Abstract
Cationic homo- and co-polymerization of indene (Ind), styrene (St), limonene (Lim), and 5-ethylidene-2-norbornene (ENB) was performed with AlCl3 in dichloroethane at −20 °C under inert N2. The aim of this work is to investigate the effect of the reaction conditions on the molecular weight of polyindene (PInd) obtained by cationic polymerization with AlCl3 without an electron donor, and also to determine the effect of the use of cyclic diolefin comonomers on the polymer properties. The polymers were synthesized at a monomer/catalyst molar ratio (MR) of 100; St and Lim showed the highest and lowest reactivity, respectively. The polymers were characterized by 13C and 1H NMR spectroscopy, Fourier transform infrared spectroscopy, ultraviolet–visible spectroscopy, size exclusion chromatography, differential scanning calorimetry, and thermogravimetric analysis. PInd with a number average molecular weight (M n) of 45 × 103 g/mol and a glass transition temperature (T g) of 207 °C was obtained, whereas only oligomeric polylimonenes were obtained. Cyclic diolefins depressed both the M n and T g values of the Ind-copolymers. There was a dramatic decrease in the M n of the polymers: those obtained at a MR of 1:1 in the feed presented M n values lower than 104 g/mol, with the exception of poly(Ind-co-ENB), which is cross-linked. Poly(Ind-co-Lim) with lateral double bonds, a comonomer content of 1 to 20 mol%, and T g values in the range of 140–200 °C were obtained.
Introduction
Conventional styrene (St) cationic polymerization has been known since the early 60s and is generally initiated by Lewis or strong protonic acids. Using this method, the polymer molecular weight is not controllable due to transfer reactions Citation[1]. Indene (Ind) can be readily polymerized with various Friedel–Crafts co-initiators (e.g. SnCl4, TiCl4, or BF3) at −70 °C to give high number average molecular weight (M n) polymers Citation[2]. Ind can also be polymerized in a living fashion, although a broad polymer molecular weight distribution (MWD) is obtained Citation[3,4]. Over the last two decades, papers published on Ind polymerization with Friedel–Crafts catalysts at low temperatures have shown that the polymer average molecular weights depend on the type and amount of catalyst and the mechanism (i.e. conventional, cationic, or live) Citation[1,4–6]. Ind–St copolymers with low M n values and broad polydispersity (M w/M n) have been obtained via a cationic mechanism Citation[1,2,5,7].
Sigwalt et al. Citation[8,9] and Kennedy et al. Citation[10,11] investigated several living polymerizations of Ind with Cumyl-OCH3, TMP-Cl/TiCl4, or TiCl3(BuO) in the presence of electron donors such as DMSO, DMA, Et3N, DTBP, or Cumyl-Cl/BCl3 to produce controlled polymer structures. Polyindene (PInd) with low M n (13 × 103 g/mol) and polydispersity (1.2) was obtained with Cumyl-Cl/BCl3 in chloroform at −80 °C. On the other hand, a triblock copolymer of Ind and isobutylene (i.e. PInd-b-PIB-b-PInd) produced with TMP-Cl/TiCl4 using a sequential monomer addition method exhibited excellent thermoplastic elastomeric characteristics Citation[4].
The benefits of the inclusion of an electron donor for the cationic mechanism have been demonstrated for diene and St polymerization Citation[6,12]. Polystyrenes (PSs) with an M n of 85 × 103 g/mol and M w/M n of 1.8–2.0 were obtained with CumOH/AlCl3OBu2/Py at a low temperature Citation[13]. A key factor in achieving living polymerization behavior is the use of an AlCl3 co-initiator with an appropriate electron donor (e.g. Bu2O): the electron donor forms a complex with the Lewis acid that solubilizes in the polymerization medium, thereby decreasing its instantaneous concentration. 1,3-Pentadiene without crosslinking could also be polymerized with AlCl3 by using a bulky electron donor Citation[14]. The cationic polymerization of norbornene with TMP-Cl/TiCl4 involves the transannular rearrangement of the monomer double bonds to give a rigid tricyclic repeat structure with glass transition temperature (T g) values around 320 °C Citation[15]. The use of an electron donor for the reaction between 35 and 60 °C increases the polymer M n linearly up to a conversion rate of 45%, which is suitable for thermoplastic applications.
Plant-derived polymers (i.e. polyterpenes) obtained from the cationic polymerization of cyclic monomers such as dipentene, limonene (Lim), and α- or β-pinene have been extensively studied Citation[16,17] and commercialized for many applications. As an example of developing sustainable plastics from a renewable biomass resource, β-pinene cationic polymerization using AlCl3 modified with an electron donor (i.e. AlCl3OPh2 or (AlCl3)(EtOAc)0.8) was reported Citation[18]. Poly(β-pinene) polymers with M n values between 9 × 103 and 14 × 103 g/mol and T g values around 85 °C were obtained under conditions suitable for industrial applications. Living cationic isomerization of β-pinene using an nBu4NCl electron donor with the HCl-adduct of 2-chloroethyl vinyl ether [(CEVE-Cl)/TiCl3(OiPr)] in dichloromethane at from −40 to −78 °C has also been reported Citation[4].
In this work, cationic polymerization of Ind with AlCl3 without an electron donor was performed to investigate the effect of the reaction conditions on the molecular weight of the resultant PInd, as well as the effect of the cyclic diolefin used as a hydrocarbon comonomer on the thermal properties of the polymer. Cationic homo- and co-polymerization were carried out in dichloroethane at −20 °C and the molecular weight and thermal properties of the Ind polymer were evaluated. For comparison, St homo- and co-polymerization were also carried out. The polymers were evaluated by Fourier transform infrared (FTIR) and ultraviolet–visible (UV–vis) spectroscopy, gel permeation chromatography (GPC), thermogravimetric analysis (TGA), and differential scanning calorimetry (DSC). Cationic polymerization of Ind with AlCl3 and electron donors at higher temperatures is still under investigation since cationic polymerization below 0 °C is commercially unviable.
Experimental
Materials
Ind (95%; b.p. = 175–185 °C; 116.16 g/mol), Lim (97%; b.p. = 176 °C; m.p. = −74.35 °C; 136.24 g/mol), and aluminum trichloride (99.99%) were purchased from Aldrich. Sodium hydroxide, which was used to stop the polymerization reaction, was purchased from Synth. Tetrahydrofuran (THF; 99%), which was used in the polymer analyses, was purchased from Aldrich. 1,2-dichloroethane (C2H4Cl2; 99%) and ethanol (96%) were purchased from Quimex. Polymerization-grade (St; 99%; b.p. = 145 °C; 104.15 g/mol) and 5-ethylidene-2-norbornene (ENB; 99%; m.p. = 42–46 °C; 94.15 g/mol) monomers were kindly donated by INNOVA Petrochemical (Brazil/RS) and DSM Elastomers (Brazil/RS), respectively. Before use, AlCl3 was soaked in dichloroethane and finely ground.
Polymerization
Polymerization reactions were performed in a 250 mL glass reactor at −20 °C under N2. The solvent (C2H4Cl2, 92 mL), monomer, comonomer, and catalyst (AlCl3) were sequentially added at the specified concentrations. A monomer/catalyst molar ratio (MR) of 100 was used for all polymerization reactions, and the reaction time was 300 min. The polymerization reaction was ceased by adding 20% NaOH solution (40 mL) and the products were recovered by precipitation in ethanol. To purify the polymers, they were dissolved in dichloroethane, re-precipitated in ethanol, and then dried in an oven at 100 °C in vacuo.
Polymer characterization
The polymers were evaluated by infrared spectroscopy (ASTM E168-06) with a PerkinElmer FTIR Spectrum 1000 using KBr pellets, and by UV–vis spectroscopy in a PG Instruments UV–vis T80 + spectrometer in chloroform. 1H NMR and 13C NMR spectra were recorded at 300 MHz and 100 MHz, respectively, on a JEOL LA-400 spectrometer in CDCl3 at 25 °C. The molecular weight averages of the polymers were determined via size exclusion chromatography in THF (flow rate: 1.0 mL/min) at 25 °C using a Waters 1515 isocratic HPLC pump equipped with a Styragel columns series (HR1-HR2-HR3-HR4) and refractive index detector (Waters Model 2414) using a PS calibration curve. The T g values of the polymers were determined by DSC with a TA Instruments DSC Q20 calorimeter at a heating rate of 10 °C/min under a nitrogen atmosphere. The thermal stability of the polymers was evaluated by TGA in a TA Instruments TGA 2050 thermobalance at a heating rate of 20 °C/min in the range of 25–1000 °C under N2.
Results and discussion
Ind was polymerized via a cationic mechanism with an initial monomer/catalyst MR of 100, as published previously Citation[5,10], leading to polymers with relatively high molecular weight. Homo- and co-polymerization of St, which is employed in the commercial production of PS, was performed as a reference for this work. In polymerizations initiated by Friedel–Crafts catalysts without electron donors, impurities of either H2O or HCl were assumed to be the initiators Citation[2] and were considered to be co-catalysts. Donor-free AlCl3 catalyst can be activated by self-ionization (Equation (1)) through the association of two AlCl3 molecules and through a catalyst system (Equation (2)) involving the molecular association of AlCl3 and H2O Citation[5].
In this reaction, AlCl3 activation was expected to occur mainly by the syn-catalyst process since the catalyst was added into the reactor feed with the monomer (M) at −20 °C under N2 and the water content of the system was approximately 2 × 10−2 mol/L. The ratio of (M = St) and CH3CH (C6H6)+ impacts the MWD of the polymer since they have different monomer affinities and chain transfer capacities. AlCl3 is a solid catalyst and is not completely soluble in the reaction medium. Thus, active centers with different chemical reactivities are expected to coexist in the reaction despite the generally high solubility of the products. The reaction products were soluble in the polymerization medium with the exception of the poly(Ind-co-ENB), which was a gel when obtained with more than 50% (v/v) ENB in the feed; the gel was produced as a result of parallel crosslinking from the lateral double bond attached to the growing chain during ENB copolymerization. All the polymers obtained were soluble in THF (i.e. the SEC solvent) and poly(Ind-co-ENB) obtained at comonomer concentrations of 20–50% was partially soluble in THF. The obtained polymers were light beige and transparent since they are amorphous materials.
Table shows the homopolymerization conversion and physical characteristics of the polymers including number average molecular weight, polydispersity, and glass transition temperature. St and Ind polymerizations presented high conversions of ∼92% and ∼82%, respectively, while that of Lim was very low (∼7%). PInd obtained at −20 °C had M n values up to 45 × 103 g/mol, which is much lower than the reported values (6 × 105 and 2 × 106 g/mol) Citation[2] for polymers obtained at −70 °C using other catalysts (i.e. SnCl4, TiCl4, or BF3). In cationic polymerization, it is known that lower reaction temperatures lead to higher M n values. Under the same conditions, PS (M n = 2.5 × 103 g/mol) and polylimonene (PLim) (M n < 500 g/mol) produced at −20 °C were oligomeric products and the average polymerization degree (PD = M n/M monomer) of PInd, PSt, and PLim was 284, 24, and 3.5, respectively. This is due to chain termination via β-elimination, which occurs much more readily for Lim; the lower PD and the polydispersity of ∼1.5 show that PLim is a more homogeneous product. PLim comprises trimer molecules with a solidification point or T g around −7 °C, which is significantly higher than that of the monomer (m.p. = −74.35 °C). The wide polydispersity of PSt, which is evident in this work and has been previously reported Citation[1,19], is potentially a consequence of competition between initiation and termination reactions. Alternatively, the high polydispersity could also be due to the variety of reactive catalytic species present since the catalyst was not completely soluble in the polymerization medium. Due to the high reactivity of St, the catalyst can also be activated via the self-ionization mechanism (Equation (1)) before the AlCl3 molecules dissociate to produce AlCl3–H2O complexes. Even with a low M n, the oligomeric PSt had a T g of the same order as commercial PS samples (90–100 °C).
Table 1. Reaction conversion and homopolymer physical characteristics.
As expected for cationic polymerization, the polydispersity of PInd was around 2.0. Its unique structure renders it a high T g of around 207 °C, which is due to the chain stiffness imparted by the five-membered ring structure of the monomeric unit in the polymer (see Figure ), resulting in a semi-ladder structural arrangement. The aromatic rings attached to the five-membered rings are equidistant in the side chain and the PInd molecules assume a nearly planar structure. The T g of PInd obtained in this work was in the same order or higher than those of other reported PInds, which were also determined by DSC Citation[10,20,21]. For example, a 27 × 103 g/mol PInd presented a T g of 204 °C Citation[10] while PInds obtained with CCl3COOH/SnCl4 in dichloromethane at temperatures between 0 and −78 °C with M n values of 60 × 103–110 × 103 g/mol have T g values around 183 °C Citation[20]. Similarly, PInd obtained by living cationic polymerization was reported to have a T g of 195 °C Citation[21]. Due to the high T g and intermediate M n, the obtained PInd produced brittle films; this can be remedied by producing PInd with higher M n or even producing new compounds such as block copolymers with rubbery or polar monomers Citation[20].
Figure 1 (a) 13C NMR spectra of PInd and Ind-copolymers with St (poly(Ind-co-St)), Lim (poly(Ind-co-Lim)), and ENB (poly(Ind-co-ENB)) obtained using AlCl3 without an electron donor in C2H4Cl2 at −20 °C and (b) 1H NMR spectra of the Ind-copolymers.
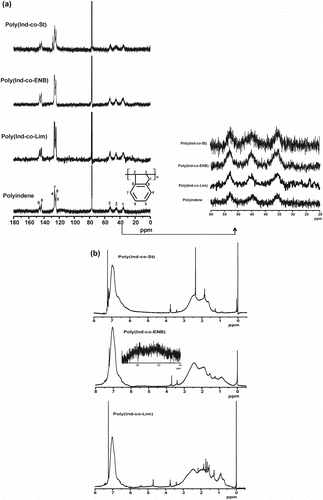
Figure shows the 13C and 1H NMR spectra, respectively, of the PInd and Ind-copolymers obtained in this study. As expected, two distinct resonances corresponding to the aromatic and aliphatic carbons are evident in the 13C NMR spectrum of PInd Citation[10,20,21]. The two ring junction carbons (labeled 8 and 9) appear as broad multiple resonances centered at 145.6 and 147 ppm, respectively. The methine carbons of the aromatic ring (labeled 4–7) range from 123.5 to 126 ppm and have more complex resonance signals. The three distinct broad resonances at a higher field correspond to the environment of the cyclopentene ring carbons labeled 1–3. The less electronegative methylene carbon (1) presents a resonance at 36 ppm and the benzylic β (2) and (3) methine carbons in the main chain present chemical shifts at 45 and 54 ppm, respectively. As reported for hydrogenated PInd Citation[22], Ind can be incorporated with the adjacent repeat units in either cis or trans configuration.
The 13C NMR spectrum of the poly(Ind-co-St) (Ind/St feed = 1:1) presented chemical shifts in the region from 39 to 40 ppm, which were attributed to the methylene carbon of the St Citation[21,22]. As shown in Figure , the 13C NMR spectrum of the CDCl3-soluble fraction of poly(Ind-co-ENB) obtained with an ENB feed of 20% features resonances in the region from 145 to 148 ppm, which were assigned to the ethylidene double bond carbons Citation[23,24]. The chemical shifts due to the aromatic ring carbons can be detected in the region from 123.5 to 127.5 ppm Citation[10,23], while the resonance peaks from 45.0 to 53.0 ppm are due to the cyclic chain methine carbons. The peaks in the regions of 35.0–38.0 ppm and 33.0–35.0 ppm can be assigned to the secondary carbon resonances of the cyclic chain methylene carbons, while those in the region of 28.2–33.0 ppm can be assigned to the ethylene units Citation[21,23–25]. The chemical shifts in the range of 36.0–54.0 ppm overlap those of poly(Ind) and Ind-copolymer with slight differences and small shifts depending on the type of C–C bond. The 13C NMR spectrum of poly(Ind-co-Lim) presented chemical shifts at 48.3, 31.8, 138, and 127 ppm due to the methine, methylene, and unsaturated >C= and =CH– carbons, respectively Citation[26,27]. The resonances with chemical shifts from 148 to 145 ppm and 127.5–123.5 ppm are due to the Ind aromatic ring carbons Citation[23]. The resonances corresponding to the methine and methylene carbons in the spectra of the copolymers are broader than those in the homopolymers because both comonomers are incorporated in the main chain.
The 1H NMR spectrum of poly(Ind-co-St) (Figure ), with regard to that of PInd Citation[10,21,23], shows chemical shifts in the region of 6.8–7.2 ppm, which were assigned to the aromatic protons, and in the region from 2.2 to 2.9 ppm, which were assigned to the aliphatic protons. The methylene protons are represented by a sharp singlet at 1.2 ppm Citation[20] while the resonance at 3.4–3.7 ppm was assigned to the methine protons adjacent to two aromatic rings; this latter signal is associated with the chain termination reactions, and the chemical shift at 0.8 ppm is due to the end-chain methyl groups Citation[23,28]. Comparison of the poly(Ind-co-ENB) and comonomer 1H NMR spectra shows that, in the former, there is no chemical shift due to the endocyclic double bond protons in the region from 5.8 to 6.0 ppm although there is a resonance due to the ethylidene proton at 4.8–5.5 ppm Citation[24]. This implies that the ENB comonomer copolymerizes through the endocyclic double bond, and the ethylidene groups remain unreacted due to the ring strain of the endocyclic double bond. The spectra of both copolymers show a singlet at 6.5–7.2 ppm due to the Ind phenyl protons. The emergence of peaks at 2 and 3 ppm is due to the protons attached to the double-bonded carbon of the main chain Citation[24,29] and the peak at 3.4–3.7 ppm was assigned to the methine protons neighboring two aromatic rings. A comparison of the poly(Ind-co-Lim) and Lim 1H NMR spectra reveals that the chemical shift at 4.7 ppm is due to the Lim exo-olefinic bond that is involved in the polymerization reaction, whereas the chemical shift at 5.4 ppm is due to the endo bond that remains in the copolymer Citation[27,30]. The multiplets in the region of 2.3–1.7 ppm are related to the methine protons and the singlets at 1.5 and 0.8 ppm are associated with the methylene protons and Lim methyl group protons Citation[23,27], respectively. The peaks that emerged in the region of 2–3 ppm are due to the protons attached to the double-bonded carbons that remain in the resin structure. Thus, both poly(Ind-co-Lim) and soluble poly(Ind-co-ENB) can be used as unsaturated resins or as an intermediates in other syntheses.
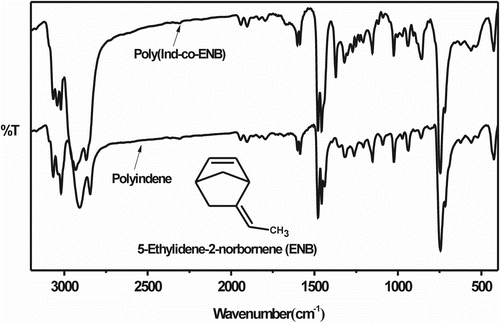
Figure shows the FTIR spectra of the homopolymers and Ind-copolymers with St (poly(Ind-co-St)) and Lim (poly(Ind-co-Lim)), respectively. Aromatic hydrocarbon resins exhibit absorption peaks in the region of 3040 cm−1 due to aromatic CH stretching vibrations, and absorption peaks at ∼1500 cm−1 due to aromatic C=C stretch bands and below 900 cm−1 attributed to CH stretching vibrations out-of-plane. The strong absorption bands in the region of 2925–2850 cm−1 are characteristic of stretching vibrations of CH2 groups. The absorptions bands at 1610, 1595, 1565, 1490, and 1450 cm−1 correspond with the stretching modes of CH groups while the weaker absorptions at 1812, 1915, and 1955 cm−1 may be associated with the out-of-plane CH bond, which was confirmed by absorptions in the region of 700–1000 cm−1. The C=C aliphatic bond is responsible for the weak absorption in the region between 1600 and 1670 cm−1 Citation[5,31–33]. PInd shows a characteristic and strong absorption band from 425 to 435 cm−1, while that of PSt appears at 540 cm−1 (Figure ). The spectrum of poly(Ind-co-St) shows absorptions related to the Ind and St moieties. The poly(Ind-co-Lim) FTIR spectrum (Figure ) presents absorption bands at 1715 and 3000 cm−1 due to the Lim tri-substituted carbon, which confirms the corresponding assignation of the 5.4 ppm peak in the 1H NMR spectrum of the copolymer (Figure ) Citation[27]. The absorption bands at approximately 2930 and 2870 cm−1 are CH2 asymmetric and symmetric deformations, respectively, and the absorptions at approximately 1455 and 1380 cm−1 are CH2 symmetric deformations in- and out-of-plane, respectively Citation[31]. The Ind-co-Lim copolymer spectrum also presents absorption bands at approximately 3024 and 910 cm−1, which are indicative of C–H aromatic ring stretching. Both poly(Ind-co-Lim) and PLim show an absorption band at 803 cm−1 due to the tri-substituted carbon. The FTIR spectrum of poly(Ind-co-ENB) is shown in Figure . The absorption peaks observed in the range of 3060–2995 cm−1 are due to the stretching of the side unsaturated carbon bond (=CH), while those in the range of 1010–985 cm−1 are due to out-of-plane CH vibrations. The absorption peak at 1372 cm−1 may be associated with CH2= bond vibrations in polyenes (CH2=CH–), which are due to norbornadiene incorporation in the main chain that is not detected in the PInd spectrum Citation[31].
Figure 2 FTIR spectra of the homopolymers and Ind-copolymers with (a) St [(poly(Ind-co-St)/Ind:St MR = 0.9] and (b) Lim [(poly(Ind-co-Lim)/Ind:Lim MR = 1.4].
![Figure 2 FTIR spectra of the homopolymers and Ind-copolymers with (a) St [(poly(Ind-co-St)/Ind:St MR = 0.9] and (b) Lim [(poly(Ind-co-Lim)/Ind:Lim MR = 1.4].](/cms/asset/f3d40d33-1082-41d6-b2c4-532b4a4c92bc/tdmp_a_747145_o_f0002g.gif)
Figure shows the UV–vis spectra of the Ind-copolymers (Ind:St MR = 0.9; Ind:Lim MR = 1.4; Ind:ENB MR = 4.3) relative to those of PInd and Ind. The Ind spectrum (Figure ) features a characteristic sharp π–π ∗ absorption peak at 267 nm Citation[20,34], whereas the spectra of PInd and the Ind-copolymers show this absorption peak at approximately 236 nm (Figure ); this difference is related to the overlap of the π-conjugated bonds of the Ind phenyl groups linked in the polymer backbone. Absorption peaks in the range of 260–280 nm and 270–310 nm are due to the St and Ind phenyl groups, respectively, linked in the polymer backbone, as reported in the literature Citation[31]. These observations coupled with those from the FTIR spectra corroborate the proposed structures of the Ind-copolymers.
Figure 4 (a) UV–vis spectra of PInd (2) and Ind-copolymers: poly(Ind-co-St)/Ind:St MR = 0.9 (1), poly(Ind-co-Lim)/Ind:Lim MR = 1.4 (3), and poly(Ind-co-Nb)/Ind:ENB MR = 4.3 (4) and (b) UV–vis spectrum of Ind.
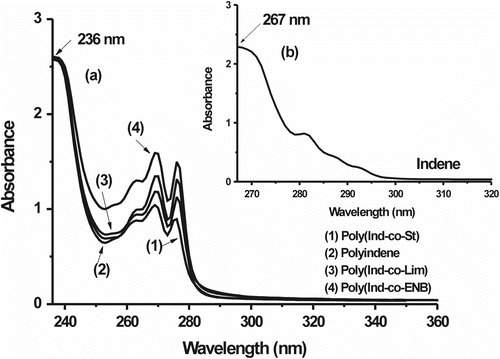
Figure shows the homopolymerization conversion and PInd molar mass (M n) as a function of Ind concentration, and the copolymerization conversion and poly(Ind-co-St) molar mass (M n) as a function of catalyst concentration, respectively. As expected, both reaction conversion and polymer M n are dependent on the catalyst and monomer concentration. A PInd reaction conversion of approximately 20% was achieved (Figure ) using Ind concentrations from 2.0 to 3.0 mol/L. In this concentration range, the M n of the PInd increased from 30 × 103 to 45 × 103 g/mol. In the graphs, the values in parentheses correspond to the polydispersity of the PInd samples; as the M n increases, the polydispersity also increases. The poly(Ind-co-St) reaction conversion was higher than 90% (Figure ) at an Ind:St MR of 0.94 and a monomer/catalyst MR of 100. The dielectric constant (ϵ) of the polymerization medium decreased since the relative concentration of dichloroethane (ϵ = 10.3) is lower and there is an increasing amount of Ind (ϵ = 2) in the solution. The chain growing and transfer reactions are also affected by ion pair proximity (i.e. proximity of an ion and its counterion) that depends on the solvent polarity. A lower dielectric constant hinders ion pair separation, thereby favoring chain termination by β-elimination and decreasing the polymer M n Citation[21,35]. Additionally, chain transfer to St is significantly higher than to Ind, and catalyst concentrations higher than 2.5 × 10−2 mol/L further depressed the copolymer M n due to the high St concentration in the reactor.
Figure 5 (a) Homopolymerization conversion and PInd molar mass (M n) as function of monomer concentration. (b) Copolymerization conversion and poly(Ind-co-St) molar mass (M n) as a function of catalyst concentration
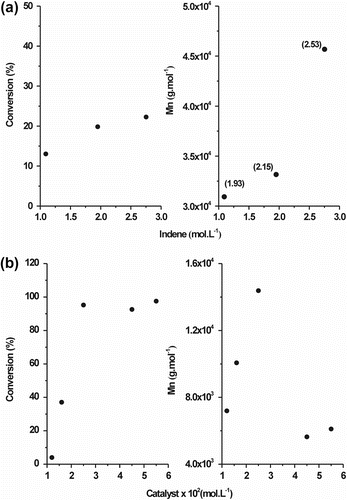
Figure shows the SEC chromatograms of PInd and the Ind-copolymers obtained with St (Ind:St MR = 3.3), Lim (Ind:Lim MR = 4.8), and ENB (Ind:ENB MR = 4.3) that illustrate the relative MWD profiles. PInd presented a unimodal MWD curve with a small fraction of low molecular weight molecules with retention times of 25–30 min; it also had a higher number average molecular weight (33 × 103 g/mol; reaction conditions shown in Table ) than the Ind-copolymers. The Ind-copolymers presented broad bimodal MWD curves with different profiles depending on the type of comonomer. A bimodal MWD profile indicates the presence of at least two substantial fractions of molecules with very similar molecular weights and can be a consequence of the type of active species and the chain propagation and chain transfer rate constants. The THF-soluble fraction of poly(Ind-co-ENB) presented a low M n (8.5 × 103 g/mol) and a small fraction of molecules with higher molecular weights (retention time <20 min) due to the simultaneous low extension crosslinking reaction. From the molecular weight curve profiles, it is possible to conclude that changes in the comonomer type enable the production of different Ind polymers.
Figure 6 SEC chromatogram curves of PInd (1) and Ind-copolymers: poly(Ind-co-St)/Ind:St MR = 3.3 (2), poly(Ind-co-ENB)/Ind:Nb MR = 4.3 (3), and poly(Ind-co-Lim)/Ind:Lim MR = 4.8 (4).
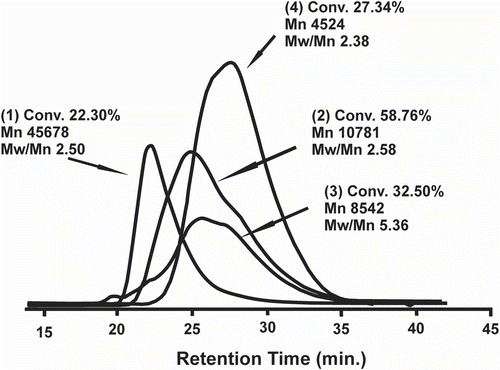
Table shows the St and Lim content of some Ind-copolymer samples that were determined by FTIR quantitative analysis Citation[36,37] and their T g values as a function of comonomer concentration in the feed. The comonomer content in the copolymers was evaluated using the absorption peaks at 1479 and 1493 cm−1 for Ind and St, respectively, in the poly(Ind-co-St) spectrum, and the absorption peaks at 3066 and 881 cm−1 for Ind and Lim, respectively, in the poly(Ind-co-Lim) spectrum. The comonomer content in poly(Ind-co-ENB) was not determined since the lateral double bond underwent a crosslinking reaction. Copolymers obtained with 10 mol% comonomer in the feed had a low comonomer content and those obtained with 20–50 mol% in the feed presented higher comonomer content when obtained with St Despite poly(Ind-co-Lim) copolymers presenting the lowest M n values and lower comonomer content in the chain, the decrease of the T g values of the Ind-copolymers was of the same order. The T g values of the Ind-copolymers were lower than that of PInd (207 °C); those with lower comonomer content had the same T g values. The comonomer contents obtained from an Ind/comonomer MR of 1 in the feed were approximately 25 and 70 mol% for Lim and St, respectively. Ind reactivity (r Ind) of 3.7 ± 0.2 and St reactivity (r St) of 0.6 ± 0.1 have been reported Citation[29,30] for the copolymerization of Ind/St with BF3:Et2O/dichloroethane. Lim has a non-aromatic and more flexible structure than St; therefore, poly(Ind-co-Lim) with 7 mol% of Lim had the same T g as poly(Ind-co-St) with 16 mol% of St.
Table 2. Comonomer content and T g values of the Ind-copolymer with St and Lim.
Figure shows the M n, polydispersity (M w/M n) and T g values of the Ind-copolymers with St, Lim, and ENB (soluble in THF), respectively, as a function of comonomer concentration in the feed. The comonomer depresses the polymer M n, and its effect on the polymer polydispersity depends on the feed. Although the poly(Ind-co-ENB) obtained with 10% ENB in the feed was completely soluble in THF, while that obtained with 20 and 50% ENB in the feed was only partially soluble in THF; therefore, the M n values of the latter polymers that are displayed in Figure correspond only to the THF-soluble fraction. Isomerization and cyclization reactions may occur even if a high norbornene composition is used. Norbornene polymerization has been investigated by others and the polymer T g can reach 320 °C Citation[3,4]. Lim presented a high chain termination capacity through the β-elimination reaction; accordingly, 10% Lim depressed the PInd M n from 33 × 104 to less than 1 × 104 g/mol (Figure ), and poly(Ind-co-St) presented the lowest M n and M w/M n values Citation[3]. Lim narrows the polymer polydispersity as well as reducing the Ind conversion compared to Ind–St copolymerization. The T g values of poly(Ind-co-EBN) and poly(Ind-co-Lim) of 160 and 140 °C, respectively, were lower than that of PInd (210 °C) when the comonomer concentration in the feed increased up to 50%. Thus, a different Ind product can be obtained by changing the comonomer type and reaction conditions.
Figure 7 M n (–▪–) and T g (–▲–) values of the Ind-copolymers as a function of the comonomer concentration: (a) poly(Ind-co-St), (b) poly(Ind-co-ENB), and (c) poly(Ind-co-Lim).
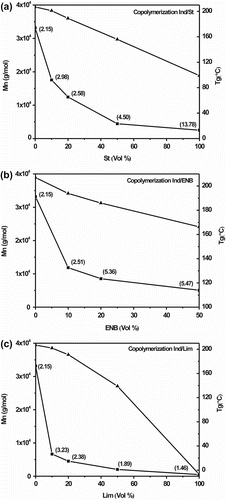
Figure shows the DSC endothermic curve profiles of PInd and the Ind-copolymers (Ind:St MR = 0.9; Ind:Lim MR = 1.4; Ind:ENB MR = 1.0). A baseline deviation in the endothermic curves is used to determine the glass transition temperature of the samples and the absence of a melting point corroborates the amorphous nature of the Ind-copolymers produced by cationic polymerization Citation[11,21]. Since they are amorphous, the Ind-copolymers are transparent and rigid and have high T g values. A decrease in the T g value indicates additional flexibility in the copolymer chain relative to the homopolymer. The lower T g values of the Ind-copolymers can also be related to their M n values since they are significantly lower than that of PInd, which has a higher density of end chains or higher entropy. As the T g of the PSt with an M n of 2.5 × 103 g/mol (99 °C) was similar to that of commercial high molecular weight PS, it is possible to attribute the T g reduction in the Ind-copolymer to the comonomer structure rather than the size of the polymers. The comonomer has a less constrained structure than Ind, which favors chain flexibility at lower temperatures. As was previously reported Citation[4] for ENB carbocationic polymerization, both double bonds undergo a transannular rearrangement to give a rigid tricyclic repeating structure with a resulting high T g. Thus, Ind polymers with T g values higher than 100 °C can be obtained by using a comonomer with a cyclic structure.
Figure 8 DSC endothermic curves of PInd and Ind-copolymers: (a) poly(Ind-co-St)/Ind:St MR = 0.9 and poly(Ind-co-Nb)/Ind:ENB MR = 1.0; (b) poly(Ind-co-Lim)/Ind:Lim MR = 1.4.
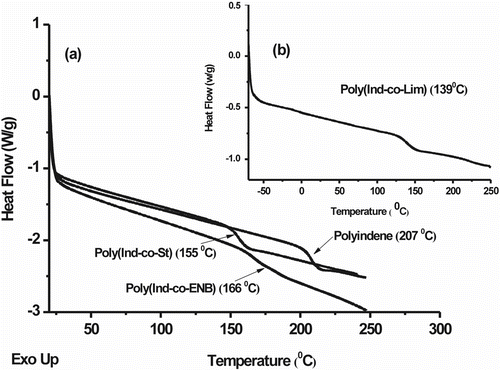
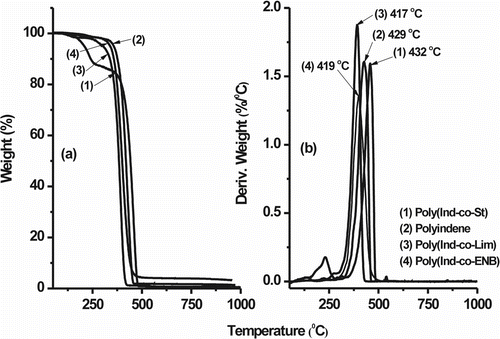
Figure shows the TGA mass loss and derivative curve profiles, respectively, of the Ind-copolymer samples relative to those of PInd. All the polymers are thermally stable up to about 300 °C under a nitrogen atmosphere. PInd and the Ind–St copolymer (poly(Ind-co-St)) were the most thermally stable and reached an apex decomposition peak (T dmax) at approximately 429 and 432 °C, respectively. Poly(Ind-co-St) underwent mass loss in the interval of 130–250 °C due to residual monomer and oligomeric products. Poly(Ind-co-Lim) and poly(Ind-co-ENB) show slightly lower average decomposition temperatures with T dmax values of 417 and 419 °C, respectively. At 1000 °C, the residue of the Ind-copolymers with St, Lim, and ENB were 0.5, 1.3, and 3.4%, respectively. The higher residue for poly(Ind-co-ENB) must be the result of cyclization reactions that occurred due to the structure of the comonomer and the residual lateral double bond as the sample was progressively heated to higher temperatures.
Conclusions
Amorphous rigid homo- and Ind-copolymers with Lim and ENB were produced via cationic polymerization with a Friedel–Craft catalyst. PInd with average M n values as high as 45 × 103 g/mol and a T g of 207 °C was obtained at −20 °C using AlCl3 without an electron donor, whereas the same reaction conditions yielded only oligomeric products of PLim and PS. The comonomer Lim was the most active chain-transfer agent followed by St and ENB. The MWD of the Ind-copolymer broadened as the concentration of St and ENB in the feed increased. On the other hand, an increase in the Lim concentration in the feed reduced the polymer polydispersity and molecular weight. The Ind-copolymers presented high T g values, which decreased with the comonomer content. The incorporation of cyclic diolefin (i.e. Lim and ENB) in the main chain depressed both the M n and T g of the Ind-copolymers, with the exception of poly(Ind-co-ENB) obtained with a MR of 1:1 in the feed, which was a cross-linked product due to the simultaneous reaction of the lateral double bond. Poly(Ind-co-ENB) obtained with ENB concentrations in the feed up to 20 mol% did not undergo a crosslinking reaction and were either soluble or partially soluble in THF depending on the ENB concentration. The Lim lateral double bond did not react during the copolymerization reaction. Both poly(Ind-co-Lim) and soluble poly(Ind-co-ENB) can be used as unsaturated resins or as an intermediates in other syntheses. All the Ind-polymers were thermally stable up to approximately 300 °C and thus a cyclic diolefin can be used as a comonomer without affecting the thermal stability of the polymer.
Acknowledgments
The financial support of the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES/PROCAD Project 200/2007) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq Projeto Universal 490845/2008-8) is gratefully acknowledged. The authors are grateful to Dr. Jairton Dupont of the Chemical Institute/UFRGS for his NMR analysis support and Dr. Ivo Vedana and Dr. Rosane Lighabue of the Chemical Faculty/PUC-RS for their GPC analysis support.
References
- Goethals , EJ and Du Prez , F . 2007 . Carbocationic polymerizations . Prog. Polym. Sci. , 32 : 220 – 246 .
- Sigwalt , P and Moreau , M . 2006 . Carbocationic polymerization: mechanisms and kinetics of propagation reactions . Prog. Polym. Sci. , 31 : 44 – 120 .
- Puskas , JE and Kaszas , G . 2000 . Living carbocationic polymerization of resonance-stabilized monomers . Prog. Polym. Sci. , 25 : 403 – 452 .
- Aoshima , S and Kanaoka , SA . 2009 . Renaissance in living cationic polymerization . Chem. Rev. , 109 : 5245 – 5287 .
- Salari , D and Jodaei , A . 2006 . Petroleum resin preparation by cationic polymerization of pyrolysis gasoline . Iran Polym. J. , 15 : 55 – 64 .
- Wang , H , Bennevault-Celton , V , Cheng , B and Cheradame , H . 2007 . Cationic polymerization of 1,3-pentadiene and 2-methylpropene: direct initiation is a general mechanism with AlCl3 in polar medium . Eur. Polym. J. , 43 : 1083 – 1090 .
- Kaputskii , FN , Mardykin , VP , Gaponik , LV , Lesnyak , VP , Kostyuk , SV and Mil’chanina , TL . 2002 . Synthesis of petroleum polymeric resin by cationic polymerization of the C9 fraction . Russ. J. Appl. Chem. , 75 : 1006 – 1008 .
- Thomas , L , Polton , A , Tardi , M and Sigwalt , P . 1992 . “Living” cationic polymerization of indene. 1. Polymerization initiated with cumyl methyl ether/titanium tetrachloride and cumyl methyl ether/n-butoxytrichlorotitanium initiating systems . Macromolecules. , 25 : 5886 – 5892 .
- Thomas , L , Polton , A , Tardi , M and Sigwalt , P . 1995 . “Living” cationic polymerization of indene. 3. Kinetic investigation of the polymerization of indene initiated with cumyl methyl ether and cumyl chloride in the presence of titanium derivatives . Macromolecules. , 28 : 2105 – 2111 .
- Kennedy , JP , Midha , S and Keszler , B . 1993 . Living carbocationic polymerization. 55. Living polymerization of indene . Macromolecules. , 26 : 424 – 428 .
- Kennedy , JP , Midha , S and Tsunogae , Y . 1993 . Living carbocationic polymerization. 56. Polyisobutylene-containing block polymers by sequential monomer addition. 8. Synthesis, characterization, and physical properties of poly(indene-b-isobutylene-b-indene) thermoplastic elastomers . Macromolecules. , 26 : 429 – 435 .
- Delfour , M , Bennevault-Celton , V , Anh Nguyen , H , Macedo , A and Cheradame , H . 2004 . Cationic polymerization of dienes VII. New electron donors in the polymerization of 1,3-pentadiene initiated by aluminum trichloride in non-polar solvent . Eur. Polym. J. , 40 : 1387 – 1398 .
- Frolov , AN , Kostjuk , SV , Vasilenko , IV and Kaputsky , FN . 2010 . Controlled cationic polymerization of styrene using AlCl3OBu2 as a coinitiator: toward high molecular weight polystyrenes at elevated temperatures . J. Polym. Sci., Part A: Polym. Chem. , 48 : 3736 – 3743 .
- Bennevault-Celton , V , Badi , N and Cheradame , H . 2009 . Cationic polymerization of dienes VIII: is the elimination of cross-linking by a bulky electron donor a general behavior in the presence of aluminium trichloride? . Eur. Polym. J. , 45 : 837 – 845 .
- Peetz , RM , Moustafa , AF and Kennedy , JP . 2003 . Cationic polymerization of norbornadiene . J. Polym. Sci., Part A: Polym. Chem. , 41 : 732 – 739 .
- Lu , J , Kamigaito , M , Sawamoto , M , Higashimura , T and Deng , YX . 1997 . Living cationic isomerization polymerization of β-Pinene. 2. Synthesis of block and random copolymers with styrene or p-methylstyrene . Macromolecules. , 30 : 27 – 31 .
- Liu , S , Xie , C , Yu , S and Liu , F . 2009 . Polymerization of α-pinene using Lewis acidic ionic liquid as catalyst . Catal. Commun. , 10 : 986 – 988 .
- Kukhta , NA , Vasilenko , IV and Kostjuk , SV . 2011 . Room temperature cationic polymerization of β-pinene using modified AlCl3 catalyst: toward sustainable plastics from renewable biomass resources . Green Chem. , 13 : 2362 – 2364 .
- Givehchi , M , Tardi , M , Polton , A and Sigwalt , P . 2000 . Influence of monomer concentration and dielectric constant on transfer reactions in the carbocationic polymerization of indene . Macromolecules. , 33 : 710 – 716 .
- Goel , S , Mazumdar , NA and Gupta , A . 2010 . Synthesis and characterization of poly (indene-co-pyrrole) nanofibers . Polym. Adv. Technol. , 21 : 888 – 895 .
- Shokyoku , K , Nobuyuki , I , Akira , T , Hitoshi , Y and Toshinobu , H . 2002 . Cationic copolymerization of indene with styrene derivatives: synthesis of random copolymers of indene with high molecular weight . J. Polym. Sci., Part A: Polym. Chem. , 40 : 2449 – 2457 .
- Soga , K and Monoi , T . 1989 . Copolymerization of styrene with indene by the Ti(OiPr)4–methylaluminoxane catalysts . Macromolecules. , 22 : 3823 – 3824 .
- Hahn , SF and Hillmyer , MA . 2002 . High glass transition temperature polyolefins obtained by the catalytic hydrogenation of polyindene . Macromolecules. , 36 : 71 – 76 .
- Li , H , Li , J , Zhang , Y and Mu , Y . 2008 . Homo- and copolymerization of 5-ethylidene-2-norbornene with ethylene by [2-C5Me4–4,6- t Bu2C6H2O]TiCl2/Al i Bu3/Ph3CB(C6F5)4 catalyst system and epoxidation of the resulting copolymer . Polymer. , 49 : 2839 – 2844 .
- Ricci , G , Leone , G , Rapallo , A , Biagini , P , Guglielmetti , G and Porri , L . 2011 . Syndiospecific oligomerization and polymerization of norbornene with titanium catalysts . Polymer. , 52 : 5708 – 5715 .
- Maślińska-Solich , J and Rudnicka , I . 1988 . Optically active polymers – I. Copolymerization of β-pinene with maleic anhydride . Eur. Polym. J. , 24 : 453 – 456 .
- Sharma , S and Srivastava , AK . 2007 . Azobisisobutyronitrile-initiated free-radical copolymerization of limonene with vinyl acetate: synthesis and characterization . J. Appl. Polym. Sci. , 106 : 2689 – 2695 .
- Kostjuk , SVD . 2007 . Alexei YuVasilenko, Irina V. Frolov, Alexander N. Kaputsky, Fyodor N. Kinetic and mechanistic study of the quasiliving cationic polymerization of styrene with the 2-phenyl-2-propanol/AlCl3·OBu2 initiating system . Eur. Polym. J. , 43 : 968 – 979 .
- Blank , F , Scherer , H and Janiak , C . 2010 . Oligomers and soluble polymers from the vinyl polymerization of norbornene and 5-vinyl-2-norbornene with cationic palladium catalysts . J. Mol. Catal. A: Chem. , 330 : 1 – 9 .
- Saroj , S and Srivastava , AK . 2004 . Synthesis and characterization of copolymers of limonene with styrene initiated by azobisisobutyronitrile . Eur. Polym. J. , 40 : 2235 – 2240 .
- Socrates , G . 1994 . Infrared characteristic group frequencies tables and charts , 2nd ed. , New York , NY : John Wiley & Sons .
- Priyadarsi , D and Sathyanarayana , DN . 2002 . Synthesis and characterization of copolyperoxides of indene with styrene, α-methylstyrene, and α-phenylstyrene . J. Polym. Sci. Part B: Polym. Phys. , 40 : 2004 – 2017 .
- Noskov , AM . 1969 . IR spectra and structure of polymers of indene and coumarone . J. Appl. Spectrosc. , 10 : 403 – 406 .
- Le Person , A , Eyglunent , G , Daële , V , Mellouki , A and Mu , Y . 2008 . The near UV absorption cross-sections and the rate coefficients for the ozonolysis of a series of styrene-like compounds . J. Photochem. Photobiol. A. , 195 : 54 – 63 .
- Sakurada , I , Ise , N , Hayashi , Y and Nakao , M . 2002 . Cationic copolymerization of styrene with indene or α-methylstyrene catalyzed with boron trifluoride etherate under an electric field . Macromolecules. , 1 : 265 – 270 .
- Cole , KC , Thomas , Y , Pellerin , E , Dumoulin , MM and Paroli , RM . 1996 . New approach to quantitative analysis of two-component polymer systems by infrared spectroscopy . Appl. Spectrosc. , 50 : 774 – 780 .
- ASTM E168-06. Standard practices for general techniques of infrared QUANTITATIVE analysis. 2006.