Abstract
In order to understand the mechanism of narrow particle size distribution of the final latex during particle coagulation, a series of experiments were performed to investigate the effect of polymer nature on particle coagulation capability. In particular, thermodynamics and kinetics in aqueous phase were considered to illustrate the detail process of particle coagulation. The final particle size decreased with the increasing side chain length of alkyl methacrylate from 181.5 nm in MMA to 131.6 nm in EMA, 119.3 nm in PMA, and 115.1 nm in BMA, indicating that the particle coagulation capability was proportional to the hydrophilicity of polymer. With increasing polymer hydrophilicity, the affinity between surfactant molecules and particle surface decreased, thus enhancing the particle coagulation capability. Moreover, the critical length of oligomer radical also increased with increasing hydrophilicity and the efficiency of radical capture decreased, thus increasing the saturation of monomer concentration in the inner part of particle, promoting particle coagulation. Combining these results and the La Mer Diagram, a novel approach was developed to prepare large-scale, narrow-dispersed, and high solid content polymer latex based on particle coagulation mechanism. Three criteria, namely, rapid nucleation, fast coagulation, and a long growth period, should be met to produce latex with a narrow particle size distribution.
1. Introduction
In the past several decades, many studies on the particle coagulation mechanism have been reported in the heterogeneous polymerization system owing to its significance in controlling the particle size distribution and morphology.[Citation1–7] However, determining the relationship between particle coagulation and particle size distribution of final latex particles is still a matter of debate, for instance, Ishii et al. [Citation8] considered that a steady growth without the aggregation of particles was necessary for preparing monodisperse polymer particles; In contrast, Telford et al. [Citation9] considered that the monodisperse polymer particles could be obtained in the presence of particle coagulation.
To investigate this phenomenon, some investigators carried out a series of experiments or simulation studies. Dobrowolska et al. [Citation10,11] obtained direct evidence of particle coagulation by tracking the evolution of particle morphology during the polymerization using high-resolution scanning electron microscopy and pointed that the ionic strength played an important role in determining particle coagulation capability. Chou and Chiu [Citation12] proposed that initiator systems were pivotal in controlling the extent of particle coagulation. Chern et al. [Citation13,14] considered that surfactant systems also played an important role in determining the particle coagulation capability. In addition to these, Gilbert et al. [Citation15,16] proposed the modified coagulation theory based on the standard Smoluchowski equation and simulated the evolution of particle size distribution during the particle coagulation.
However, some important factors were ignored in these studies, namely, (i) varying capability of particle coagulation for different latex particles, (ii) ways to control the particle coagulation, and (iii) preparing narrow distribution latex particles.
To overcome these problems, the particle coagulation behavior of different polymers was investigated in detail by tracking the evolution of particle size and number as a function of monomer conversion under the same reaction conditions. The particle coagulation behavior was explained by different mechanisms such as thermodynamics and radical reaction kinetics in aqueous phase. Furthermore, combining with the La Mer Diagram and the competitive growth kinetics, a novel physical model based on particle coagulation mechanism was proposed.
2. Experimental
2.1. Materials
Methyl methacrylate (MMA), ethyl methacrylate (EMA), propyl methacrylate (PMA), butyl methacrylate (BMA), and styrene (St) were purchased from the Fuchen Chemical Reagent Factory (Tianjin, China) and distilled under reduced pressure to remove the inhibitor prior to use as the reaction monomers. Potassium persulphate (KPS) (≥99.0%), sodium dodecyl sulfate (SDS) (≥98%), potassium carbonate (K2CO3), and methanol (≥99.5%) were purchased from the Fuyu Fine Chemical Factory (Tianjin, China) and used without any further purification. Methanol solution containing 1 wt% hydroquinone was selected as the polymerization inhibitor to terminate the polymerization reaction. Distilled deionized water (DDI) was used for all the experiments.
2.2. Polymerization reaction
Polymerization reactions were performed in a 500-mL three-necked round-bottom flask equipped with a water bath, anchor stirrer, and reflux condenser. The initiator (KPS), surfactant (SDS), and electrolyte (K2CO3) concentrations were kept at 0.6, 1.0, and 0.6 for aqueous phases, respectively. Polymerization experiments were performed as follows: the electrolyte, surfactant, and DDI were added to the reactor. After the dissolution of electrolyte and surfactant in DDI, the monomer was added to the reactor. Nitrogen gas was ventilated to remove the oxygen. Finally, the initiator dissolved in DDI was charged into the reactor. The time of the addition of initiator was noted as time zero. The polymerization temperature was set at 65 °C. The polymerization reaction times for different aqueous phase compositions were set as 40 and 180 min (runs 1–1, 1–2, 1–3, 2–1, 2–2, and 2–3 were 40 min; runs 3–1, 3–2, and 3–3 were 180 min). All the experimental recipes are listed in Table .
Table 1. Standard recipe used in different reaction systems.
2.3. Characterizations
2.3.1. Particle size and distribution
The particle size and distribution of the polymer latex particles were characterized using a 90 plus particle size analyzer (Brookhaven, US). Five measurements were made for each sample at 10 min, and the mean value of these five measurements was considered as the actual value. The particle size distribution of polymer latex was expressed by the polydispersity index (PDI). At the same time, particle size and distribution was also obtained by transmission electron microscopy (TEM, JEOL-1210). The detailed procedure of the TEM for particle size distribution is as follows: first, the latex particles were directly diluted in DDI to approximately 1/10,000, then the diluted latex was removed to the copper mesh covered by a carbon film using a pipette; and the samples on the copper mesh was dried at room temperature. Finally, the particle size and distribution of the latex particles were observed by TEM at 100 kV at 100,000× magnification.
2.3.2. Contact angle and surface tension
The contact angle between the polymer and aqueous phase were measured using a Drop shape analysis instrument DSA-30 (KRÜSS, Germany) used to characterize the hydrophilicity (or polarity) of polymer. The polymer film was obtained by the solvent (toluene) split membrane. The surface tension of the polymeric latexes was also measured using this machine.
2.3.3. Glass translation temperature
Glass translation temperature (Tg) was determined using a dynamic mechanical analyzer (DMA242, Germany) at a frequency of 1 Hz and a heating rate of 3 °C/min in the temperature ranged −40 to 140 °C. A point of tan δ was recorded as a function of temperature. The Tg was taken as the maximum temperature in tan δ plots.
2.3.4. Monomer conversion
The monomer conversion (X) was defined as the ratio of polymer content to the monomer fraction in the reaction mixture at t = 0, and could be calculated using the following equation:(1)
Where the solid include the initiator, emulsifier, and electrolyte in the sample.
2.3.5. Particle number
The particle number (Np) was obtained according to the following expression:(2)
Where M0 was the monomer concentration in the unit aqueous phase (g/L), ρp was the polymer density (g/cm3), which was directly obtained from the polymer density instrument. And the Dp (nm) was the average size of the latex particles.
3. Results and discussion
3.1. Effect of polymer nature on particle coagulation
Alkyl methacrylates with different side chain lengths were selected as the reaction monomer to investigate the effect of polymer nature on particle coagulation capability. Figure shows that the evolution of particle size and number as a function of monomer conversion for emulsion polymerization reactions of alkyl methacrylates with different side chain lengths, clearly indicating that the particle size increased with increasing monomer conversion as expected, but the final latex particle size decreased with increasing side chain length from 181.5 nm in MMA to 131.6 nm in EMA, 119.3 nm in PMA, and 115.1 nm in BMA. Moreover, a rapid increase in particle size between 0 and 0.24 was observed in MMA system. It was interesting to note that the trend of Np in all the systems was found to be governed by the monomer nature. With increasing side chain length, the final particle number increased from 1.51 × 1017 L−1 in MMA to 4.62 × 1017, 6.23 × 1017, and 8.0 × 1017 L−1 in EMA, PPM, and MMA, respectively. In contrast to the normal trend of particle number, the particle number density in excess of 8.0 × 1017 L−1 was observed in the early nucleation period and dropped to a constant value in the monomer conversion range 0.21–0.35. The above results suggest that a large amount of particles was generated at the beginning of the reaction by micelle nucleation mechanism, and the unstable particles coagulated until the critical stability of particle was achieved. Kemmere et al. [Citation17] investigated the coagulation behavior during the polymerization of styrene and vinyl acetate, indicating that the particle coagulation would take place if the kinetic energy of the particles was sufficiently high to overcome the potential energy barrier. In our reaction systems, the potential energy was mainly attributed to the adsorption of surfactant molecules on the particle surface, because of the large amount surfactant. The adsorption capability of surfactant molecules on the particle surface not only depended on the surfactant, but also on the nature of polymer particles.
Figure 1. Plot of diameter and particle number versus reaction time in the batch emulsion polymerization of different alkyl methacrylate in pure water system (
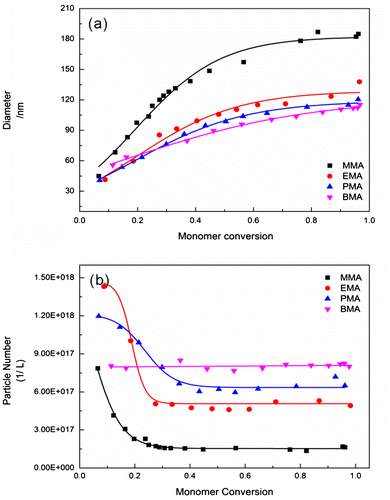
The polarity of polymer decreases with the increasing side chain length, thus decreasing the hydrophilicity of polymer. Piirma et al. investigated the adsorption of surfactant molecules on latex particles and found that the affinity between the surfactant and polymer surface decreased with increasing hydrophilicity of polymer,[Citation18] leading to the tight packing of the surfactant molecules on the weak hydrophilicity polymer surface such as PBMA. As a result, the stability of particles decreased with increasing polymer hydrophilicity. Further, the coagulation capability of latex particles would improve with decreasing side chain length. Certainly, the source of particle stability could also be explained by the interfacial tension view according to the theory of Vijayendran [Citation19], i.e. the free energy of adsorption was controlled by the interfacial tension and was proportional to that. For the weak hydrophilicity polymer such as PBMA, the interfacial tension between the polymer and the aqueous phase was much more than that between the strong hydrophilicity one (ca. PBMA: 36.7 mN/m; PMMA: 26.0 mN/m). Thus, the adsorption capability of surfactant molecules on the polymer surface increased with decreasing hydrophilicity of polymer, thus the stability of particles increased with increasing side chain length.
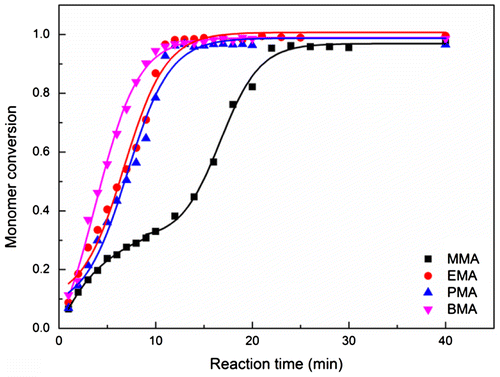
In addition to these, particle coagulation behavior was examined by tracking the monomer conversion as a function of polymerization time, as shown in Figure , clearly indicating an obviously slow growth in the MMA system in the early reaction period from 5 to 11 min. This indicates that the particle number in this period was significantly less among all the systems (polymerization rate ∝ Np). The promoted particle coagulation decreased the particle number and increased the particle size. Besides, the critical radical length also could be considered as another factor determining the particle number. The critical oligomeric radical length would increase with the increasing hydrophilicity of polymer (e.g. ~65 for PMMA, [Citation20], ~5 for PS [Citation21]), thus increasing the solubility of growing chains and delaying the precipitation, further, decreasing the particle number in the nucleation period. Therefore, the initial particle size was largest for PMMA latex particles.
Figure 2. Plots of monomer conversion versus reaction time in the batch emulsion polymerization of different alkyl methacrylate in pure water system (
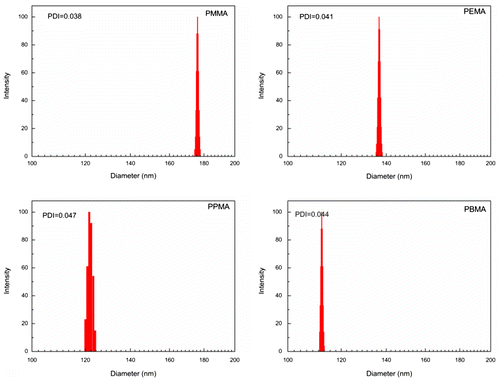
Based on the above-mentioned analysis, we concluded that the capability of particle coagulation was controlled by polymer nature such as hydrophilicity by affecting the adsorption of surfactant molecules on the polymer surface and the critical oligomeric radical length in the nucleation period. Subsequently, the effect of polymer nature on particle size distribution of latex particles was investigated. As shown in Figure , the particle size distribution of latex particles was narrow for all latex systems, because the PDI of the latexes was ~0.1, [Citation22] attributed to the reversibility of the particle coagulation [Citation23] and the competitive growth mechanism.[Citation24]
Figure 3. The particle size distribution of final latexes prepared by emulsion polymerization of alkyl methacrylate with different side chain lengths under same conditions.
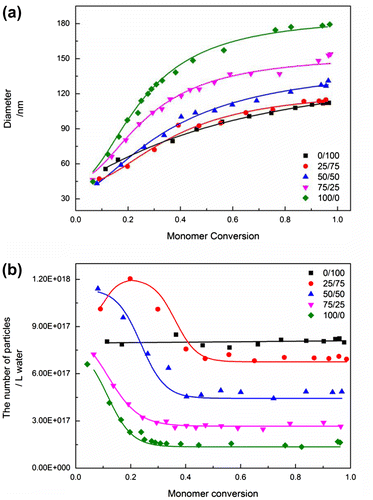
The polymer nature is known to be controlled by copolymerizing with different monomers. Next, the effect of copolymerizing different monomers such as BMA and MMA was investigated. A series of copolymerization reactions with different MMA/BMA ratios were performed to prove this assumption. Figure shows that the curves of tan δ as a function of temperature for the copolymer prepared at different monomer compositions. The temperature corresponding to the maximum value of tan δ is well known to be the glass translation temperature of the copolymer. Thus, the glass translation temperature of the copolymer was found to increase from 41.6 °C at 0/100 to 50.9, 79.6, 103.5, and 126.4 °C at 25/75, 50/50, 75/25, and 100/0 with increasing MMA content as listed in Table , indicating that the copolymer was formed by random copolymerization of MMA and BMA. The contact angle of copolymer decreased with increasing MMA content from 89.2 °C at 0/100 to 85, 80.4, 75.1, and 69.2 °C at 25/75, 50/50, 75/25, and 100/0, respectively.
Figure 4. The curves of tan δ as a function of temperature for the polymers prepared by batch emulsion polymerization at different monomer compositions (BMA/MMA).
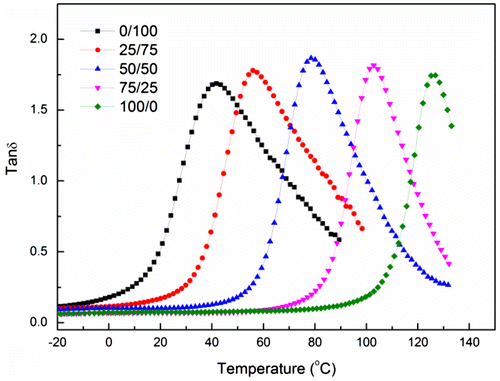
Table 2. The detailed parameters of final latexes prepared by batch emulsion polymerization using different BMA/St ratios.
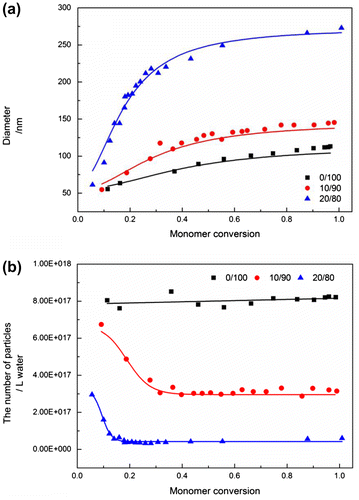
Figure shows the evolution of particle size and number as a function of monomer conversion for the emulsion copolymerization of MMA and BMA at different ratios. Similar to the effect of polymer nature on particle coagulation as discussed before, the particle size of the final latex particles increased with increasing MMA content from 115.1 nm at 0/100 MMA/BMA ratio to 119.6, 136.8, 154.3, and 181.5 nm at 25/75, 50/50, 75/25, and 100/0 MMA/BMA ratio. Moreover, particle number decreased with increasing monomer conversion, and the extent of the decrease in particle number increased with increasing MMA/BMA ratios, indicating that the capability of particle coagulation increased with increasing MMA content, and the particle size of the final latex particles could also be controlled by varying monomer composition.
3.2. Effect of reaction kinetics in aqueous phase on particle coagulation
Interface phenomenon is known to play an important role in determining the extent of particle coagulation in the emulsion polymerization system. However, the interfacial behavior between polymer latex particles and aqueous phases not only depend on the polymer nature as discussed above but also on the aqueous phase composition. To further investigate the effect of cosolvent content on particle coagulation, methanol was added as the cosolvent to the reaction recipe to adjust the reaction kinetics in the aqueous phase. Kim et al. [Citation25] investigated the polymerization mode of MMA in methanol solution and found that a transition from emulsion to dispersion occurred when methanol content exceeded 30 wt% in a soap-free system. Therefore, the methanol concentration was controlled at the 30 wt% in our study for maintaining the emulsion system.
Figure shows the evolution of particle size and number as a function of monomer conversion for the emulsion polymerization of BMA at different methanol contents. This clearly indicated that the particle size of the final latex particles increased with increasing methanol content from 115.1 nm in pure water medium to 132.6 and 272.1 nm in 10 and 20 wt% methanol solution, respectively. Based on the trend of particle size and number, the capability of particle coagulation increased with increasing methanol content in the medium. The effect of cosolvent (methanol) on particle coagulation could be understood by two aspects.
Figure 6. The evolutions of particle size and number as functions of monomer conversion in emulsion polymerization of BMA in solutions of different methanol content (methanol/water =
From the view of the reaction kinetics in aqueous phase, the addition of methanol could enhance the solubility of growing chains and delay their precipitation, further prolonging the nucleation period. Smeets et al. [Citation26] investigated the critical length of oligomers (jcrit) in methanol solution and found that the jcrit increased with increasing methanol content and temperature. The value of jcrit is known to govern the rate of particle coagulation and the rate of the radical entering the polymer particles according to the Smoluchowski theory of rapid coagulation. With increasing methanol content, the jcrit increased, and the rate of the radical entering the polymer particles decreased, increasing the saturated monomer concentration. Therefore, the particle stability reduced, and the extent of particle coagulation also increased. At the same time, the polymerization rate decreased because of the decrease in the rate of the radical entering the polymer particles. This interpretation was analogous to the modified Morton equation reported by Nomura et al. [Citation27]
In contrast, from the thermodynamics view, the addition of methanol could reduce the interfacial tension between the polymer particles and aqueous phase.[Citation28] With increasing methanol content, the surface tension of medium phase decreased from 71.98 mN/m in pure water to 58.3 and 49.05 mN/m in 10 and 20 wt% methanol solution, respectively. According to the Derjaguin–Landu–Verwey–Overbeek theory, the particle stability is derived from the sum of the attractive potential (UA) energy and the repulsive energy (UR) and can be expressed by the following equation: [Citation29](3)
where A and H are the Hamaker constant and the distance of neighboring particle surface in the system, respectively; and Dv is the diameter of latex particle.(4)
where s = 2 + H/R = 2 + 2H/Dv, the ψ0 is the surface potential proportional to the surface charge density (σ), depending on the amount of emulsifier and initiator residues adsorbed on the particle surface; ε is the dielectric constant of water; kB is the Boltzmann constant; T is the absolute temperature; e is the charge of a single electron; NA is the Avagadro’s number; γ and Z are the ionic concentration and the charge of the cations in water, respectively; R12 = 2 R1 R2/(R1 + R2) and s = 2 + (H/R12). Based on this theory, the addition of methanol increased the attractive energy and decreased the repulsive energy, promoted particle coagulation.
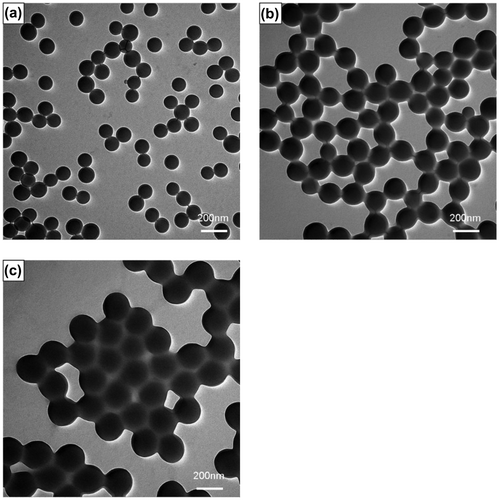
Styrene, a hydrophobic monomer, is well known to be the most common monomer in emulsion polymerization systems. Thus, the emulsion polymerization reactions of styrene at different methanol contents were performed to investigate the theory discussed above. The morphologies of the final polystyrene latex particles prepared at different methanol concentrations are shown in Figure , clearly indicating the formation of monodisperse and spherical polystyrene microspheres, and the particle size increased from 95.9 nm in pure water to 163.4 and 271.5 nm in 15 and 30 wt% methanol solution, respectively, as expected with increasing methanol concentration. This proved the particle coagulation could be induced by methanol in the emulsion polymerization of styrene.
3.3. Preparation of large-scale and narrow-distributed latex particles based on particle coagulation
In order to understand the formation of monodispersed polymer latex particles under the particle coagulation condition, a schematic illustration of the particle coagulation growth in the batch emulsion polymerization was proposed as shown in Figure and is analogous to the La Mer Diagram.[Citation29] The primary particles were formed in a short period of nucleation time, and the particle coagulation occurred at the same time because the particle stability decreased with increasing particle size. Notably, the process of particle coagulation was reversible and controlled by the balance between the clustering of primary particles and the rate of particle detachment as argued by Dobrowolska and Koper [Citation11] The particle coagulation process (aggregation process) could be considered as two steps: (1) the association of multiple latex particles formed a reversible aggregated particle cluster, in which the reversibility was controlled by the reaction kinetics in aqueous phase such as the rate of the radical entering the polymer particles and the collision frequency of the particles; (2) the latter one was the merging process, also called flocculation, which is irreversible and controlled by the polymer nature such as the Tg.
Figure 8. Schematic illustration of the relationship between the particle coagulation growth and the preparation of the narrowly dispersed polymer latex in the batch emulsion polymerization.
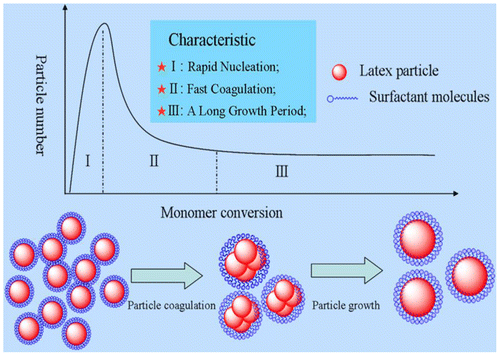
The reversible balance during the particle aggregation process narrowed the difference in the particle cluster size and promoted a narrow particle size distribution. The disaggregation of the particle cluster would occur if the particle cluster is much larger than others because the particle cluster stability was not enough to maintain the increase in the surface tension. Sajjadi [Citation30] demonstrated that the critical surface coverage of surfactant on the particle surface was different at different reaction periods. The reversible balance during the particle aggregation process was only necessary for preparing monodispersed polymer latex particles, and the time of the particle aggregation was another important factor for determining particle size distribution of the final latex particles attributed to the coalescence of the particle cluster. When the particles are soft, they easily merge to form an actual particle. However, as the polymerization reaction proceeds, the particle gradually transforms to hard from soft, because the monomer concentration in the inner side of particle decreases, resulting in the formation of a cross-linked structure. Certainly, this process could be understood by the particle description or adsorption on the particle surface as described by Herrera-Ordonez et al. [Citation31] Thus, the polymerization condition to prepare monodispersed polymer based on particle coagulation was obtained, the particle coagulation occurred in the early nucleation period and could be expressed by rapid nucleation (Step I) and fast coagulation (Step II).
In addition to these, competitive growth mechanism was another factor promoting narrow dispersed latex particles. When the particle cluster was formed by particle aggregation, the relative particle surface decreased. In other words, the ratio of the surface and volume (S/V) decreased, further decreasing the rate of monomer adsorption, thus decreasing the rate of large particle growth, and favoring the formation of monodispersed latex particles. Han et al. also proved that the process mentioned above (competitive growth mechanism) was responsible for the monodispersed latex obtained by surfactant-free polymerization system.[Citation24] This process was similar to the self-sharpening in particle growth.[Citation8,32,33] Thus, the second condition to prepare monodispersed polymerization based on particle coagulation was obtained, i.e. the competitive growth mechanism, indicating that the time for competitive particle growth should be long enough to overcome the self-sharpening of particle growth, as shown the step III in Figure .
This model provided an approach to prepare monodispersed, large-scale, and relatively high solid content latex based on particle coagulation mechanism in conventional emulsion polymerization and explained the occurrence of particle coagulation and the formation of monodispersed latex. A quantitative study on the relationship between the PDI of the final latex particles and polymerization recipe is in progress and will be reported in the near future.
4. Conclusions
In this study, a series of emulsion polymerization reactions of alkyl methacrylate with different side chain lengths were performed to investigate the effect of polymer nature on particle coagulation. With increasing side chain length, the final latex particle size decreased from 181.5 nm in MMA to 131.6, 119.3, 115.1 nm in EMA, PMA, and BMA, respectively, and was inversely proportional to the final particle number. These phenomena proved that the capability of particle coagulation was determined by the polymeric nature. By the copolymerization of MMA and BMA or the addition of methanol into the system, the polymer or aqueous phase was adjusted to control the extent of particle coagulation. The experimental results show that the capability of particle coagulation was proportional to the hydrophilicity of the polymer and inversely proportional to the polarity of the aqueous phase. The electrostatic repulsion, surfactant adsorption, and the kinetic of the aqueous phase could be the natural factors leading to particle coagulation. In addition, a physical model to explain the narrow dispersed latex particles was obtained in the presence of particle coagulation. Certainly, this physical model could also be considered as a novel approach to prepare large-scale narrow-dispersed polymer latex particles.
Acknowledgments
The authors acknowledge the financial support from the National Natural Scientific Foundation of China (No. 51573022).
Funding
This work was financially supported by the National Natural Scientific Foundation of China [grant number 51573022].
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Zhang Q, Yu G, Wang WJ, et al. Preparation of CO2/N2-triggered reversibly coagulatable and redispersible polyacrylate latexes by emulsion polymerization using a polymeric surfactant. Macromol. Rapid Commun. 2012;33:916–921.10.1002/marc.201200033
- Fowler CI, Muchemu CM, Miller RE, et al. Emulsion polymerization of styrene and methyl methacrylate using cationic switchable surfactants. Macromolecules 2011;44:2501–2509.10.1021/ma102936a
- Behniafar H, Yousefzadeh D. Chemical synthesis of PEDOT/Ag nanocomposites via emulsion technique in silver colloid. Des. Monomers Polym. 2015;18:6–11.10.1080/15685551.2014.918018
- Zhang Q, Wang WJ, Lu Y, et al. Reversibly coagulatable and redispersible polystyrene latex prepared by emulsion polymerization of styrene containing switchable amidine. Macromolecules 2011;44:6539–6545.10.1021/ma201056g
- Jiang W, Li W, Chen L. Synthesis and characterization of novel acrylate emulsion containing phosphorus and silicon prepared via semi-continuous seeded emulsion polymerization. Des. Monomers Polym. 2015;18:180–184.10.1080/15685551.2014.971393
- Carro S, Herrera-Ordonez J, Castillo-Tejas J. On the evolution of the rate of polymerization, number and size distribution of particles in styrene emulsion polymerization above CMC. J. Polym. Sci., Part A: Polym. Chem. 2010;48:3152–3160.10.1002/pola.v48:14
- Kazantsev OA, Kamorin DM, Orekhov DV, et al. Study of amphiphilic properties of amine- and oligo(ethylene glycol)-containing (meth)acrylic monomers. Des. Monomers Polym. 2015;18:378–384.10.1080/15685551.2015.1012627
- Ishii H, Ishii M, Nagao D, et al. Advanced synthesis for monodisperse polymer nanoparticles in aqueous media with sub-millimolar surfactants. Polymer 2014;55:2772–2779.10.1016/j.polymer.2014.04.011
- Telford AM, Pham BT, Neto C, et al. Micron-sized polystyrene particles by surfactant-free emulsion polymerization in air: synthesis and mechanism. J. Polym. Sci., Part A: Polym. Chem. 2013;51:3997–4002.10.1002/pola.26841
- Dobrowolska ME, van Esch JH, Koper GJM. Direct visualization of “coagulative nucleation” in surfactant-free emulsion polymerization. Langmuir 2013;29:11724–11729.10.1021/la4027927
- Dobrowolska ME, Koper GJM. Optimal ionic strength for nonionically initiated polymerization. Soft Matter 2014;10:1151–1154.10.1039/c3sm51998h
- Chou IC, Chiu WY. Novel synthesis of multi-scaled, surfactant-free monodisperse latexes via alcoholic dispersion polymerization in a mixed ionic/nonionic initiation system. Macromolecules 2013;46:3561–3569.10.1021/ma400277s
- Chern CS, Lee C. Emulsion polymerization of styrene stabilized with an amphiphilic PEG-containing graft copolymer. Macromol. Chem. Phys. 2001;202:2750–2759.10.1002/(ISSN)1521-3935
- Krishnan S, Klein A, El-Aasser MS, et al. Effect of surfactant concentration on particle nucleation in emulsion polymerization of n-butyl methacrylate. Macromolecules 2003;36:3152–3159.10.1021/ma021120p
- Feeney PJ, Napper DH, Gilbert RG. Coagulative nucleation and particle size distributions in emulsion polymerization. Macromolecules 1984;17:2520–2529.10.1021/ma00142a010
- Coen EM, Gilbert RG, Morrison BR, et al. Modelling particle size distributions and secondary particle formation in emulsion polymerisation. Polymer 1998;39:7099–7112.10.1016/S0032-3861(98)00255-9
- Kemmere MF, Meuldijk J, Drinkenburg AAH, et al. Aspects of coagulation during emulsion polymerization of styrene and vinyl acetate. J. Appl. Polym. Sci. 1998;69:2409–2421.10.1002/(ISSN)1097-4628
- Piirma I, Chen SR. Adsorption of ionic surfactants on latex particles. J. Colloid Interface Sci. 1980;74:90–102.10.1016/0021-9797(80)90173-3
- Vijayendran BR. Polymer polarity and surfactant adsorption. J. Appl. Polym. Sci. 1979;23:733–742.10.1002/app.1979.070230308
- Fitch RM, Tsai CH. Particle formation in polymer colloids, III: prediction of the number of particles by a homogeneous nucleation theory. In: Fitch RM, editor. Polymer colloids. Chicago, IL: Springer; 1971. p. 73–102.10.1007/978-1-4684-1920-7
- Ganeva DE, Sprong E, de Bruyn H, et al. Particle formation in ab initio RAFT mediated emulsion polymerization systems. Macromolecules 2007;40:6181–6189.10.1021/ma070442w
- Camli ST, Buyukserin F, Balci O, et al. Size controlled synthesis of sub-100nm monodisperse poly(methylmethacrylate) nanoparticles using surfactant-free emulsion polymerization. J. Colloid Interface Sci. 2010;344:528–532.10.1016/j.jcis.2010.01.041
- Ohshima H. Approximate analytic expression for the stability ratio of colloidal dispersions. Colloid Polym. Sci. 2014;292:2269–2274.10.1007/s00396-014-3257-1
- Li Z, Cheng H, Han CC. Mechanism of narrowly dispersed latex formation in a surfactant-free emulsion polymerization of styrene in acetone-water mixture. Macromolecules 2012;45:3231–3239.10.1021/ma202535j
- Kim G, Lim S, Lee BH, et al. Effect of homogeneity of methanol/water/monomer mixture on the mode of polymerization of MMA: soap-free emulsion polymerization versus dispersion polymerization. Polymer 2010;51:1197–1205.10.1016/j.polymer.2009.12.038
- Smeets NM, Hutchinson RA, McKenna TF. Determination of the critical chain length of oligomers in dispersion polymerization. ACS Macro Lett. 2011;1:171–174.
- Nomura M, Tobita H, Suzuki K. Emulsion polymerization: kinetic and mechanistic aspects. In: Okubo M, editor. Polymer particles. Berlin: Springer; 2005. Vol. 175. p. 1–128. 10.1007/b14102
- Liu BJ, Zhang MY, Gui Y, et al. Effect of aqueous phase composition on particle coagulation behavior in batch emulsion polymerization of styrene. J. Colloids Surf., A 2014;452:159–164.10.1016/j.jcis.2013.11.053
- La Mer VK, Dinegar RH. Theory, production and mechanism of formation of monodispersed hydrosols. J. Am. Chem. Soc. 1950;72:4847–4854.10.1021/ja01167a001
- Sajjadi S. Particle formation and coagulation in the seeded semibatch emulsion polymerization of butyl acrylate. J. Polym. Sci., Part A: Polym. Chem. 2000;38:3612–3630.10.1002/(ISSN)1099-0518
- Herrera-Ordonez J, Rivera O, Maldonado-Textle H, et al. Kinetics of styrene emulsion polymerization above the critical micelle concentration: effect of the initial monomer concentration on the molecular weight. J. Polym. Sci., Part A: Polym. Chem. 2005;43:1963–1972.10.1002/(ISSN)1099-0518
- Nagao D, Yamada Y, Inukai S, et al. Quantitative understanding of the self-sharpening of growing polymer particle size distributions in soap-free emulsion polymerization. Polymer 2015;68:176–182.10.1016/j.polymer.2015.05.020
- Suwabe C, Nagao D, Ishii H, et al. Chemical bonding heterocoagulation of nanoparticles onto polymeric spheres by two-step addition of polymerizable coupling agent. Colloid Polym. Sci. 2015;293:2095–2100.10.1007/s00396-015-3603-y