ABSTRACT
Glyceollins are the major pathogen- and stress-inducible natural products (phytoalexins) of soybean that possess broad-spectrum anticancer and neuroprotective properties. Yet like other phytoalexins, glyceollins are difficult to obtain because they are typically biosynthesized only transiently and in low amounts in plant tissues. We recently identified acidity stress (pH 3.0 growth medium) as an elicitor that exerted prolonged (week-long) inductive effects on glyceollin biosynthesis and identified the NAC family TF gene GmNAC42-1 that activates glyceollin biosynthesis in response to acidity stress or WGE from the soybean pathogen Phytophthora sojae. GmNAC42-1 was annotated as an SAR gene and SAR genes were statistically overrepresented in the transcriptomic response to acidity stress suggesting that acidity stress triggers the systemic elicitation of glyceollin biosynthesis. Here, we demonstrate that acidity stress acts as a systemic elicitor when provided to soybean roots. Acidity stress preferentially elicited specific glyceollins in different soybean organs with exceptionally high yields of glyceollin I in root tissues.
The value of the botanical- and plant-derived drugs market worldwide was estimated to be ~$25 billion in 2016 and is expected to increase to almost $42 billion by 2023. Yet, phytoalexins represent a vast array of inducible molecules in plants that remain a mainly untapped drug resource. Phytoalexins are antimicrobial plant metabolites that are not readily accessible as drugs since they are biosynthesized only in response to pathogens or specific abiotic stresses. The chemical structures of phytoalexins vary widely among plant species and include alkaloids, terpenoids, lactones, and phenolics. With roles in plant defense, phytoalexins are naturally bioactive and have diverse activities in human cells. Mounting evidence of their value to human health and agriculture has spurred renewed interest in identifying elicitors of their biosynthesis, biosynthetic genes, and the TFs that could be used to enhance their bioproduction via bioengineering.Citation1–Citation5
Glyceollins are the major phytoalexins of soybean that are biosynthesized from the isoflavonoid pathway. For decades, the study of glyceollins has served as a model for understanding the molecular aspects of elicitation and the role of inducible secondary metabolites in plant defense. Biochemical and genetic evidence suggests that glyceollins are essential for providing race-specific resistance to Phytophthora sojae, one of the most devastating pathogens in soybean agriculture.Citation6–Citation8 They also exhibit anticancer, antidiabetic, and neuroprotective properties that have been the subject of recent reviews.Citation9–Citation11 Glyceollin I, in particular, inhibits the proliferation and survival of a broad range of cancer types by inhibiting ER signaling and by other unclear mechanism(s).Citation12–Citation19 Conversely, glyceollins promote the survival of challenged neurons by stimulating the Nrf2/HO-1 signaling pathway.Citation20
The critical barriers that limit the accessibility of glyceollins are that they cannot be synthesized economicallyCitation21–Citation23 or obtained in sufficient amounts from soybean tissues.Citation24–Citation26 Efforts to improve accessibility have included (semi)-synthesis,Citation21-Citation23 fermentation of soybeans,Citation27 combined treatments of soybean seeds with pathogens and malting,Citation26 pathogens and environment stress,Citation28 and pathogens and chemicals.Citation25 A combined treatment of P. sojae WGE and the inorganic chemical silver nitrate had an additive effect, eliciting 1.5-fold more glyceollin I than the WGE treatment alone.Citation25 Yet, overexpressing the isoflavonoid biosynthesis gene encoding IFR increased glyceollin amounts more than 3-fold,Citation29 demonstrating that bioengineering can provide greater enhancements in glyceollin yields. However, overexpressing one rate-limiting biosynthetic enzyme will indefinitely uncover another. Since upregulating the expressions of all biosynthesis genes could provide greater increases in yield, we recently set-out to identify the TF gene(s) that regulate glyceollin biosynthesis.
By screening a panel of abiotic stresses, we identified acidity (pH 3.0 medium) to be a potent elicitor and dehydration to be a suppressor of glyceollin biosynthesis.Citation30 Using a comparative transcriptomics approach we found that all known glyceollin biosynthesis genes were oppositely regulated by acidity stress and dehydration, and that their expressions mirrored that of the NAC family TF gene GmNAC42-1.Citation30 Overexpressing and suppressing GmNAC42-1 in soybean hairy roots increased and decreased, respectively, the levels of glyceollin biosynthesis gene transcripts and metabolites.Citation30 GmNAC42-1 was annotated as an SAR gene and SAR genes were significantly overrepresented among those upregulated by acidity stress. SAR is a component of the plant immune system whereby tissues distant from a pathogen infection site become primed to more rapidly activate resistance during subsequent encounters with the pathogen. Yet, whether localized acidity stress could stimulate systemic phytoalexin biosynthesis remained untested.
Here, we grew seedlings in vermiculite to the first trifoliate leaf stage, then transferred them to pH 3.0 growth medium as reported previously with roots in contact with the acidic medium.Citation30 Following 9 d of treatment, seedlings were harvested and organs were dissected for analysis of glyceollins.
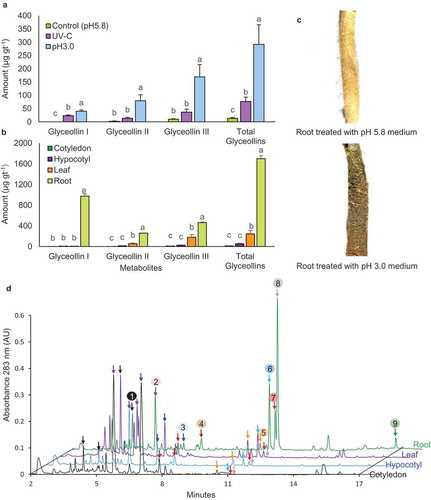
Acidity stress elicited greater amounts of total glyceollins compared to UV-C irradiation, which is classical abiotic elicitor of phytoalexins (). Transferring seedling roots to the acidic medium resulted in glyceollin accumulation in other organs with true leaves accumulating more than hypocotyls and cotyledons (). Yet, the roots were the major site of glyceollin accumulation (). Roots had an average of 1.7 mg gt−1 glyceollins, which is greater than the yield observed for the combined WGE-AgNO3 treatment of germinating seeds (837.2 µg gt−1),Citation25 or that observed for soybean sprouts that were simultaneously malted and challenged with Rhizopus microsporus (1.5 mg gt−1).Citation26 The organs preferentially accumulated specific glyceollins with glyceollin I being predominant in the roots and glyceollin III in the true leaves (). Roots exhibited a browning in color after 9 d treatment (). The phenotype was reminiscent of the localized browning observed during an incompatible interaction with P. sojae.Citation6,Citation8 Glyceollin I represented the major isoflavonoid peak in the roots (). This is uncommon since the isoflavone-conjugates 6-O-malonyldaidzin and 6-O-malonylgenistin generally remain the major isoflavonoids in pathogen-treated Williams 82 roots.
Figure 1. Elicitation of glyceollins in soybean roots by pH 3.0 medium. (a) Seven-day-old seedlings were transferred to pH 5.8 or pH 3.0 medium for 9 d, or exposed to a 30 W UV-C lamp for 1 h daily. Metabolites were extracted from intact seedlings (a) or dissected organs (b) by 80% ethanol and were quantified by UPLC-PDA by comparison to purified standards. (c) Root color phenotypes following 9 days treatment with pH 5.8 or pH 3.0 medium. (d) UPLC-PDA chromatograms of isoflavonoid extracts from cotyledons, leafs, hypocotyls and roots of Harosoy 63 seedlings elicited with pH 3.0 medium for 9 d. Peaks: 1) daidzin, 2) 6-O-malonyldaidzin, 3) 6-O-malonylgenistin, 4) daidzein, 5) genistein, 6) glyceollin III, 7) glyceollin II, 8) glyceollin I and 9) βprenyl-genistein.
In conclusion, the acidic growth medium is a potent systemic elicitor of glyceollin biosynthesis. Whether acid mine drainage or other collected environmental contaminants (e.g. acid rain) could be exploited to economically elicit the biosynthesis of these anticancer and neuroprotective natural products, or other plant phytoalexins, remains to be investigated.
Methods
Plant materials and growth conditions
Harosoy 63 seeds were a kind gift from Elroy Cober (Agriculture and Agri-Food Canada, Ottawa, Ontario). Seeds (16–20 per batch) were surface sterilized with 70% ethanol 0.2% triton X (v/v) for 5 min on a mixer wheel and grown in sterile vermiculite as indicated.Citation30
Acidity stress treatments
The roots of five unblemished, uniform seedlings at their first trifoliate leaf stage (~7-day-old) were gently wrapped together with filter paper saturated with control (pH 5.8) or acidic (pH 3.0) medium that contained half-strength MS salts, MS vitamins and 1% (w/v) sucrose and were placed on the medium inside sterile beakers.Citation30 The plant organs were harvested separately and processed for metabolite analysis as indicated.Citation30
Isoflavonoid analysis
Pulverized freeze-dried tissue powder (~12 mg) from whole seedlings, or dissected roots, hypocotyls, cotyledons and leaves were extracted with 80% ethanol (10 µL mg−1 dry tissue) as described.Citation30
UPLC-PDA
UPLC-PDA measurements were performed using an Accela System (Thermo Scientific, San Jose, CA, USA) containing a 1250 pump, Open AS autosampler, and photodiode array (PDA) as described.Citation25
Abbreviations
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Ahuja I, Kissen R, Bones AM. Phytoalexins in defense against pathogens. Trends Plant Sci. 2012;17:73–90. doi:10.1016/j.tplants.2011.11.002.
- Großkinsky DK, van der Graaff E, Roitsch T. Phytoalexin transgenics in crop protection—fairy tale with a happy end? Plant Sci. 2012;195:54–70. doi:10.1016/j.plantsci.2012.06.008.
- Jeandet P, Clément C, Cordelier S. Regulation of resveratrol biosynthesis in grapevine: new approaches for disease resistance? J Exp Bot. 2019;70:375–378. doi:10.1093/jxb/ery446.
- Jeandet P, Hébrard C, Deville M-A, Cordelier S, Dorey S, Aziz A, Crouzet J. Deciphering the role of phytoalexins in plant-microorganism interactions and human health. Molecules. 2014;19:18033–18056. doi:10.3390/molecules191118033.
- Jiang J, Xi H, Dai Z, Lecourieux F, Yuan L, Liu X, Patra B, Wei Y, Li S, Wang L. VvWRKY8 represses stilbene synthase genes through direct interaction with VvMYB14 to control resveratrol biosynthesis in grapevine. J Exp Bot. 2018;70:715–729. doi:10.1093/jxb/ery401.
- Graham TL, Graham MY, Subramanian S, Yu O. RNAi silencing of genes for elicitation or biosynthesis of 5-deoxyisoflavonoids suppresses race-specific resistance and hypersensitive cell death in Phytophthora sojae infected tissues. Plant Physiol. 2007;144:728–740. doi:10.1104/pp.107.097865.
- Hahn MG, Bonhoff A, Grisebach H. Quantitative localization of the phytoalexin glyceollin I in relation to fungal hyphae in soybean roots infected with phytophthora megasperma f. sp. glycinea. Plant Physiol. 1985;77:591–601.
- Subramanian S, Graham MY, Yu O, Graham TL. RNA interference of soybean isoflavone synthase genes leads to silencing in tissues distal to the transformation site and to enhanced susceptibility to phytophthora sojae. Plant Physiol. 2005;137:1345–1353. doi:10.1104/pp.104.057257.
- Bamji SF, Corbitt C. Glyceollins: soybean phytoalexins that exhibit a wide range of health-promoting effects. J Funct Foods. 2017;34:98–105. doi:10.1016/j.jff.2017.04.020.
- Nwachukwu ID, Luciano FB, Udenigwe CC. The inducible soybean glyceollin phytoalexins with multifunctional health-promoting properties. Food Res Int. 2013;54:1208–1216. doi:10.1016/j.foodres.2013.01.024.
- Pham T, Lecomte S, Efstathiou T, Ferriere F, Pakdel F. An update on the effects of glyceollins on human health: possible anticancer effects and underlying mechanisms. Nutrients. 2019;11:79. doi:10.3390/nu11010079.
- Bratton MR, Martin EC, Elliott S, Rhodes LV, Collins-Burow BM, McLachlan JA, Wiese TE, Boue SM, Burow ME, Wiese TE, Boue SM, Burow ME. Glyceollin, a novel regulator of mTOR/p70S6 in estrogen receptor positive breast cancer. J Steroid Biochem Mol Biol. 2015;150:17–23. doi:10.1016/j.jsbmb.2014.12.014.
- Chimezie C, Ewing A, Schexnayder C, Bratton M, Glotser E, Skripnikova E, Sá P, Boué S, Stratford RE. Glyceollin effects on MRP2 and BCRP in Caco‐2 cells, and implications for metabolic and transport interactions. J Pharm Sci. 2015;105:972–981.
- Lee SH, Jee JG, Bae JS, Liu KH, Lee YM. A group of novel HIF‐1α inhibitors, glyceollins, blocks HIF‐1α synthesis and decreases its stability via inhibition of the PI3K/AKT/mTOR pathway and Hsp90 binding. J Cell Physiol. 2015;230:853–862. doi:10.1002/jcp.24813.
- Payton-Stewart F, Schoene NW, Kim YS, Burow ME, Cleveland TE, Boue SM, Cleveland TE, Boue SM, Wang TT. Molecular effects of soy phytoalexin glyceollins in human prostate cancer cells LNCaP. Mol Carcinog. 2009;48:862–871. doi:10.1002/mc.20532.
- Rhodes LV, Tilghman SL, Boue SM, Wang S, Khalili H, Muir SE, Bratton MR, Zhang Q, Wang G, Burow ME, Collins-Burow BM. Glyceollins as novel targeted therapeutic for the treatment of triple-negative breast cancer. Oncol Lett. 2012;3:163–171. doi:10.3892/ol.2011.460.
- Salvo VA, Boue SM, Fonseca JP, Elliott S, Corbitt C, Collins-Burow BM, TJ Curiel, SK Srivastav, et al. Antiestrogenic glyceollins suppress human breast and ovarian carcinoma tumorigenesis. Clin Cancer Res. 2006;12:7159–7164. doi:10.1158/1078-0432.CCR-06-1426.
- Shin SH, Lee YM. Glyceollins, a novel class of soybean phytoalexins, inhibit SCF-induced melanogenesis through attenuation of SCF/c-kit downstream signaling pathways. Exp Mol Med. 2013;45:1–9. doi:10.1038/emm.2013.20.
- Zimmermann MC, Tilghman SL, Boue SM, Salvo VA, Elliott S, Williams KY, Skripnikova EV, Ashe H, Payton-Stewart F, et al. Glyceollin I, a novel antiestrogenic phytoalexin isolated from activated soy. J Pharmacol Exp Ther. 2010;332:35–45. doi:10.1124/jpet.109.160382.
- Seo JY, Kim BR, Oh J, Kim J-S. Soybean-derived phytoalexins improve cognitive function through activation of Nrf2/HO-1 signaling pathway. Int J Mol Sci. 2018;19:268. doi:10.3390/ijms19010268.
- Khupse RS, Sarver JG, Trendel JA, Bearss NR, Reese MD, Wiese TE, Boue SM, Burow ME, Cleveland TE, Bhatnagar D, Erhardt PW. Biomimetic syntheses and antiproliferative activities of racemic, natural (-), and unnnatural (+) glyceollin I. J Med Chem. 2011;54:3506–3523. doi:10.1021/jm101619e.
- Luniwal A, Khupse R, Reese M, Liu J, El-Dakdouki M, Malik N, Fang L, Erhardt P. Multigram synthesis of glyceollin I. Org Process Res Dev. 2011;15:1149–1162. doi:10.1021/op200112g.
- Malik N, Zhang Z, Erhardt P. Total synthesis of (±)-glyceollin II and a dihydro derivative. J Nat Prod. 2015;78:2940–2947. doi:10.1021/acs.jnatprod.5b00607.
- Boue SM, Carter CH, Ehrlich KC, Cleveland TE. Induction of the soybean phytoalexins coumestrol and glyceollin by Aspergillus. J Agric Food Chem. 2000;48:2167–2172.
- Farrell KC, Jahan MA, Kovinich N. Distinct mechanisms of biotic and chemical elicitors enable additive elicitation of the anticancer phytoalexin glyceollin I. Molecules. 2017;22:1261–1273. doi:10.3390/molecules22081261.
- Simons R, Vincken JP, Roidos N, Bovee TFH, van Iersel M, Verbruggen MA, Gruppen H. Increasing soy isoflavonoid content and diversity by simultaneous malting and challenging by a fungus to modulate estrogenicity. J Agric Food Chem. 2011;59:6748–6758. doi:10.1021/jf2010707.
- Park S, Kim Da S, Kim JH, Kim JS, Kim HJ. Glyceollin-containing fermented soybeans improve glucose homeostasis in diabetic mice. Nutrition. 2012;28:204–211. doi:10.1016/j.nut.2011.05.016.
- Aisyah S, Gruppen H, Madzora B, Vincken JP. Modulation of isoflavonoid composition of Rhizopus oryzae elicited soybean (Glycine max) seedlings by light and wounding. J Agric Food Chem. 2013;61:8657–8667. doi:10.1021/jf4020203.
- Cheng Q, Li N, Dong L, Zhang D, Fan S, Jiang L, Wang X, Xu P, Zhang S. Overexpression of soybean isoflavone reductase (GmIFR) Enhances resistance to phytophthora sojae in soybean. Front Plant Sci. 2015;6:1024. doi:10.3389/fpls.2015.01024.
- Jahan MA, Harris B, Lowery M, Coburn K, Infante AM, Percifield RJ, Ammer AG, Kovinich N. The NAC family transcription factor GmNAC42–1 regulates biosynthesis of the anticancer and neuroprotective glyceollins in soybean. BMC Genomics. 2019;20:149. doi:10.1186/s12864-018-5376-4doi.org/10.1186/s12864-019-5524-5.
