ethylene regulates a variety of physiological processes, such as flowering, senescence, abscission, and fruit ripening. In particular, leaf expansion is also controlled by ethylene in Arabidopsis. Exogenous treatment with ethylene inhibits leaf expansion, and consistently, ethylene insensitive mutants show increased leaf area. Here, we report that the RING finger-containing E3 ubiquitin ligase HIGH EXPRESSION OF OSMOTICALLY RESPONSIVE GENES 1 (HOS1) regulates leaf expansion in an ethylene signaling pathway. The HOS1-deficient mutant showed reduced leaf area and was insensitive to ethylene perception inhibitor, silver thiosulfate (STS). Accordingly, genes encoding ethylene signaling components were significantly up-regulated in hos1-3. This study demonstrates that the HOS1 protein is involved in ethylene signal transduction for the proper regulation of leaf expansion possibly under environmentally stressful conditions.
Keywords:
Abbreviations
| CTR1 | = | CONSTITUTIVE TRIPLE RESPONSE 1 |
| EIN | = | ETHYLENE INSENSITIVE |
| ERF | = | ETHYLENE-RESPONSE FACTOR |
| ERS | = | ETHYLENE RESPONSE SENSOR |
| ESE1 | = | ETHYLENE AND SALT INDUCIBLE 1 |
| ETR | = | ETHYLENE RECEPTOR |
| HOS1 | = | HIGH EXPRESSION OF OSMOTICALLY RESPONSIVE GENES 1 |
| STS | = | silver thiosulfate |
Leaf expansion is a crucial process that not only determines the shape and size of mature leaves but also ensures its photosynthetic capacity for plant growth and development.Citation1,2 It is influenced by multiplicity of environmental factors, such as water availability and day length.Citation3,4 In addition, endogenous hormone signaling is also intensively involved in the control of leaf expansion. Promotive roles of auxin, brassinosteroid, and gibberellin in leaf expansion have been demonstrated.Citation5-7
The simple hydrocarbon ethylene is a gaseous plant hormone that regulates a variety of developmental processes, such as seed germination, cell elongation, abscission, senescence, sex determination, and fruit ripening.Citation8-13 It has been also proposed that ethylene negatively regulates leaf expansion process. Exogenous ethylene treatment results in reduced leaf area.Citation14 Furthermore, ethylene insensitive mutants, such as ethylene receptor 1 (etr1) and ethylene response sensor 1 (ers1), show increased leaf size.Citation15,16
Five receptor isoforms are responsible for ethylene perception: ETR1, ETR2, ERS1, ERS2, and ETHYLENE INSENSITIVE 4 (EIN4).Citation17 Their ethylene perception is integrated into a negative regulator of ethylene signaling, CONSTITUTIVE TRIPLE RESPONSE 1 (CTR1) Ser/Thr protein kinase,Citation18 which in turn regulates a series of transcriptional cascades in order to control ethylene-dependent physiological processes. EIN2 is a representative positive signaling regulator and subsequently activates genes encoding EIN3 and related EIN3-LIKE (EIL), which further regulate expression of ETHYLENE-RESPONSE FACTORs (ERFs).Citation19,20 In the presence of ethylene, ethylene receptors inactivate kinase activity of CTR1 so that subsequent transcriptional cascades are activated.Citation21 Although ethylene signaling pathways have been intensively investigated, its intricate networks with other signaling pathways remain to be unraveled.
The RING-type E3 ligase HOS1 was originally reported as cold signaling attenuator.Citation22 The cold-activated HOS1 protein executes protein turnover of the INDUCER OF CBF EXPRESSION1 (ICE1) MYC-like basic helix-loop-helix (bHLH) transcription factor,Citation23,24 which activates the C-REPEAT BINDING FACTOR (CBF)-COLD-REGULATED (COR) pathway,Citation23,25,26 indicating the homeostatic role in plant adaptation to long-term cold stress. In addition to its role in cold response, the HOS1 protein also mediates plant hormone signaling. For instance, HOS1 negatively regulates auxin biosynthesis in the control of hypocotyl elongation.Citation27
It has been previously reported that hos1 mutants show reduced leaf area,Citation28 although underlying molecular mechanism is elusive. Thus, we wanted to know how HOS1 regulates leaf expansion. We hypothesized that HOS1 is associated with ethylene signaling, because the hos1-3 mutant showed similar phenotypes in leaf expansion to the ctr1 mutant that exhibits constitutive ethylene responses.Citation14
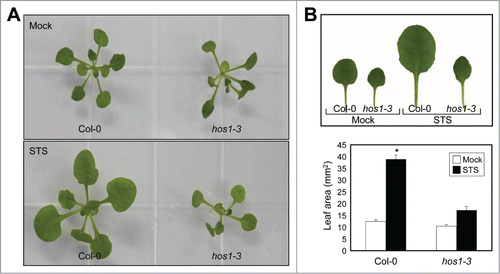
To investigate whether alterations in ethylene signaling underlie reduced leaf expansion of hos1-3, we employed a chemical inhibitor of ethylene receptors, STS. The ethylene receptors require the copper ion for high-affinity ethylene binding.Citation29 Silver can substitute for copper as a cofactor and thereby inhibit ethylene responses.Citation30 In the presence of STS, wild-type plants showed increased leaf expansion as previously reported.Citation31 However, the hos1-3 mutant was insensitive to STS and showed constitutively reduced leaf area (). Quantitative analysis revealed that wild-type leaves exhibited approximately 4-fold increase in the leaf area upon the treatment with STS, whereas the hos1-3 mutant only showed 1.5-fold increase in its leave size ().
Figure 1. The reduced leaf expansion of hos1-3 is insensitive to STS. The effects of 10 μM STS on leaf expansion were examined using Arabidopsis wild-type (Columbia-0; Col-0) and hos1-3 seedlings. Biological triplicates were averaged and statistically analyzed by Student's t-test (*P < 0.05). Bars indicate standard error of the mean.
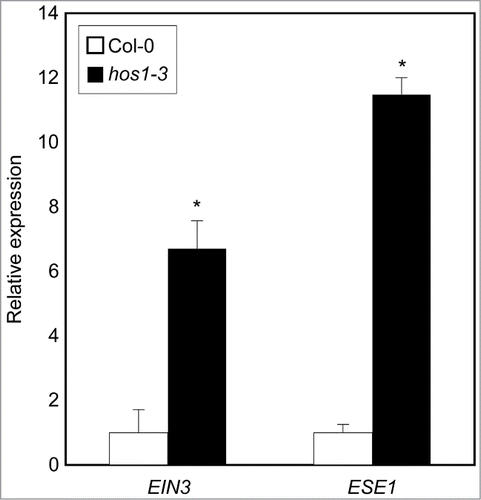
To convince the constitutive ethylene response in hos1-3, we analyzed expression of ethylene signaling genes, such as EIN3 and ETHYLENE AND SALT INDUCIBLE 1 (ESE1), in wild-type and hos1-3 seedlings. Consistent with its leaf expansion, ethylene-responsive genes were significantly upregulated in hos1-3 (), indicating that HOS1 negatively regulates ethylene signaling.
Figure 2. Ethylene signaling genes are up-regulated in hos1-3 mutant. Ten-day-old seedlings grown under long day conditions were used to analyze expression of genes encoding ethylene signaling components. Transcript accumulation was analyzed by quantitative RT-PCR (RT-qPCR). The EUKARYOTIC TRANSLATION INITIATION FACTOR 4A1 (eIF4a) gene (At3g13920) was used as an internal control. Biological triplicates were averaged and statistically analyzed by Student's t-test (*P<0.05). Bars indicate standard error of the mean.
Ethylene regulates leaf expansion largely through cell expansion.Citation15 Ethylene-insensitive ers1 mutant shows increased cell expansion in leaf epidermal cells,Citation15 whereas the ctr1 mutation leads to reduced cell expansion without substantial alterations in cell division.Citation18 Consistently, HOS1 also regulates cell expansion in the control of hypocotyl elongation,Citation27 supporting the probable role of HOS1 in ethylene-controlled cell elongation for leaf expansion process.
HOS1 is a key signaling component that regulates multiple hormone signaling pathways. We previously reported that HOS1 regulates auxin biosynthesis in the control of hypocotyl elongation.Citation27 In addition, this study provides evidence that HOS1 also plays a role in ethylene regulation of leaf expansion. Given that auxin and ethylene signaling is elaborately intertwined,Citation32-34 HOS1 might establish an important crosstalk. Auxin biosynthesis and ethylene signaling would be coordinated to fine-tune HOS1-mediated developmental processes.
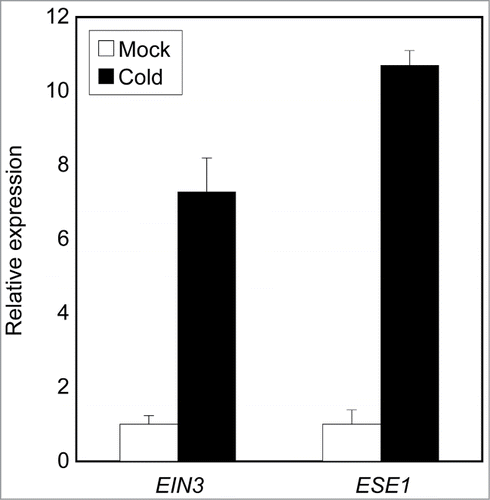
HOS1 was originally identified as cold signaling attenuator. Considering that HOS1 regulates ethylene signaling, we postulated that cold stress may stimulate ethylene signaling to control leaf expansion. Indeed, ethylene signaling was activated at low temperature (). Moreover, leaf area is also significantly reduced with lower photosynthetic activity under long-term cold conditions.Citation35 Collectively, HOS1 may underlie the processes that balance growth and environmental tolerance, ensuring plant fitness.
Figure 3. Ethylene signaling genes are induced at low temperature. Ten-day-old seedlings grown under long day conditions were exposed to cold (4°C) for 1h. Transcript accumulation was analyzed by RT-qPCR. The eIF4a gene was used as an internal control. Biological triplicates were averaged. Bars indicate standard error of the mean.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interests were disclosed.
Funding
This work was supported by the Basic Science Research (NRF-2013R1A1A1004831) and Global Research Network (NRF-2014S1A2A2028392) programs provided by the National Research Foundation of Korea and by the Next-Generation BioGreen 21 Program (PJ011192042015) provided by the Rural Development Administration. K.L. was supported by the BK21 PLUS program in the Department of Bioactive Material Sciences.
References
- Gonzalez N, Vanhaeren H, Inzé D. Leaf size control: complex coordination of cell division and expansion. Trends Plant Sci 2012; 17: 332–40; PMID:22401845; http://dx.doi.org/10.1016/j.tplants.2012.02.003
- Kalve S, De Vos D, Beemster GT. Leaf development: a cellular perspective. Front Plant Sci 2014; 5: 362; PMID:25132838; http://dx.doi.org/10.3389/fpls.2014.00362
- Qin M, Kuhn R, Moran S, Quail PH. Overexpressed phytochrome C has similar photosensory specificity to phytochrome B but a distinctive capacity to enhance primary leaf expansion. Plant J 1997; 12: 1163–72; PMID:9418054; http://dx.doi.org/10.1046/j.1365-313X.1997.12051163.x
- Tang AC, Boyer JS. Growth-induced water potentials and the growth of maize leaves. J Exp Bot 2002; 53: 489–503; PMID:11847248; http://dx.doi.org/10.1093/jexbot/53.368.489
- Keller CP, Stahlberg R, Barkawi LS, Cohen JD. Long-term inhibition by auxin of leaf blade expansion in bean and Arabidopsis. Plant Physiol 2004; 134: 1217–26; PMID:14988474; http://dx.doi.org/10.1104/pp.103.032300
- Mitchum MG, Yamaguchi S, Hanada A, Kuwahara A, Yoshioka Y, Kato T, Tabata S, Kamiya Y, Sun TP. Distinct and overlapping roles of two gibberellin 3-oxidases in Arabidopsis development. Plant J 2006; 45: 804–18; PMID:16460513; http://dx.doi.org/10.1111/j.1365-313X.2005.02642.x
- Zhiponova MK, Vanhoutte I, Boudolf V, Betti C, Dhondt S, Coppens F, Mylle E, Maes S, González-García MP, Caño-Delgado AI, et al. Brassinosteroid production and signaling differentially control cell division and expansion in the leaf. New Phytol 2013; 197: 490–502; PMID:23253334; http://dx.doi.org/10.1111/nph.12036
- Smalle J, Haegman M, Kurepa J, Van Montagu M, Straeten DV. Ethylene can stimulate Arabidopsis hypocotyl elongation in the light. Proc Natl Acad Sci USA 1997; 94: 2756–61; PMID:11038610; http://dx.doi.org/10.1073/pnas.94.6.2756
- Yamasaki S, Fujii N, Matsuura S, Mizusawa H, Takahashi H. The M locus and ethylene-controlled sex determination in andromonoecious cucumber plants. Plant Cell Physiol 2001; 42: 608–19; PMID:11427680; http://dx.doi.org/10.1093/pcp/pce076
- Butenko MA, Patterson SE, Grini PE, Stenvik GE, Amundsen SS, Mandal A, Aalen RB. Inflorescence deficient in abscission controls floral organ abscission in Arabidopsis and identifies a novel family of putative ligands in plants. Plant Cell 2003; 15: 2296–307; PMID:12972671; http://dx.doi.org/10.1105/tpc.014365
- Jing HC, Schippers JH, Hille J, Dijkwel PP. Ethylene-induced leaf senescence depends on age-related changes and OLD genes in Arabidopsis. J Exp Bot 2005; 56: 2915–23; PMID:16172137; http://dx.doi.org/10.1093/jxb/eri287
- Kevany BM, Tieman DM, Taylor MG, Cin VD, Klee HJ. Ethylene receptor degradation controls the timing of ripening in tomato fruit. Plant J 2007; 51: 458–67; PMID:17655616; http://dx.doi.org/10.1111/j.1365-313X.2007.03170.x
- Linkies A, Müller K, Morris K, Turecková V, Wenk M, Cadman CS, Corbineau F, Strnad M, Lynn JR, Finch-Savage WE, et al. Ethylene interacts with abscisic acid to regulate endosperm rupture during germination: a comparative approach using Lepidium sativum and Arabidopsis thaliana. Plant Cell 2009; 21: 3803–22; PMID:20023197; http://dx.doi.org/10.1105/tpc.109.070201
- Chao Q, Rothenberg M, Solano R, Roman G, Terzaghi W, Ecker JR. Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ETHYLENE-INSENSITIVE3 and related proteins. Cell 1997; 89: 1133–44; PMID:9215635; http://dx.doi.org/10.1016/S0092-8674(00)80300-1
- Hua J, Chang C, Sun Q, Meyerowitz EM. Ethylene insensitivity conferred by Arabidopsis ERS gene. Science 1995; 269: 1712–4; PMID:7569898; http://dx.doi.org/10.1126/science.7569898
- Tholen D, Voesenek LA, Poorter H. Ethylene insensitivity does not increase leaf area or relative growth rate in Arabidopsis, Nicotiana tabacum, and Petunia x hybrida. Plant Physiol 2004; 134: 1803–12; PMID:15064382; http://dx.doi.org/10.1104/pp.103.034389
- Guo H, Ecker JR. The ethylene signaling pathway: new insights. Curr Opin Plant Biol 2004; 7: 40–9; PMID:14732440; http://dx.doi.org/10.1016/j.pbi.2003.11.011
- Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR. CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the raf family of protein kinases. Cell 1993; 72: 427–41; PMID:8431946; http://dx.doi.org/10.1016/0092-8674(93)90119-B
- Zhu Z, Guo H. Genetic basis of ethylene perception and signal transduction in Arabidopsis. J Integr Plant Biol 2008; 50: 808–15; PMID:18713391; http://dx.doi.org/10.1111/j.1744-7909.2008.00710.x
- An F, Zhao Q, Ji Y, Li W, Jiang Z, Yu X, Zhang C, Han Y, He W, Liu Y, et al. Ethylene-induced stabilization of ETHYLENE INSENSITIVE3 and EIN3-LIKE1 is mediated by proteasomal degradation of EIN3 binding F-box 1 and 2 that requires EIN2 in Arabidopsis. Plant Cell 2010; 22: 2384–401; PMID:20647342; http://dx.doi.org/10.1105/tpc.110.076588
- Chang C, Shockey JA. The ethylene-response pathway: signal perception to gene regulation. Curr Opin Plant Biol 1999; 2: 352–8; PMID:10508761; http://dx.doi.org/10.1016/S1369-5266(99)00004-7
- Ishitani M, Xiong L, Lee H, Stevenson B, Zhu JK. HOS1, a genetic locus involved in cold-responsive gene expression in Arabidopsis. Plant Cell 1998; 10: 1151–61; PMID:9668134; http://dx.doi.org/10.1105/tpc.10.7.1151
- Chinnusamy V, Ohta M, Kanrar S, Lee BH, Hong X, Agarwal M, Zhu JK. ICE1: a regulator of cold-induced transcriptome and freezing tolerance in Arabidopsis. Genes Dev 2003; 17: 1043–54; PMID:12672693; http://dx.doi.org/10.1101/gad.1077503
- Dong CH, Agarwal M, Zhang Y, Xie Q, Zhu JK. The negative regulator of plant cold responses, HOS1, is a RING E3 ligase that mediates the ubiquitination and degradation of ICE1. Proc Natl Acad Sci USA 2006; 103: 8281–6; PMID:16702557; http://dx.doi.org/10.1073/pnas.0602874103
- Gilmour SJ, Zarka DG, Stockinger EJ, Salazar MP, Houghton JM, Thomashow MF. Low temperature regulation of the Arabidopsis CBF family of AP2 transcriptional activators as an early step in cold-induced COR gene expression. Plant J 1998; 16: 433–42; PMID:9881163; http://dx.doi.org/10.1046/j.1365-313x.1998.00310.x
- Lee H, Xiong L, Gong Z, Ishitani M, Stevenson B, Zhu JK. The Arabidopsis HOS1 gene negatively regulates cold signal transduction and encodes a RING finger protein that displays cold-regulated nucleo-cytoplasmic partitioning. Genes Dev 2001; 15: 912–24; PMID:11297514; http://dx.doi.org/10.1101/gad.866801
- Lee K, Seo PJ. The Arabidopsis E3 ubiquitin ligase HOS1 contributes to auxin biosynthesis in the control of hypocotyl elongation. Plant Growth Regul 2014; http://dx.doi.org/10.1007/s10725-014-9985-xx
- Lazaro A, Valverde F, Piñeiro M, Jarillo JA. The Arabidopsis E3 ubiquitin ligase HOS1 negatively regulates CONSTANS abundance in the photoperiodic control of flowering. Plant Cell 2012; 24: 982–99; PMID:22408073; http://dx.doi.org/10.1105/tpc.110.081885
- Rodríguez FI, Esch JJ, Hall AE, Binder BM, Schaller GE, Bleecker AB. A copper cofactor for the ethylene receptor ETR1 from Arabidopsis. Science 1999; 283: 996–8; PMID:9974395; http://dx.doi.org/10.1126/science.283.5404.996
- McDaniel BK, Binder BM. ethylene receptor 1 (etr1) is sufficient and has the predominant role in mediating inhibition of ethylene responses by silver in Arabidopsis thaliana. J Biol Chem 2012; 287: 26094–103; PMID:22692214; http://dx.doi.org/10.1074/jbc.M112.383034
- Lee SH, Reid DM. The role of endogenous ethylene in the expansion of Helianthus annuus leaves. Can J Bot 1997; 75: 501–8; PMID:11541081; http://dx.doi.org/10.1139/b97-054
- Rahman A, Hosokawa S, Oono Y, Amakawa T, Goto N, Tsurumi S. Auxin and ethylene response interactions during Arabidopsis root hair development dissected by auxin influx modulators. Plant Physiol 2002; 130: 1908–17; PMID:12481073; http://dx.doi.org/10.1104/pp.010546
- Stepanova AN, Yun J, Likhacheva AV, Alonso JM. Multilevel interactions between ethylene and auxin in Arabidopsis roots. Plant Cell 2007; 19: 2169–85; PMID:17630276; http://dx.doi.org/10.1105/tpc.107.052068
- Swarup R, Perry P, Hagenbeek D, Van Der Straeten D, Beemster GT, Sandberg G, Bhalerao R, Ljung K, Bennett MJ. Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation. Plant Cell 2007; 19: 2186–96; PMID:17630275; http://dx.doi.org/10.1105/tpc.107.052100
- Gorsuch PA, Pandey S, Atkin OK. Thermal de-acclimation: how permanent are leaf phenotypes when cold-acclimated plants experience warming? Plant Cell Environ 2010; 33: 1124–37; PMID:20199622; http://dx.doi.org/10.1111/j.1365-3040.2009.02074.x
