ABSTRACT
Mutations in BIG gene not only produce pleiotropic phenotypes of plant development but also impair plant adaptive responses under various stresses. However, the role of BIG gene in sugar signaling is not known. In this study, we first found that BIG deficiency significantly sensitized the sugar-induced anthocyanin accumulation and the sugar-inhibited primary root growth, suggesting BIG is an important component of the sugar signaling pathway. Then we found that big mutant plants had higher sugar levels compared with the wild type, indicating the involvement of BIG gene in regulating plant sugar homeostasis. Importantly, we also found that the relative ratio of carbon to nitrogen (C/N) was greatly enhanced by BIG deficiency. Overall, our work expands the known functionality of BIG and reveals its role in regulating sugar response and C/N balance. It is likely that BIG connects nutrient, light, and hormone signaling networks for regulating plant development and adaptive responses.
Text
Sugars in particular sucrose (Suc) and glucose (Glc) which serve as the main sources of carbon-skeleton and energy are increasingly recognized as signaling molecules involved in various developmental processes such as seed germination, cotyledons expansion, root growth, flowering, senescence, and stress responses in plants.Citation1BIG gene encoding a putative calossin-like protein with a molecular weight of 560 KD, was identified originally as a regulator of light signaling,Citation2 auxin transport, apical dominance,Citation3 and later was shown to control multiple hormone signaling pathways,Citation4 vesicle trafficking and endocytosis ,Citation5,Citation6 and recently was implicated in participating in the dynamic adjustment of circadian period which is profoundly affected by the availability of sucrose.Citation7,Citation8 Mutations in BIG as well as the homologous Pushover produce pleiotropic phenotypes.Citation9,Citation10 More recently, works by our lab have suggested that BIG is a component required for elevated CO2-induced stomatal closure and plant defense,Citation11,Citation12 two processes that are both modulated by sugars,Citation13,Citation14 prompting us to investigate whether BIG deficiency affects cellular sugar homeostasis and sugar signaling.
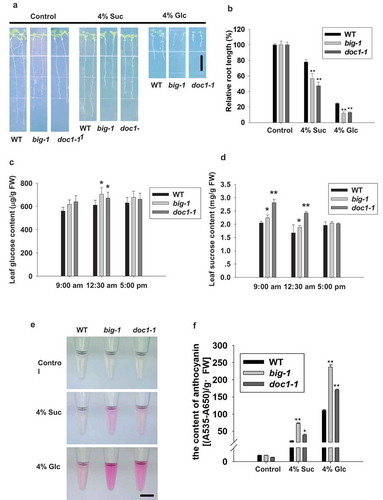
High concentration of sugars arrests plant primary root growth.Citation15 To examine whether BIG participates in sugar response, we first assayed big mutant and wild-type Col-0 (WT) seedlings in parallel and found that when higher concentration (4%) of sugars was presented in the half-strength Murashige and Skoog (MS) media, big mutants showed enhanced sensitivity to sucrose- and glucose-mediated inhibition of primary root growth (,b). We then examined whether the enhanced sensitivity to sugars was due to altered sugar contents by monitoring the glucose and sucrose levels of big mutant and WT plants, we observed a deviation of the dynamics of sugar accumulations. For example, as shown in c,, at all three test time points during the artificial day (when the light is on) mutant leaves consistently accumulated more glucose than WT, in particular, at 12:30 pm the difference was statistically significant, and the elevation of sucrose levels in big mutant was more pronounced as even at 9:00am (one hour after the onset of the artificial day) we observed a significant difference between mutant and WT plants. Intriguingly, the elevated sugar levels in big mutants dropped in the afternoon and even returned to the WT level at 5 pm (one hour before the onset of night). Whether the enhanced soluble sugars were a consequence of the enhanced photosynthesis and/or of the disturbed distribution/utilization of the photosynthates awaits further investigation. Notably, big mutant seedlings produced significantly more anthocyanin than those of WT when grown with 4% glucose or sucrose (), in accordance with the reported stimulatory effect of sugar on anthocyanin biosynthesis.Citation16,Citation17 These results strongly indicate that BIG is involved in regulating sugar accumulation and responses in Arabidopsis.
Figure 1. BIG negatively regulates sugar responses.
(a) Representative images showing BIG gene mutants are more sensitive to sugar-mediated inhibition of primary root elongation than that wild-type Col-0 (WT). Seedlings of indicated genotypes were grown on half-strength MS medium for 4 days, then transferred to half-strength MS media with 1% sucrose (control) or with 4% sucrose or 4% glucose and grown for another 5 days before measurement. Scale bar, 1 cm. (b) Relative inhibition of primary root length was analyzed from (a). (c) Glucose levels of leaves from four-week-old WT and big mutant plants were measured. (d) Leaf sucrose content was measured in four-week-old WT and big mutants. (e) Representative images showing that big mutants are more sensitive to glucose-induced anthocyanin accumulation than WT. Seedlings were grown on half-strength medium with 1% sucrose (control) or with 4% sucrose or 4% glucose for 11 days. Scale bar, 1 cm. (f) Anthocyanin contents were measured in (e). For (b), (c), (d) and (f), asterisks indicate significant differences detected using Student’s t-test (*P < .05, **P < .01) when compared to WT.
To sustain optimal growth and development for plants, cellular carbon (C) and nitrogen (N) metabolism must be tightly coordinated.Citation18 For example, when the C/N ratio is too high, the number and density of lateral root would decrease dramatically in Arabidopsis,Citation19 whereas when the C/N ratio is too low, the whole plant growth and photosynthesis are both inhibited in maize.Citation20 Having shown that mutations in BIG gene perturbed the accumulations of sucrose and glucose, we next investigated whether BIG impacts on the concentrations of C and N as well as the C/N ratio. We first determined the carbon content and found that the carbon level in the leaves of the mutants was significantly higher than that of WT plants (). In contrast to the increased C levels, the nitrogen concentration in mutant plants was markedly reduced (). Accordingly, the C/N ratio in big mutants was greatly elevated (), in agreement with the phenotype of much reduced lateral roots (LR) of big mutants as a high C/N ratio inhibits LR initiation in Arabidopsis.Citation4,Citation19,Citation21 Given plant growth at elevated CO2 stimulates carbohydrate production that will activate sugar sensing systems but lead to lower N concentration ,Citation22 and BIG is required for stomatal responses to CO2 which is an indispensable substrate that plants need to carry out photosynthesis ,Citation11 we wondered whether BIG has a role to play in adjusting C and N metabolism under elevated CO2 condition. Therefore, the C and N contents of both WT and big mutant plants grown under elevated CO2 (1000 ppm) conditions were determined. Results presented in d, show that in WT elevated CO2 caused a significant increase of C level but decrease of N level, resulting in an increase of C/N ratio, whereas big mutant plants exhibited reduced sensitivity to elevated CO2-induced elevation of C/N ratio ().
Figure 2. BIG is important for regulating carbon/nitrogen balance.
(a) and (b) The percentage of C content was significantly increased in big mutants compared to WT, whereas the percentage of N content was significantly decreased in big mutants than WT. The C/N contents of rosette leaves from five-week-old plants were determined by elemental analyzer. (c) The C/N ratio was significantly increased in big mutants. (d) BIG gene mutants are less sensitive to elevated CO2-induced C content increase. (e) BIG gene mutants are less sensitive to elevated CO2-induced N content decrease. (f) BIG gene mutants are less sensitive to elevated CO2-induced increase of C/N ratio. For (a), (b) and (c), asterisks indicate significant differences detected using Student’s t-test (*P < .05, **P < .01) when compared to WT.
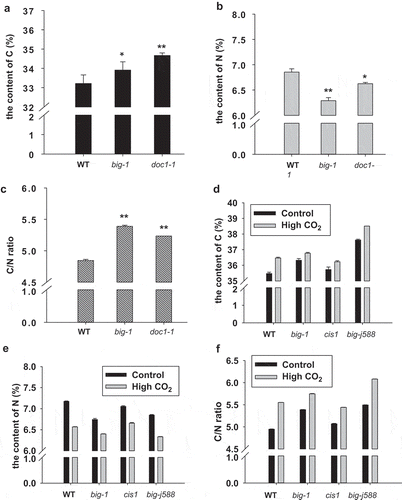
Rising CO2 levels profoundly impact not only plant physiology but also defense responses.Citation23,Citation24 For example, CO2 enrichment experiments have consistently shown that when fed on elevated CO2-grown plants, most leaf-chewing insects exhibit reduced growth and survival rates because of increased C/N ratios and reduced N contents.Citation25,Citation26 In a recent study, we found that BIG deficiency allowed more growth of pathogens when challenged with both avirulent and virulent bacteria, and interestingly big mutant plants retained significantly fewer larvae of pest S. exigua when larvae were given the choices between big mutant and WT plants, suggesting that BIG is required for plant defense.Citation12 Our observation that N concentration in big mutant plants was markedly lower than that in WT () which is suggestive of lacking proteins and nutritional values might partly account for the feeding deterrent effects of big mutants. Whether BIG plays essential roles in plant defense against insect feeding on elevated CO2-grown plants merits further investigation, as the findings might provide new insights into the feeding habit of phytophagous insects and the future of agriculture in a high-CO2 world.
Many research efforts have been made to understand the links between BIG gene and auxin pathway, as big mutants show typical defects in lateral root growth, organ elongation and apical dominance that have long been associated with auxin action.Citation3,Citation6,Citation27,Citation28 Two recent reports have suggested that sucrose itself, rather than auxin, is acting in regulating root growth and apical dominance.Citation29,Citation30 If this is the case, the decrease in apical dominance and some growth arrests observed in big mutants could be at least partly attributed to the perturbed sugar homeostasis/partitioning. Of note, mutations in BIG gene could greatly alter JA responses including JA-induced anthocyanin biosynthesis,Citation12 which is also modulated by sugars.Citation17,Citation31Citation33 Taking our findings and the well-documented roles of BIG in conveying light information and hormonal signaling into consideration, it is tempting for us to speculate that BIG connects light, nutrient and hormone signaling networks to regulate plant development and defense response, of which the mechanistic basis merits further investigation.
Disclosure of potential conflicts of interest
The authors declare that they have no conflicts of interest in the research.
Acknowledgments
This work was supported by the National Natural Science Foundation of China under grant numbers 31171356 and 31470360.
Additional information
Funding
References
- Smeekens S, Ma J, Hanson J, Rolland F. Sugar signals and molecular networks controlling plant growth. Curr Opin Plant Biol. 2010;13:1–5. doi:10.1016/j.pbi.2009.12.002.
- Li HM, Altschmied L, Chory J. Arabidopsis mutants define downstream branches in the phototransduction pathway. Genes Dev. 1994;8:339–349. doi:10.1101/gad.8.3.339.
- Gil P, Dewey E, Friml J, Zhao Y, Snowden KC, Putterill J, Palme K, Estelle M, Chory J. BIG: a calossin-like protein required for polar auxin transport in Arabidopsis. Genes Dev. 2001;15:1985–1997. doi:10.1101/gad.905201.
- Kanyuka K, Praekelt U, Franklin KA, Billingham OE, Hooley R, Whitelam GC, Halliday KJ. Mutations in the huge Arabidopsis gene BIG affect a range of hormone and light responses. Plant J. 2003;35:57–70. doi:10.1046/j.1365-313X.2003.01779.x.
- Yamaguchi N, Suzuki M, Fukaki H, Morita-Terao M, Tasaka M, Komeda Y. CRM1/BIG-mediated auxin action regulates Arabidopsis inflorescence development. Plant Cell Physiol. 2007;48:1275–1290. doi:10.1093/pcp/pcm094.
- Paciorek T, Zažímalová E, Ruthardt N, Petrášek J, Stierhof YD, Kleine-Vehn J, Morris DA, Emans N, Jürgens G, Geldner N, et al. Auxin inhibits endocytosis and promotes its own efflux from cells. Nature. 2005;435:1251–1256. doi:10.1038/nature03633.
- Haydon MJ, Mielczarek O, Robertson FC, Hubbard KE, Webb AA. Photosynthetic entrainment of the Arabidopsis thaliana circadian clock. Nature. 2013;502:689–692. doi:10.1038/nature12603.
- Hearn TJ, Marti Ruiz MC, Abdul-Awal SM, Wimalasekera R, Stanton CR, Haydon MJ, Theodoulou FL, Hannah MA, Webb AAR. BIG regulates dynamic adjustment of circadian period in Arabidopsis thaliana. Plant Physiol. 2018;178:358–371. doi:10.1104/pp.18.00571.
- Richards S, Hillman T, Stern M. Mutations in the Drosophila pushover gene confer increased neuronal excitability and spontaneous synaptic vesicle fusion. Genetics. 1996;142:1215–1223.
- Sekelsky JJ, McKim KS, Messina L, French RL, Hurley WD, Arbel T, Chin GM, Deneen B, Force SJ, Hari KL, et al. Identification of novel Drosophila meiotic genes recovered in a P-element screen. Genetics. 1999;152:529–542.
- He J, Zhang RX, Peng K, Tagliavia C, Li S, Xue S, Liu A, Hu H, Zhang J, Hubbard KE, et al. The BIG protein distinguishes the process of CO2-induced stomatal closure from the inhibition of stomatal opening by CO2. New Phytol. 2018;218:232–241. doi:10.1111/nph.14957.
- Zhang R-X, Ge S, He J, Li S, Hao Y, Du H, Liu Z, Cheng R, Feng Y, Xiong L, et al. BIG regulates stomatal immunity and jasmonate production in Arabidopsis. New Phytol. 2019;222:335–348. doi:10.1111/nph.15568.
- Tauzin AS, Giardina T. Sucrose and invertases, a part of the plant defense response to the biotic stresses. Front Plant Sci. 2014;5:293. doi:10.3389/fpls.2014.00293.
- Daloso DM, Anjos LD, Fernie AR. Roles of sucrose in guard cell regulation. New Phytol. 2016;211:809–818. doi:10.1111/nph.2016.211.issue-3.
- Mishra BS, Singh M, Aggrawal P, Laxmi A. Glucose and auxin signaling interaction in controlling Arabidopsis thaliana seedlings root growth and development. PLoS One. 2009;4:e4502. doi:10.1371/journal.pone.0004502.
- Solfanelli C, Poggi A, Loreti E, Alpi A, Perata P. Sucrose-specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiol. 2006;140:637–646. doi:10.1104/pp.105.071290.
- Das PK, Shin DH, Choi S, Park Y. Sugar-hormone cross-talk in anthocyanin biosynthesis. Mol Cell. 2012;34:501–507. doi:10.1007/s10059-012-0151-x.
- Nunes-Nesi A, Fernie AR, Stitt M. Metabolic and signaling aspects underpinning the regulation of plant carbon nitrogen interactions. Mol Plant. 2010;3:973–996. doi:10.1093/mp/ssq049.
- Malamy JE, Ryan KS. Environmental regulation of lateral root initiation in Arabidopsis. Plant Physiol. 2001;127:899–909. doi:10.1104/pp.010406.
- Saizfernandez I, De Diego N, Brzobohatý B, Munozrueda A, Lacuesta M. The imbalance between C and N metabolism during high nitrate supply inhibits photosynthesis and overall growth in maize (Zea mays L.). Plant Physiol Bioch. 2017;213–222. doi:10.1016/j.plaphy.2017.10.006.
- Remans T, Nacry P, Pervent M, Girin T, Tillard P, Lepetit M, Gojon A. A central role for the nitrate transporter NRT2.1 in the integrated morphological and physiological responses of the root system to nitrogen limitation in Arabidopsis. Plant Physiol. 2006;140:909–921. doi:10.1104/pp.105.071290.
- Ainsworth EA, Long SP. What have we learned from 15 years of free-air CO2 enrichment (FACE)? a meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2005;165:351–372. doi:10.1111/j.1469-8137.2004.01224.x.
- Noctor G, Mhamdi A. Climate change, CO2, and defense: the metabolic, redox, and signaling perspectives. Trends Plant Sci. 2017;22:857–870. doi:10.1016/j.tplants.2017.07.007.
- Zavala JA, Casteel CL, Nabity PD, Berenbaum MR, DeLucia EH. Role of cysteine proteinase inhibitors in preference of Japanese beetles (Popillia japonica) for soybean (Glycine max) leaves of different ages and grown under elevated CO2. Oecologia. 2009;161:35–41. doi:10.1007/s00442-009-1360-7.
- Wu G, Chen FJ, Sun YC, Ge F. Response of successive three generations of cotton bollworm, Helicoverpa armigera (Hubner), fed on cotton bolls under elevated CO2. J Environ Sci. 2007;19:1318–1325. doi:10.1016/S1001-0742(07)60215-0.
- Cease AJ, Elser JJ, Ford CF, Hao S, Kang L, Harrison JF. Heavy livestock grazing promotes locust outbreaks by lowering plant nitrogen content. Science. 2012;335:467–469. doi:10.1126/science.1214433.
- Guo X, Lu W, Ma Y, Qin Q, Hou S. The BIG gene is required for auxin-mediated organ growth in Arabidopsis. Planta. 2013;237:1135–1147. doi:10.1007/s00425-012-1834-4.
- Ruegger M, Dewey E, Hobbie L, Brown D, Bernasconi P, Turner J, Muday G, Estelle M. Reduced naphthylphthalamic acid binding in the tir3 mutant of Arabidopsis is associated with a reduction in polar auxin transport and diverse morphological defects. Plant Cell. 1997;9:745–757. doi:10.1105/tpc.9.1.97.
- Mason MG, Ross J, Babst BA, Wienclaw BN, Beveridge CA. Sugar demand, not auxin, is the initial regulator of apical dominance. Proc Natl Acad Sci USA. 2014;111:6092–6097. doi:10.1073/pnas.1322045111.
- Kircher S, Schopfer P. Photosynthetic sucrose acts as cotyledon-derived long-distance signal to control root growth during early seedling development in Arabidopsis. Proc Natl Acad Sci USA. 2012;109:11217–11221. doi:10.1073/pnas.1203746109.
- Loreti E, Povero G, Novi G, Solfanelli C, Alpi A, Perata P. Gibberellins, jasmonate and abscisic acid modulate the sucrose-induced expression of anthocyanin biosynthetic genes in Arabidopsis. New Phytol. 2008;179:1004–1016. doi:10.1111/j.1469-8137.2008.02511.x.
- Belhadj A, Telef N, Saigne C, Cluzet S, Barrieu F, Hamdi S, Mérillon JM. Effect of methyl jasmonate in combination with carbohydrates on gene expression of PR proteins, stilbene and anthocyanin accumulation in grapevine cell cultures. Plant Physiol Biochem. 2008;46:493–499. doi:10.1016/j.plaphy.2007.12.001.
- Shan XY, Zhang YS, Peng W, Wang ZL, Xie DX. Molecular mechanism for jasmonate-induction of anthocyanin accumulation in Arabidopsis. J Exp Bot. 2009;60:3849–3860. doi:10.1093/jxb/erp223.
