ABSTRACT
By regulating the levels of nitric oxide (NO) in a cell and tissue specific fashion, Phytoglobins (Pgbs), plant hemoglobin-like proteins, interfere with many NO-mediated pathways participating in developmental and stress-related responses. Recent evidence reveals that one of the functions of Pgbs is to protect the root apical meristem (RAM) from stress conditions by retaining the viability and function of the quiescent center (QC), required to maintain the stem cells in an undifferentiated state and ensure proper tissue patterning and root viability. Based on this and other evidence, it is suggested that Pgbs regulate cell fate by modulating NO homeostasis.
Strategies for coping with adverse environmental conditions can be broadly classified as: acclimation involving metabolic or molecular adjustments to tolerate and survive through defined periods of stress; or, avoidance where plants circumvent the stress.Citation1 Regardless of the strategy employed, survival is often associated with the ability of the plant to circumscribe a stress response in defined cells, tissues and/or organs, by altering their developmental behaviour and fate. Localized stress-induced behavioural changes have been documented during both abiotic and biotic interactions. Well characterized examples include the formation of aerenchyma from the execution of programmed cell death (PCD) in adjacent cortical cells of waterlogged plantsCitation2 or the generation of necrotic tissue around the infection sites during the hypersensitive response in plant pathogen interaction.Citation3
If the dismantling and elimination of specific cells by death is needed for the well-being of the organism, survival is ultimately dependent on the ability of plants to ensure that sensitive, meristematic regions do not succumb to the stress. For example, the prevention of death in root meristematic cells ensures root growth and enhances plant performance under conditions of limitedCitation4 and excessiveCitation5,Citation6 moisture. Furthermore, cells destined to die are often in close proximity to those that need to remain viable, as shown in corn where PCD-targeted cells forming aerenchyma are separated by only a few layers of cells from the apical meristem.Citation5,Citation6 Thus a thin boundary separates the death/life fate. A fundamental question in plant biology is how this boundary is regulated and which molecular mechanisms need to be activated (or de-activated) to modulate this opposite cellular behaviour. A common denominator influencing the life/death decision is nitric oxide (NO), a ubiquitous signal molecule operating in diverse responses.Citation3 It acts as a secondary messenger producing selective local and/or systemic responses to regulate plant metabolism and modify anatomical features during different developmental stages or in response to biotic or abiotic stresses.Citation6 Alterations in NO homeostasis are often sufficient to influence the behaviour of a cell and ultimately its fate.Citation7
Among several factors modulating cellular NO levels, and thus cell behaviour, are phytoglobins (Pgbs), well characterized stress-related proteins with effective NO-scavenging properties.Citation8 It has been suggested that Pgbs might act as universal molecular switches governing cell fate through NO.Citation9 A recent paperCitation4 provides evidence to suggest that the expression of Pgb during environmental stress may have a profound influence on how meristematic regions respond to the stress.
The root apical meristem: keeping meristematic cells functional
Plant meristems guide and support continuous growth and development as they consist of a reservoir of mitotically active pluripotent stem cells generating all tissues and organs of the organism. The regulation of “dynamic homeostasis”, that is, the balance between division of the stem cells and differentiation of their derivatives, is very susceptible to environmental perturbations and must be maintained to ensure growth. Examples of stress factors altering this balance and compromising plant performance include salinity, drought, and excess moisture. These types of stress have pronounced effects on the root apical meristem (RAM), which is directly exposed to the soil. The structure of the RAM is elaborated around a quiescent center (QC) composed of mitotically inactive cells with the primary functions to 1) maintain the surrounding stem (initial) cells in an undifferentiated state, and 2) act as a reservoir for the short-lived adjacent stem cellsCitation10 (). Following asymmetric divisions, the stem cells produce two cells types: one retaining a stem fate in proximity of the QC, and the other undergoing several rounds of divisions generating derivatives which are displaced away from the QC. Ablation of one or more quiescent cell results in the precocious differentiation of the adjacent stem cell.Citation10 The unique role of the QC as an “organizing center” of meristem function is governed by the PIN-mediated establishment of an auxin maximum in the stem cell nicheCitation11,Citation12 required for the activation of QC-specific markers, such as WOX5.Citation13 Perturbations of the apical-basal gradient of auxin affect QC functionCitation14 ().
Figure 1. Schematic representation of Phytoglobin (Pgb) in the root apical meristem (RAM) conditions of stress, The function of the RAM relies on the PIN-mediated basipetal flow of auxin specifying the quiescent center (QC), which suppresses the differentiation of the surrounding stem cells. Conditions of stress produce NO which destabilizes the auxin flow and the viability of the QC resulting in the precocious differentiation of the stem cells leading to root growth arrest. Over-accumulation of NO also results in ROS-mediated cell death. By scavenging NO, Pgbs retain QC and meristem function during conditions of stress.
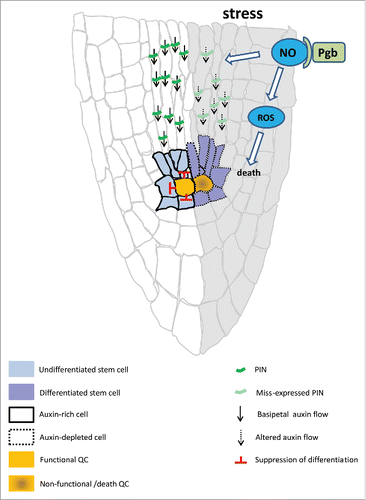
Factors influencing meristem behaviour and ultimately its viability involve reactive oxygen species (ROS) and NO, the levels of which are known to increase during conditions of stress. While triggering death mechanisms when over-accumulating, ROS have been associated with the regulation of division-differentiation patterns at the root meristem, thus affecting the size and functionality of the RAM.Citation15 This observation is consistent with the emerging function of ROS in the maintenance of animal stem cells.Citation16 Like ROS, NO accumulates at the root tip under adverse conditions where it reduces the auxin maximum in the stem cell niche through PIN-1 mediated mechanisms leading to severe defects in the RAMCitation17 and reduced size of the meristem.Citation18 Evidence below supports the notion that through their cell-specific expression pattern, Pgbs modulate NO and ROS levels and maintain meristem functionality under conditions of stress.
Cell-specific phytoglobin expression during plant development
While it is probable that Pgbs have metabolic roles in plant metabolism, there is increasing evidence that they have distinct functions in plant development. As NO scavengers, Pgbs have the capacity to interrupt the action of NO and manage plant developmental and stress responses in which NO is a component of the signal transduction pathway orchestrating hormone responses and PCD.Citation4,Citation5,Citation19,Citation20,Citation21,Citation22 More importantly, the expression of Pgbs can be cell-specific, allowing cells adjacent to one another to respond differently to a given perturbation.
During in vivo development, Pgbs are mainly expressed in juvenile tissue such as the apical meristems and in emerging root primordia,Citation23 while it is absent or expressed at low levels in more mature domains. This expression pattern, by itself, indicates the likelihood of an involvement of the protein in developmental processes and a possible function in protecting meristematic cells, which are the most susceptible to environmental perturbations.
The selective effect of Pgb is best exemplified during embryo development in culture. The spatio-temporal expression of Pgbs in Zea mays modulates NO resulting in the selective elimination of specific cells by PCD. Two maize Pgbs, ZmPgb1.1 and ZmPgb1.2, regulate the cell fate decision through their specific localization patterns. The repression of ZmPgb1.2 “localized in the anchoring cells between the embryos” allows the embryos to separate and develop further, whereas repression of ZmPgb1.1 “localized in several embryonic cells” results in massive PCD leading to embryo abortion.Citation24 Cell and tissue specificity of Pgbs has also been reported in dicotyledonous plants where they regulate developmental processes.Citation19,Citation21,Citation25
Suppression of an Arabidopsis Pgb gene, AtPgb2, that is normally expressed at the base of the cotyledons of zygotic embryos during the induction phase of somatic embryogenesis, increases auxin and its distribution due to repression of MYC2, a condition enhancing somatic embryo production.Citation20 Accumulation of jasmonic acid as a result of the elevated NO following suppression of Pgbs is also implicated in the suppression of MYC2.Citation19 Note the distinction and the different regulatory mechanisms controlled by Pgbs during somatic embryogenesis in monocots (corn) and dicots (Arabidopsis). In addition, while dicots have three types (Class 1–3) of Pgbs, monocots only express Class 1 and 3 Pgbs.
Root acclimation to, or avoidance of, abiotic stress is crucial in determining the survival of the whole plant.Citation26 Under hypoxia, the cortical cells of fully differentiated tissues die and subsequently form aerenchyma, a process involving PCD that facilitates gas movement within the hypoxic root.Citation27 No change in Pgb expression has been observed in the maize root cortical cells during aerenchyma formation.Citation28 consistent with what would be expected in a process involving PCD. Programmed cell death is averted in the RAMs exposed to severe hypoxia or drought stress conditions where Pgb expression is up-regulatedCitation4,Citation5 enabling root viability and survival to the stress (). This cell-specific Pgb action is mediated by NO levels, ethylene biosynthesis, and ROS accumulation.Citation4,Citation5,Citation9 Thus, fluctuations in Pgbs affect cell fate and ultimately the death/live decision.
Phytoglobins have also been implicated in biotic stress responses. The coincident action of NO and ROS to induce PCD has been found to regulate necrotic lesion formation during the hypersensitive response (HR)Citation29 restricting pathogen spread.Citation30 Pgbs have been implicated in a number of plant responses to pathogensCitation31,Citation32 with the effect of the expression on NO and ROS being foremost in the mechanism of the response.
Phytoglobins act as molecular switches that control NO homeostasis
Well known as oxygen carriers and NO scavengers, Pgbs are expressed in various plant tissues/organs and can be induced by different external stimuli.Citation8 These unique molecules are present at different stages of plant development, such as embryogenesis,Citation20 seed germination,Citation33 de novo organogenesis,Citation34 root differentiation and flower development.Citation22 Induced by abiotic stress conditions such as hypoxia, cold and heat, Pgbs interfere with hormone signaling pathways actively and trigger a multitude of cellular phenotypes via the refinement of NO signaling.Citation9 The observation that there are as many as five Pgb genes, as well as a truncated form that codes for a protein that essentially performs the same chemical reactions, suggests that the protein can potentially be strategically expressed to switch on and off distinct NO-mediated functions in specific plant tissues.
In rice, for instance, Pgbs are expressed in both vegetative and reproductive organs. In a scan of the Rice Genome Annotation Database, the five class 1 rice Pgbs showed organ-specific expression.Citation35 OsPgbs were preferentially localized in the ovary, embryo, roots, reproductive stems and in response to stress. However, within the reproductive tissue, OsPgb1.5 expression could only be found in the lemma, while OsPgb1.3 was expressed in the palea. Cell and tissue specific localization of Pgbs was also documented in ArabidopsisCitation20,Citation22 and maize.Citation24 In the same studies it was demonstrated that domains expressing Pgbs were generally depleted of NO while those suppressing Pgbs accumulated NO. In addition, the unique NO accumulation pattern, modulated by Pgbs, influenced cell fate and triggered specific morphogenic and developmental events.
Managing cellular NO is crucial not only during plant development, but also in response to stress responses. For example, under abiotic stress conditions, the protective action of Pgb1 through NO removal can be achieved at different scales, ranging from the whole plant level, to organ-specific or cell-specific levels.Citation6,Citation36 However, organ-specific or cell-specific NO scavenging by Pgb1 seems to be the first wave of stress alleviation. For instance, introduction of spinach Pgb1 into Arabidopsis enhances salt and osmotic tolerance by reducing stress-generated cellular NO in the root.Citation37 Predominant expression of Pgb1 in the protoderm and cortical cells has also been shown to positively correlate with the level of flooding-tolerance in oak roots, likely through NO degradation.Citation38 Similarly, expression of AtPgb1 in the RAM alleviates Arabidopsis root growth retardation during PEG-induced water deficit.Citation4 Scavenging of NO by Pgbs also minimizes cellular ROS and ethylene that can accumulate in the RAM as a result of the stress. Elevated levels of ROS and ethylene induce PCD and disrupt the function of the QC, causing the precocious differentiation of the stem cellsCitation4 (see also ).
Phytoglobins may act as early positional regulators in cell behaviour
The global behaviour of a plant is a comprehensive integration of hormone-mediated local or cellular activities, such as cell division, differentiation or death.Citation39 NO-hormone interactions are one of the most prominent features of these cellular activities in developmental processes and environmental responses.Citation40,Citation41 In addition to its involvement in PCD, NO has a key role in hormonal cross-talk.Citation7 It is more and more evident that the site-specific expression of Pgbs is one of the ingenious mechanisms employed to ensure the fine tuning of this crosstalk, through regulation of cellular NO levels, to achieve definitive responses in specific cell types.Citation9,Citation24 Formation and maintenance of the RAM is likely the best example of a homeostatic balancing between cell division and differentiation controlled by hormones such as auxin, cytokinin and ethyleneCitation41 which can be modulated by Pgbs.
Auxin is directly implicated in root cell and tissue patterning by specifying the identity of the epidermis, ground tissue precursors, and vascular stem cell initials during early embryogenesis.Citation42 A unique auxin response, independent of SHORTROOT and mediated by MONOPTEROS, has been recently identified as the first step in establishing ground tissue in Arabidopsis embryos.Citation43 Within the ground tissue, SCARECROW regulates asymmetric cell divisions, and contributes to endodermis and cortex specification.Citation44 The PIN-mediated basipetal flow of auxin and its accumulation at the root tip is also a prerequisite for the formation and specification of the “stem niche” in both conifers and angiosperms.Citation7,Citation45 The maintenance of the QC by auxin is mediated by WOX5Citation46 and SCARECROWCitation47 which regulate the rate of progeny cell differentiation in the transition zone by repressing ARR1.Citation48,Citation49 In addition, the establishment of an auxin minimum that positions the boundary between dividing and differentiating cells has been suggested to act as the trigger in controlling meristem size.Citation50 Thus minute perturbations in auxin synthesis and transport compromise the function of the RAM. By managing NO levels, Pgbs influence PIN localization and auxin transportCitation4,Citation20 and ultimately the function of the root meristem. Severe drought stress alters the PIN-flow of auxin at the RAM, thus compromising the function of the QC, which miss-express WOX5, inducing the precocious differentiation of the surrounding stem cells (). Through their NO scavenging properties, Pgbs are needed to retain a normal flow of auxin and protect the QC, thus maintaining a functional RAM under conditions of water stress. The suppression of NO by Pgbs is also required to limit the over-accumulation of ROS, leading to cell death ().
The Pgb protection of meristematic cells through regulation of NO homeostasis could potentially apply to any stress conditions threatening the meristems. Hypoxic root meristems lose their functionality as a result of an over-accumulation of NO at the root tip increasing production of ethylene, an inhibitor of meristematic cell proliferationCitation51 and inducer of cell death through ROS.Citation6 By scavenging NO, Pgbs repress these events allowing hypoxic roots to survive and grow.Citation5
Interaction between NO and hormones also influences the behaviour of cells in the aleurone layer, which is controlled by the balance between gibberellins, triggering PCD, and abscisic acid suppressing PCD.Citation52 The crosstalk between these two hormones with NO has been well established in many fundamental processes, including the execution of death in the aleurone cellsCitation53 where Pgb1 is transcriptionally induced under oxygen depletion.Citation54 Interestingly, the GA-elicited promotion of PCD in the aleurone layer is mediated by ROS, the levels of which are also influenced by the expression of Pgbs.Citation24
In conclusion, it is evident that by managing NO in a cell and tissue specific fashion, Pgbs interfere with NO-mediated pathways. Because of the considerable involvement of NO in hormone signal transduction pathways and in the initiation of cell death processes, Pgb expression can have a profound influence on plant cell development and on the response of plants to stress. The function of Pgbs might be particularly relevant in juvenile and meristematic tissues, where the proteins are preferentially present, and in response to adverse environmental conditions compromising meristem viability. Compelling evidence reveals that a key function of Pgbs might be to protect the root meristems from stress conditions, by retaining the QC and stem cells in a functional state, thus ensuring proper tissue patterning and root growth.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Chaves MM, Pereira JS, Maroco J, Rodrigues ML, Ricardo CP, Osório ML, Carvalho I, Faria T, Pinheiro C. How plants cope with water stress in the field. Photosynthesis and growth. Ann Bot. 2002;89:907–16. doi:10.1093/aob/mcf105.
- Bailey-Serres J, Voesenek LA. Flooding stress: acclimations and genetic diversity. Annu Rev Plant Biol. 2008;59:313–39. doi:10.1146/annurev.arplant.59.032607.092752.
- Wendehenne D, Durner J, Klessig DF. Nitric oxide: a new player in plant signalling and defence responses. Curr Opin Plant Biol. 2004;7:449–55. doi:10.1016/j.pbi.2004.04.002.
- Mira MM, Huang S, Kapoor K, Hammond C, Hill RD, Stasolla C. Expression of Arabidopsis class 1 phytoglobin (AtPgb1) delays death and degradation of the root apical meristem during severe PEG-induced water deficit. J Exp Bot. 2017;68:5653–68. doi:10.1093/jxb/erx371.
- Mira MM, Hill RD, Stasolla C. Phytoglobins improve hypoxic root growth by alleviating apical meristem cell death. Plant Physiol. 2016;172:2044–56. doi:10.1104/pp.16.01150.
- Mira MM, El-Khateeb EA, SayedAhmed HI, Hill RD, Stasolla C. Are avoidance and acclimation responses during hypoxic stress modulated by distinct cell-specific mechanisms? Plant Signal Behav. 2017;12:e1273304. doi:10.1080/15592324.2016.1273304.
- Sanz L, Albertos P, Mateos I, Sánchez-Vicente I, Lechón T, Fernández-Marcos M, Lorenzo O. Nitric oxide (NO) and phytohormones crosstalk during early plant development. J Exp Bot. 2015;66:2857–68. doi:10.1093/jxb/erv213.
- Hill RD. Non-symbiotic hemoglobins-What's happening beyond nitric oxide scavenging? AoB Plants. 2012;2012:pls004. doi:10.1093/aobpla/pls004.
- Stasolla C, Hill RD. Determining cellular responses: Phytoglobins may direct the traffic. Trends Plant Sci. 2017;22:820–22. doi:10.1016/j.tplants.2017.08.002.
- van den Berg C, Willemsen V, Hendriks G, Weisbeek P, Scheres B. Short-range control of cell differentiation in the Arabidopsis root meristem. Nature. 1997;390:287–89. doi:10.1038/36856.
- Grafi G, Florentin A, Ransbotyn V, Morgenstern Y. The stem cell state in plant development and in response to stress. Front Plant Sci. 2011;2–53. doi:10.3389/fpls.2011.00053.
- Lee Y, Lee WS, Kim SH. Hormonal regulation of stem cell maintenance in roots. J Exp Bot. 2013;64:1153–65. doi:10.1093/jxb/ers331.
- Kong X, Lu S, Tian H, Ding Z. WOX5 is shining in the root stem cell niche. Trends Plant Sci. 2015;20:601–3. doi:10.1016/j.tplants.2015.08.009.
- Friml J, Vieten A, Sauer M, Weijers D, Schwarz H, Hamann T, Offringa R, Jürgens G. Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature. 2003;426:147–53. doi:10.1038/nature02085.
- Tsukagoshi H, Busch W, Benfey PN. Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell. 2010;143:606–16. doi:10.1016/j.cell.2010.10.020.
- Bigarella CL, Liang R, Ghaffari S. Stem cells and the impact of ROS signaling. Development. 2014;141:4206–18. doi:10.1242/dev.107086.
- Fernández-Marcos M, Sanz L, Lewis DR, Muday GK, Lorenzo O. Nitric oxide causes root apical meristem defects and growth inhibition while reducing PIN-FORMED 1 (PIN1)-dependent acropetal auxin transport. Proc Natl Acad Sci USA. 2011;108:18506–11. doi:10.1073/pnas.1108644108.
- Liu W, Li RJ, Han TT, Cai W, Fu ZW, Lu YT. Salt stress reduces root meristem size by nitric oxide-mediated modulation of auxin accumulation and signaling in Arabidopsis. Plant Physiol. 2015;168:343–56. doi:10.1104/pp.15.00030.
- Mira MM, Wally OSD, Elhiti M, El-Shanshory A, Reddy DS, Hill RD, Stasolla C. Jasmonic acid is a downstream component in the modulation of somatic embryogenesis by Arabidopsis class 2 phytoglobin. J Exp Bot. 2016;67:22–31. doi:10.1093/jxb/erw022.
- Elhiti M, Hebelstrup KH, Wang A, Li C, Cui Y, Hill RD, Stasolla C. Function of the type-2 Arabidopsis hemoglobin in the auxin-mediated formation of embryogenic cells during morphogenesis. Plant J. 2013;74:946–58. doi:10.1111/tpj.12181.
- Mira M, Hill RD, Stasolla C. Regulation of programmed cell death by phytoglobins. J Exp Bot. 2016;67:5901–20. doi:10.1093/jxb/erw259.
- Wally OS, Mira MM, Hill RD, Stasolla C. Hemoglobin regulation of plant embryogenesis and plant pathogen interaction. Plant Signal and Behav. 2013; 8:e25264. doi:10.4161/psb.25264.
- Hebelstrup KH, Jensen EO. Expression of NO scavenging hemoglobin is involved in the timing of bolting in Arabidopsis thaliana. Planta. 2008;227:917–27. doi:10.1007/s00425-007-0667-z.
- Huang S, Hill RD, Wally OS, Dionisio G, Ayele BT, Jami SK, Stasolla C. Hemoglobin control of cell survival/death decision regulates in vitro plant embryogenesis. Plant Physiol. 2014;165:810–25. doi:10.1104/pp.114.239335.
- Mira M, Adel ES, Stasolla C. Ethylene is integrated into nitric oxide regulation of Arabidopsis somatic embryogenesis. J Genet Eng Biotechnol. 2015;13:7–17. doi:10.1016/j.jgeb.2015.01.001.
- Lipka E, Muller S. Nitrosative stress triggers microtubule reorganization in Arabidopsis thaliana. J Exp Bot. 2014;65:4177–89. doi:10.1093/jxb/eru194.
- Drew MC, He II, Morgan PW. Programmed cell death and aerenchyma formation in roots. Trends Plant Sci. 2000;5:123–7. doi:10.1016/S1360-1385(00)01570-3.
- Rajhi I, Yamauchi T, Takahashi H, Nishiuchi S, Shiono K, Watanabe R, Mliki A, Nagamura Y, Tsutsumi N, Nishizawa NK, et al. Identification of genes expressed in maize root cortical cells during lysigenous aerenchyma formation using laser microdissection and microarray analyses. New Phytol. 2011;190:351–68. doi:10.1111/j.1469-8137.2010.03535.x.
- Delledonne M, Xia Y, Dixon RA, Lamb CJ. Nitric oxide functions as a signal in plant disease resistance. Nature. 1998;394:585–88. doi:10.1038/29087.
- Delledonne M, Zeier J, Marocco A, Lamb CJ. Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proc Natl Acad Sci USA. 2001;98:13454–59. doi:10.1073/pnas.231178298.
- Gross F, Durner J, Gaupels F. Nitric oxide, antioxidants and prooxidants in plant defence responses. Frontiers in Plant Science. 2013;4:419. doi:10.3389/fpls.2013.00419.
- Qu ZL, Zhong NQ, Wang HY, Chen AP, Jian GL, Xia GX. Ectopic expression of the cotton non-symbiotic hemoglobin gene GhHbd1 triggers defense responses and increases disease tolerance in Arabidopsis. Plant Cell Physiol. 2006;47:1058. doi:10.1093/pcp/pcj076.
- Guy PA, Sidaner J-P, Schroeder S, Edney M, MacGregor AW, Hill RD. Embryo phytoglobin gene expression as a measure of germination in cereals. J Cereal Sci. 2002;36:147–56. doi:10.1006/jcrs.2002.0460.
- Wang Y, Elhiti M, Hebelstrup KH, Hill RD, Stasolla C. Manipulation of hemoglobin expression affects Arabidopsis shoot organogenesis. Plant Physiol Biochem. 2011;49:1108–06. doi:10.1016/j.plaphy.2011.06.005.
- Arredondo-Peter R, Moran JF, Sarath G. Rice (Oryza) hemoglobins. F1000Research. 2014;3:253. doi:10.12688/f1000research.5530.2.
- Montilla-Bascon G, Rubiales D, Hebelstrup KH, Mandon J, Harren FJM, Cristescu SM, Mur LAJ, Prats E. Reduced nitric oxide levels during drought stress promote drought tolerance in barley and is associated with elevated polyamine biosynthesis. Sci Rep. 2017;7:13311. doi:10.1038/s41598-017-13458-1.
- Bai X, Long J, He X, Yan J, Chen X, Tan Y, Li K, Chen L, Xu H. Overexpression of spinach non-symbiotic hemoglobin in Arabidopsis resulted in decreased NO content and lowered nitrate and other abiotic stresses tolerance. Sci Rep. 2016;6:26400. doi:10.1038/srep26400.
- Parent C, Crevecoeur M, Capelli N, Dat JF. Contrasting growth and adaptive responses of two oak species to flooding stress: role of non-symbiotic haemoglobin. Plant Cell Environ. 2011;34:1113–26. doi:10.1111/j.1365-3040.2011.02309.x.
- Ubeda-Tomás S, Beemster GTS, Bennett MJ. Hormonal regulation of root growth: integrating local activities into global behaviour. Trends Plant Sci. 2012;17;326–331. doi:10.1016/j.tplants.2012.02.002.
- Serrano I, Romero-Puertas MC, Sandalio LM, Olmedilla A. The role of reactive oxygen species and nitric oxide in programmed cell death associated with self-incompatibility. J Exp Bot. 2015;66:2869–76. doi:10.1093/jxb/erv083.
- Lee Y, Lee WS, Kim SH. Hormonal regulation of stem cell maintenance in roots. J Exp Bot. 2013;64:1153–65. doi:10.1093/jxb/ers331.
- Miyashima S, Sebastian J, Lee JY, Helariutta Y. Stem cell function during plant vascular development. EMBO J. 2013;32:178–93. doi:10.1038/emboj.2012.301.
- Moller BK, Ten Hove CA, Xiang D, Williams N, Lopez LG, Yoshida S, Smit M, Datla R, Weijers D. Auxin response cell-autonomously controls ground tissue initiation in the early Arabidopsis embryo. Proc Natl Acad Sci USA. 2017;114:E2533–39. doi:10.1073/pnas.1616493114.
- Pernas M, Ryan E, Dolan L. SCHIZORIZA controls tissue system complexity in plants, Curr. Biol. 2010;20:818–23.
- Palovaara J, de ZT, Weijers D. Tissue and organ initiation in the plant embryo: A first time for everything. Annu Rev Cell Dev Biol. 2016;32:47–75. doi:10.1146/annurev-cellbio-111315-124929.
- Forzani C, Aichinger E, Sornay E, Willemsen V, Laux T, Dewitte W, Murray JA. WOX5 suppresses CYCLIN D activity to establish quiescence at the center of the root stem cell niche. Curr Biol. 2014;24:1939–44. doi:10.1016/j.cub.2014.07.019.
- Sabatini S, Heidstra R, Wildwater M, Scheres B. SCARECROW is involved in positioning the stem cell niche in the Arabidopsis root meristem. Genes Dev. 2003;17:354–58. doi:10.1101/gad.252503.
- Moubayidin L, Di MR, Sozzani R, Pacifici E, Salvi E, Terpstra I, Bao D, van Dijken A, Dello Ioio R, Perilli S, et al. Spatial coordination between stem cell activity and cell differentiation in the root meristem. Dev Cell. 2013;26:405–15. doi:10.1016/j.devcel.2013.06.025.
- Zhang W, Swarup R, Bennett M, Schaller GE, Kieber JJ. Cytokinin induces cell division in the quiescent center of the Arabidopsis root apical meristem. Curr Biol. 2013;23:1979–89. doi:10.1016/j.cub.2013.08.008.
- Di MR, De RM, Pacifici E, Salvi E, Sozzani R, Benfey PN, Busch W, Novak O, Ljung K, Di Paola L, et al. Auxin minimum triggers the developmental switch from cell division to cell differentiation in the Arabidopsis root. Proc Natl Acad Sci USA. 2017;114:E7641–49. doi:10.1073/pnas.1705833114.
- Street IH, Aman S, Zubo Y, Ramzan A, Lost Data, Shakeel SN, Kieber JJ, Schaller GE. Ethylene inhibits cell proliferation of the Arabidopsis root meristem. Plant Physiol. 2015;169:338–50. doi:10.1104/pp.15.00415.
- Arc E, Galland M, Godin B, Cueff G, Rajjou L. Nitric oxide implication in the control of seed dormancy and germination. Front Plant Sci. 2013;4:346. doi:10.3389/fpls.2013.00346.
- Bethke PC, Libourel IG, Aoyama N, Chung YY, Still DW, Jones RL. The Arabidopsis aleurone layer responds to nitric oxide, gibberellin, and abscisic acid and is sufficient and necessary for seed dormancy. Plant Physiol. 2007;143:1173–88. doi:10.1104/pp.106.093435.
- Nie XZ, Hill RD. Mitochondrial respiration and hemoglobin gene expression in barley aleurone tissue. Plant Physiol. 1997;114:835–40. doi:10.1104/pp.114.3.835.
