Abstract
Poaceae plants release phytosiderophores into the rhizosphere in order to chelate iron (Fe), which often exists in insoluble forms especially under high pH conditions. The impact of phytosiderophore treatment at the physiological and molecular levels in vivo remains largely elusive, although the biosynthesis of phytosiderophores and the transport of phytosiderophore-metal complexes have been well studied. We recently showed that the application of 30 μM of the chemically synthesized phytosiderophore 2′-deoxymugineic acid (DMA) was sufficient for apparent full recovery of otherwise considerably reduced growth of hydroponic rice seedlings at high pH. Moreover, unexpected induction of high-affinity nitrate transporter gene expression as well as nitrate reductase activity indicates that the nitrate response is linked to Fe homeostasis. These data shed light on the biological relevance of DMA not simply as a Fe chelator, but also as a trigger that promotes plant growth by reinforcing nitrate assimilation.
Abbreviations
| DMA | = | 2′-deoxymugineic acid |
| Fe | = | iron |
| NA | = | nicotianamine |
| NR | = | nitrate reductase |
| NRT2 | = | high-affinity nitrate transporter |
| SPAD | = | soil-plant analysis development |
| YSL | = | yellow stripe-like |
Iron (Fe) is the fourth most abundant element in soil, and is an essential micronutrient for plants and various other organisms. However, since the majority of Fe in the soil is in the form of insoluble Fe(III) especially under calcareous conditions, which account for approximately 30% of the arable land worldwide,Citation1 plants need to have evolved mechanisms to solubilize Fe(III), which is otherwise unavailable for assimilation. Various negative impacts of Fe-deficient conditions are represented by chlorosis,Citation2 highlighting the importance of maintaining Fe homeostasis for optimal growth and development in plants. Plants have acquired two distinct strategies for improving Fe uptake under Fe-limited conditions, namely Strategy I and Strategy II.Citation3-Citation5 The two key features of Strategy I, which operates in most non-graminaceous plant species for Fe uptake from the rhizosphere, are i) the reduction of Fe(III) to Fe(II), and ii) the selective uptake of the reduced Fe(II) into the cytoplasm via the iron transporter IRT1.Citation6-Citation10 Strategy II, on the other hand, functions only in graminaceous species and is characterized by the biosynthesis and the release of mugineic acids (MAs) including 2′-deoxymugineic acid (DMA).Citation11 Biological function of DMA has been well established as a phytosiderophore that chelates and mobilizes insoluble Fe(III). The resulting Fe-DMA complex is absorbed by, and transported through, specific YELLOW STRIPE 1-LIKE (YSL) transporters that are localized in the plasma membrane.Citation3-5,12,13
Extensive studies in the last two decades uncovered the molecular basis for the biosynthesis and exudation of MAs, and various genes encoding enzymes and transporters that are responsible for MA biosynthesis have been identified (reviewed in Kobayashi and Nishizawa5). The positive effect of MAs as phytosiderophores upon growth of Poaceae plants under calcareous conditions has been appreciated since their discovery.Citation14,15 For example, rice plants overexpressing MA biosynthetic genes show tolerance to Fe-limited conditions.Citation16,17 These in vivo evidences show that enhanced availability of MAs leads to improved Fe uptake under high pH conditions. However, since the prime focus on the effect of MAs in previous studies has been the enhancement of Fe uptake rather than growth recovery, it has not been clearly demonstrated whether the acquired ‘tolerance’ of transgenic rice lines overexpressing MA biosynthesis–related genes to Fe-deficiency was sufficient to restore the growth of rice plants to the level of plants grown under normal conditions. Furthermore, it has only been poorly discussed how the growth improvement of those plants was achieved at the physiological and molecular levels and what are the limiting factors, if any, for plants producing increased levels of MAs when the plant growth is not significantly improved.
In order to address above issues, a method for the chemical synthesis of DMA from tert-butoxycarbonyl-L-allylglycine was developed and this allowed us to unravel novel biological roles that phytosiderophores play.Citation18,19 The results indicate that the application of 3 to 30 μM DMA to rice seedlings for two weeks under Fe-deficient condition (pH 8.0) led to almost complete recovery of growth of rice seedlings compared to those grown under normal conditions with respect to shoot height and soil-plant analysis development (SPAD) values.Citation18 This type of approach has been essentially unavailable, since the only source of MAs has been the extraction of MAs from root exudates, which is not easily scalable to obtain enough amounts of MAs for feeding studies.
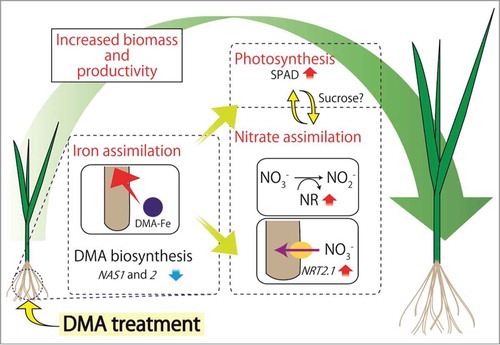
Gene expression analysis showed that the recovered growth of rice seedlings was accompanied by selective uptake of Fe, prompt translocation of Fe throughout the plant body, and an unexpected increase in the expression of NITRATE REDUCTASE gene (NIA) and HIGH-AFFINITY NITRATE TRANSPORTER 2.1 (NRT2.1) as well as nitrate reductase (NR) activity under high pH conditions, despite the preference of rice for ammonium over nitrate.Citation18 The expression of NRT2.1 has been shown to be induced by nitrate after nitrogen starvation treatment or sucrose.Citation20 Since nitrogen level did not change considerably in our experimental conditions, the increased expression of NRT2.1 might be caused by increased level of sucrose. On the other hand, the increase in SPAD values and Fe assimilation by DMA treatment supports the view that DMA treatment promotes photosynthetic activity that leads to the increased rate of synthesis of sucrose and other photosynthates ().Citation18 These collectively demonstrate that DMA links the processes of Fe and nitrate assimilations, both of which are required for maximal growth under given soil conditions. Moreover, the biological role that DMA plays in maximizing the growth of rice seedlings seems to be fairly substantial regardless of pH or Fe status, since the expression of NIA, NRT2.1 or other nitrate transporter genes was increased by DMA supplementation even at normal pH.18
Figure 1. Supplementation with DMA significantly promotes the growth of rice seedlings. Fe that is assimilated in root as a complex with DMA is readily transported to aerial parts and contributes to the increase in SPAD values. Exogenous DMA supplementation contributes to increased levels of Fe and SPAD values in rice tissues through up-regulation of Fe assimilation. Because of sufficient levels of Fe within rice tissues, the treatment consequently triggers down-regulation of Fe assimilation including NAS1 and 2. While the treatment triggers down-regulation of Fe assimilation-related genes NAS1 and 2 as a consequence of the sufficient levels of Fe in rice tissues, the increased availability of Fe allows rice seedlings to maximize the rate of photosynthesis as well as nitrate assimilation by up-regulating NRT2 and other nitrate assimilation-related genes as well as NR activity that all collectively contribute to increased biomass and productivity of rice plants.
It is noteworthy that the addition of EDTA only partially mimics the positive effect of DMA on growth of rice seedlings at the physiological and molecular levels. For example, supplementation with DMA, but not EDTA, down-regulates the expression of Fe assimilation-related genes YSL15 and NICOTIANAMINE SYNTHASE (NAS), which would otherwise be upregulated under high pH conditions. Additionally, compared to EDTA, DMA substantially enhanced the transport of55Fe in the medium to the shoot was observed only when DMA was applied. These results are in accordance with the previous notions that Fe needs to be presented as proper forms in appropriate tissues through the integral use of various Fe transporters and multiple ligands.Citation21
Plants secrete up to 40% of photosynthate such as polysaccharides and organic acids in establishing optimal growth conditions in the rhizosphere.Citation22 Therefore, exogenous supplementation with DMA should minimize the amount of photosynthate as well as nitrogen required for de novo biosynthesis of DMA starting from the methionine cycle at the expense of ATP.1 In addition, induced nitrate uptake processes triggered by exogenous DMA application should also help plants promote the overall metabolic capacity that leads to growth enhancement. For the above reasons, supplementation with DMA seems to be feasible for maximizing the growth of Poaceae plants, particularly in the early stages of seedling development, when all the leaves are still developing as sink organs. This might be especially the case when plants are under Fe-limited conditions caused by calcareous soil, thus cannot fully perform photosynthesis. In addition, supplementation of exogenous DMA is advantageous over transgenic approaches in that it is readily applicable to a wide variety of Poaceae plants without a need, for example, to establish elite transgenic lines. Indeed, the potential uses of phytosiderophores and bacterial siderophores for improved management of crops and environment has been discussed, ranging from growth and pathogen bio-control of plants to bioremediation and other environmental processes.Citation23 Interestingly, nitrate supplementation to Arabidopsis seedlings grown under nitrate-deficient conditions triggers increased expression of genes involved in nitrate assimilation processes as well as those related to various other metabolic processes including NAS1 and NAS2.Citation24 This also implicates that nitrate response is linked to Fe homeostasis. Therefore, it is likely that the processes for Fe and nitrate assimilation coordinately support the optimal growth under given nutritional condition, so that various developmental and physiological processes that require both nitrate and Fe would be operated properly. Notably, our data show that DMA application significantly increases the growth of rice seedlings not only at pH 8.0 but also at pH 5.8.Citation18 These data indicate that Fe, together with nitrate, is a key factor that limits the plant growth, and in turn, suggest that a combined use of DMA with nitrogen fertilizers helps maximizing the growth potential of plants. Extensive studies have demonstrated the physiological interplays among various micro-nutrients as well as macro-nutrients and elucidated the molecular mechanisms behind. In contrast, not much is known whether the regulatory processes of assimilation and metabolism across micro- and macro-nutrients exist. Further studies with the use of chemically synthesized DMA should allow dissecting the regulatory mechanism that coordinates the physiological link between Fe and nitrate assimilation.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported in part by JSPS Grant-in-Aid for Scientific Research Grant Number 21310148 to YM.
References
- Mori S. Iron acquisition by plants. Curr Opin Plant Biol 1999; 2:250-53; PMID:10375565; http://dx.doi.org/10.1016/S1369-5266(99)80043-0
- Ma JF, Nomoto K. Effective regulation of iron acquisition in graminaceous plants. The role of mugineic acids as phytosiderophores. Physiol Plant 1996; 97:609-17; http://dx.doi.org/10.1111/j.1399-3054.1996.tb00522.x
- Conte SS, Walker EL. Transporters contributing to iron trafficking in plants. Mol Plant 2011; 4:464-76; PMID:21447758; http://dx.doi.org/10.1093/mp/ssr015
- Curie C, Briat JF. Iron transport and signaling in plants. Annu Rev Plant Biol 2003; 54:183-206; PMID:14509968; http://dx.doi.org/10.1146/annurev.arplant.54.031902.135018
- Kobayashi T, Nishizawa NK. Iron uptake, translocation, and regulation in higher plants. Annu Rev Plant Biol 2012; 63:131-52; PMID:22404471; http://dx.doi.org/10.1146/annurev-arplant-042811-105522
- Chaney RL, Brown JC, Tiffin LO. Obligatory reduction of ferric chelates in iron uptake by soybeans. Plant Physiol 1972; 50:208-13; PMID:16658143; http://dx.doi.org/10.1104/pp.50.2.208
- Connolly EL, Fett JP, Guerinot ML. Expression of the IRT1 metal transporter is controlled by metals at the levels of transcript and protein accumulation. Plant Cell 2002; 14:1347-57; PMID:12084831; http://dx.doi.org/10.1105/tpc.001263
- Korshunova Y, Eide D, Gregg Clark W, Lou Guerinot M, Pakrasi H. The IRT1 protein from Arabidopsis thaliana is a metal transporter with a broad substrate range. Plant Mol Biol 1999; 40:37-44; PMID:10394943; http://dx.doi.org/10.1023/A:1026438615520
- Römheld V, Marschner H. Mechanism of iron uptake by peanut plants: I. FeIII reduction, chelate splitting, and release of phenolics. Plant Physiol 1983; 71:949-54; PMID:16662934; http://dx.doi.org/10.1104/pp.71.4.949
- Vert G, Grotz N, Dédaldéchamp F, Gaymard F, Guerinot ML, Briat JF, Curie C. IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell 2002; 14:1223-33; PMID:12084823; http://dx.doi.org/10.1105/tpc.001388
- Römheld V, Marschner H. Evidence for a specific uptake system for iron phytosiderophores in roots of grasses. Plant Physiol 1986; 80:175-80; PMID:16664577; http://dx.doi.org/10.1104/pp.80.1.175
- Curie C, Cassin G, Couch D, Divol F, Higuchi K, Le Jean M, Misson J, Schikora A, Czernic P, Mari S. Metal movement within the plant: contribution of nicotianamine and yellow stripe 1-like transporters. Ann Bot 2009; 103:1-11; PMID:18977764; http://dx.doi.org/10.1093/aob/mcn207
- Curie C, Panaviene Z, Loulergue C, Dellaporta SL, Briat JF, Walker EL. Maize yellow stripe1 encodes a membrane protein directly involved in Fe(III) uptake. Nature 2001; 409:346-49; PMID:11201743; http://dx.doi.org/10.1038/35053080
- Takagi S. Naturally occurring iron-chelating compounds in oat- and rice-root washings. I. Activity measurement and preliminary characterization. Soil Sci Plant Nutr 1976:423-33; http://dx.doi.org/10.1080/00380768.1976.10433004
- Takemoto T, Nomoto K, Fushiya S, Ouchi R, Kusano G, Hikino H, et al. Structure of mugineic acid, a new amino acid possessing an iron-chelating activity from roots washings of water-cultured Hordeum vulgare L. Proc Japan Acad 1978; 54:469-73; http://dx.doi.org/10.2183/pjab.54.469
- Takahashi M, Nakanishi H, Kawasaki S, Nishizawa NK, Mori S. Enhanced tolerance of rice to low iron availability in alkaline soils using barley nicotianamine aminotransferase genes. Nat Biotech 2001; 19:466-69; PMID:11329018; http://dx.doi.org/10.1038/88143
- Suzuki M, Morikawa KC, Nakanishi H, Takahashi M, Saigusa M, Mori S, et al. Transgenic rice lines that include barley genes have increased tolerance to low iron availability in a calcareous paddy soil. Soil Sci Plant Nutr 2008; 54:77-85; http://dx.doi.org/10.1111/j.1747-0765.2007.00205.x
- Araki R, Kousaka K, Namba K, Murata Y, Murata J. 2′-Deoxymugineic acid promotes growth of rice (Oryza sativa L.) by orchestrating iron and nitrate uptake processes under high pH conditions. Plant J 2015; 81:233-46; PMID:25393516; http://dx.doi.org/10.1111/tpj.12722
- Namba K, Murata Y, Horikawa M, Iwashita T, Kusumoto S. A practical synthesis of the phytosiderophore 2′-deoxymugineic acid: a key to the mechanistic study of iron acquisition by graminaceous plants. Angew Chem Int Ed 2007; 46:7060-63; PMID:17691091; http://dx.doi.org/10.1002/anie.200702403
- Feng H, Yan M, Fan X, Li B, Shen Q, Miller AJ, Xu G. Spatial expression and regulation of rice high-affinity nitrate transporters by nitrogen and carbon status. J Exp Bot 2011; 62:2319-32; PMID:21220781; http://dx.doi.org/10.1093/jxb/erq403
- Kobayashi T, Nishizawa NK. Iron sensors and signals in response to iron deficiency. Plant Sci 2014; 224:36-43; PMID:24908504; http://dx.doi.org/10.1016/j.plantsci.2014.04.002
- Berendsen RL, Pieterse CMJ, Bakker PAHM. The rhizosphere microbiome and plant health. Trend Plant Sci 2012; 17:478-86; http://dx.doi.org/10.1016/j.tplants.2012.04.001
- Ahmed E, Holmström SJM. Siderophores in environmental research: roles and applications. Microb Biotechnol 2014; 7:196-208; PMID:24576157; http://dx.doi.org/10.1111/1751-7915.12117
- Wang R, Okamoto M, Xing X, Crawford NM. Microarray analysis of the nitrate response in Arabidopsis roots and shoots reveals over 1000 rapidly responding genes and new linkages to glucose, trehalose-6-phosphate, iron, and sulfate metabolism. Plant Physiol 2003; 132:556-67; PMID:12805587; http://dx.doi.org/10.1104/pp.103.021253
