Abstract
A high thermal stability spirobifluorene core derivative with a substituted diphenylphosphine oxide moiety was synthesized as a new electron transport material (spiro(cyclopenta[def]fluorene-1,5,9′9″-bifluorene)-2′,2″-diylbis(diphenylphosphineoxide) [DSPPO1]) for phosphorescent organic light-emitting diodes (PHOLEDs). The glass transition temperature of DSPPO1 was observed at 156°C and was higher than that of common electron transport materials. The triplet energy of DSPPO1 was 2.77 eV and DSPPO1 was appropriate as an electron transport material for PHOLEDs. The highest occupied molecular orbital and lowest unoccupied molecular orbital of DSPPO1 were −6.51 and −2.70 eV, respectively. The energy level of DSPPO1 was suitable for hole blocking and electron injection as an electron transport material for PHOLEDs.
1. Introduction
It is very important to choose appropriate electron transport materials for phosphorescent organic light-emitting diodes (PHOLEDs) because they affect electron density in the emitting layer, hole block from the emitting layer and triplet exciton quenching from the emitting layer [Citation1–4]. The electron transport materials for PHOLEDs should possess deep highest occupied molecular orbital (HOMO), moderate lowest unoccupied molecular orbital (LUMO), good electron transport properties, good thermal properties and high triplet energy so that the PHOLEDs has high external quantum efficiency [Citation5–14].
A lot of researches have been carried out to develop the electron transport materials and the electron transport materials with a spirobifluorene core structure was one of the well-known structures for good thermal stability, good electron transport properties and high triplet energy [Citation5–14]. The spirobifluorene core structure has a high triplet energy of 2.8 eV and molecular rigidity, which make it suitable to use the core structure as a moiety of thermally stable electron transport materials for PHOLEDs. Therefore, the spirobifluorene core structure can not only suppress the crystallization of organic materials at high temperature, but also provide high external quantum efficiency in PHOLEDs [Citation15–18].
The spirobifluorene core structure showed a high triplet energy of 2.8 eV because of sp3 carbon linkage which disconnects conjugation between two fluorene moieties. The spirobifluorene core structure can be further modified for additional thermal stability while keeping the high triplet energy of the core. Our group reported hole transport materials with a double spirobifluorene core structure. The spiro(cyclopenta[def]fluorene-1,5,9′,9″-bifluorene) with the double spirobifluorene core exhibited good charge transport property, good thermal stability and high triplet energy compared to the spirobifluorene core structure [Citation19–21]. However, there was any research on the electron transport material based on the double spirobifluorene core structure, yet.
In this study, we developed a new electron transport type material, spiro(cyclopenta[def]fluorene-1,5,9′9″- bifluorene)-2′,2″-diylbis(diphenylphosphineoxide) (DSP- PO1), based on the double spirobifluorene core. We attached diphenylphosphine units as a strong electron-withdrawing moiety to the double spirobifluorene core to develop the electron transport material. We synthesize the DSPPO1 from a cyclopenta[def]fluorene core structure and diphenylphosphine moiety and demonstrated a high triplet energy of 2.77 eV and a high glass transition temperature of 156°C as a potential electron transport material.
2. Experimental
2.1. General information
1,2-Dibromobenzene, n-butyllithium and chlorodiphenylphosphine (Aldrich Chem. Co, St. Louis, MO, US.) were used without further purification. Hydrogen peroxide (Duksan Sci. Co., Seoul, South Korea) was used without further purification. 2-Bromo-9H-fluorenone (P&H Co., Yongin, South Korea) was used without further purification. Tetrahydrofuran (THF) was distilled over sodium and calcium hydride.
2.2. Synthesis
2′,2″-Dibromo-spiro(cyclopenta[def]fluorene-1,5,9′9″-bifluorene) was synthesized by a route of the hole transport materials in our previous report [Citation21].
2.3. Synthesis of spiro(cyclopenta[def]fluorene-1, 5,9′9″-bifluorene)-2′,2″-diylbis(diphenylphosphine oxide) (DSPPO1)
2′,2″-Dibromo-spiro(cyclopenta[def]fluorene-1,5,9′9″-bifluorene) (1.40 g, 2.19 mmol) was dissolved in anhydrous THF (30 mL) under an inert atmosphere. The reaction two neck flask was allowed to stir at −78°C for 30 min and then n-BuLi (2.5 M in Hexane, 2.20 mL) was added drop-wise slowly by using an 18 gage needle. The reaction flask was stayed at the same condition for 3 h. A chlorodiphenylphosphine (1.21 g, 5.49 mmol) was added drop-wise slowly by the needle. The reaction flask was gently warmed to room temperature overnight. The reaction mixture was quenched by methanol (10 mL) and distilled water. The reaction mixture was extracted with dichloromethane three times. The combined organic layer was washed with distilled water, and then dried with anhydrous MgSO4. Solvent was removed under a reduced pressure, and the reaction product was purified by silica gel chromatography with a mixture of dichloromethane and n-hexane. A white powdery product was obtained. It was dissolved in dichloromethane (20 mL) and hydrogen peroxide (4 mL), which was stirred 12 h at room temperature. The combined organic layer was washed with distilled water and then dried with anhydrous MgSO4. Solvent was removed by reduced pressure. A white solid product was obtained in 51.7% yield.
1H NMR (400 MHz, CDCl3): δ 8.24–8.13 (m, 2H), 7.99–7.09 (m, 27H), 6.83–6.74 (m, 5H), 6.56–6.49 (m, 5H), 6.22 (s, 1H), MS (FAB) miz 879 [(M+H)+].
3. Results and discussion
The electron transport type material, DSPPO1, adopted a double spirobifluorene core structure to acquire high thermal stability and high triplet energy as mentioned in our previous work [Citation19–21]. The double spirobifluorene core derived hole transport material was modified with a diphenylamine moiety in order to increase hole transport property due to strong electron donating property of the diphenylamine moiety [Citation19–21]. Comparing the hole transport material with the diphenylamine moiety, DSPPO1 was introduced into a diphenylphosphine oxide group for good electron transport properties due to enhanced electron-withdrawing property compared to the diphenylamine group.
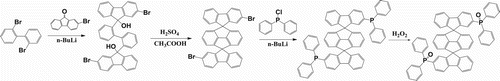
The core structure of DSPPO1 was synthesized by one-step ring closing reaction according to the method reported in our previous work [Citation21]. 2,2′-Dibromobiphenyl was reacted with 2-bromofluorenone by lithiation using 2.5 M solution of n-butyllithium in hexane with cooling bath followed by ring closing reaction with sulfuric acid. The intermediate core structure was substituted with diphenylphosphine oxide group by lithiation with n-butyllithium and oxidation using hydrogen peroxide. Synthetic scheme of DSPPO1 is shown in . Synthetic yields of the final product was 51.7% from 2,2′-dibrom-spiro(cyclopenta[def]fluorene-1,5,9′9″-bifluorene) intermediate. Identification and confirmation of the chemical structure were carried out using 1H nuclear magnetic resonance spectrometer and mass analysis. Purity level confirmed from high-performance liquid chromatography using a mixture of acetonitrile and methanol mobile phase was over 99%.
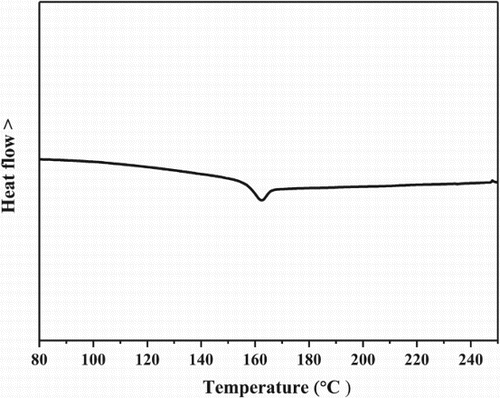
The DSPPO1 compound used a double spirobifluorene core structure because the moiety is advantageous to thermal stability. The glass transition temperature of DSPPO1 was measured using differential scanning calorimeter (DSC). DSC scan was carried out at a heating rate of 10°C/min under nitrogen atmosphere. The DSC curve of DSSPO1 is shown in . The DSPPO1 sample was heated up to 300°C followed by cooling to room temperature and then scanned again to collect second scan data for the glass transition temperature measurement. The glass transition temperature of DSPPO1 was observed at 156°C from the inflection point of the DSC scan. The glass transition temperature of DSPPO1 was higher than that of typical electron transport materials because of the rigidity of the double spirobifluorene core structure [Citation5–14]. Considering that the glass transition temperature above 100°C is required in the device application, DSPPO1 can be suitable as the thermally stable electron transport material. The double spirobifluorene core structure is difficult to be flexible by thermal energy because two central fluorene units cannot be distorted due to strain of the central fluorene units. The rigidity of the core structure raises the glass transition temperature of the DSPPO1 and a high glass transition temperature of 156°C was obtained.
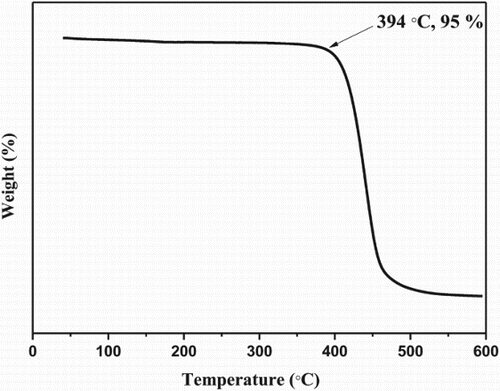
Thermal gravimetric analysis (TGA) curve of DSPPO1 was performed on TA instruments Q50. TGA scan was recorded at a heating rate of 10°C/min. The TGA curve of DSPPO1 is shown in . Onset decomposition temperature was 394°C for DSPPO1 at a weight loss of 5%.
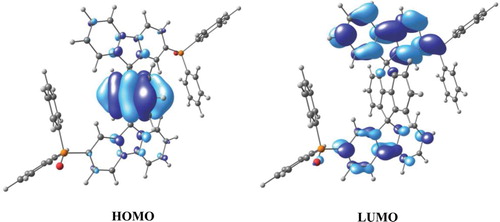
The molecular orbital distribution of DSPPO1 was calculated by using Gaussian 03 software to study the HOMO and LUMO distributions [Citation22]. The geometries in the ground state were calculated via density functional theory with the most popular functional, B3LYP, to simulate HOMO and LUMO of DSPPO1. The HOMO and LUMO images of DSPPO1 are shown in . The HOMO of DSPPO1 was situated on the cyclopenta[def]fluorene core structure and the LUMO was distributed over the fluorene unit attached to the diphenylphosphine oxide group. This simulation result implies that the electron transport property of DSPPO1 is dominated by the fluorene unit connected to diphenylphosphine oxide group which becomes electron deficient due to strong electron-withdrawing character of the phosphine oxide moiety. The electron deficient fluorene moiety can readily accept electrons and can function as an electron transport unit. As the HOMO of DSPPO1 was found on the weakly electron transporting cyclopenta[def]fluorene core structure, DSPPO1 may not be suitable as a hole transport material. Calculated HOMO and LUMO levels of DSPPO1 were −5.64 and −1.32 eV, respectively. Triplet energy and band gap from the molecular simulation were 2.95 and 4.32 eV, respectively.
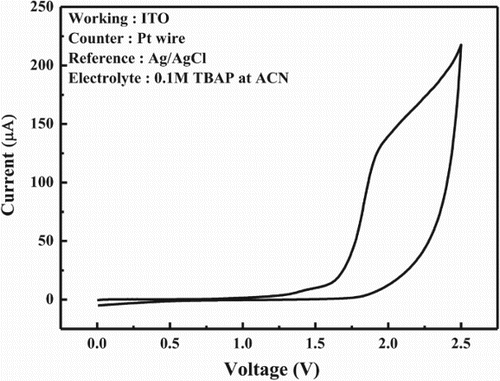
Cyclic voltammetry measurement of DSPPO1 was carried out using a cyclic current-voltage monitoring program in three electrodes with a glass-coated indium-tin-oxide working electrode, a platinum wire counter electrode and Ag/AgCl reference electrode and dipped all electrodes into 1.0 × 10–1 M acetonitrile solution of tetrabutylammonium perchlorate. Ferrocene was used as the internal standard material. shows cyclic voltammetry curve of DSPPO1. The HOMO energy level of DSPPO1 analyzed by cyclic voltammetry was −6.51 eV from the onset of electrochemical oxidation of DSPPO1, which suggests that the hole carriers from the emitting layer can be blocked by DSPPO1 because common host materials have the shallower LUMO level than that of DSPPO1. A typical host material has the HOMO level in the range from −5.80 to −6.30 eV. The difference of the HOMO level between host and DSPPO1 is at least about 0.21 eV, which can block hole leakage from the emitting layer. Therefore, DSPPO1 is suitable for the hole blocking layer. The LUMO level of DSPPO1 was −2.70 eV, which was calculated from the HOMO and optical band gap. The LUMO level of −2.70 eV of DSPPO1 is suitable for electron carrier injection into the emitting layer from the DSPPO1 electron transport material and it can play a role of a hole blocking type electron transport material.
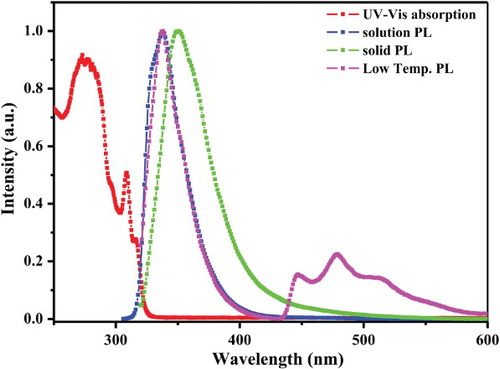
Photophysical properties of DSSPO1 were measured by using ultraviolet-visible (UV–VIS) absorption and photoluminescence (PL) spectrometers. shows UV–VIS absorption, solid, solution and low temperature PL emission spectra of DSPPO1. UV–VIS, solution PL and low temperature PL spectra were obtained using 1.0 × 10–5 M THF solution of DSPPO1 and excitation wavelength was 308 nm. Solid film of DSPPO1 was prepared by vacuum thermal evaporation at a thickness of 30 nm. The double spirobifluorene core structure mainly contributed to the light absorption and DSSPO1 showed strong broad absorption peaks at 273, 308 and 316 nm. The diphenylphosphine oxide group did not greatly affect the UV–VIS absorption spectra of DSPPO1. The band gap of DSPPO1 was 3.81 eV and that was calculated from the absorption edge of the UV–VIS spectrum. DSPPO1 showed large band gap because the UV–VIS absorption is mainly observed by the double spirobifluorene core. The solution and solid PL spectra of DSPPO1 were observed at 338 and 351 nm, respectively. The solution PL peak was at a short wavelength because the PL emission is from the double spirobifluorene core. The red shift of the solid PL emission compared to the solution PL emission is caused by intermolecular interaction in solid state. Phosphorescent emission of DSPPO1 was observed from 450 to 520 nm at low temperature. First phosphorescent peak was observed at 447 nm and vibrational peaks were detected at longer wavelength. The triplet energy of DSPPO1 was determined from phosphorescent emission spectra collected at low temperature and that was 2.77 eV [Citation23]. In general, the triplet energy is governed by the degree of conjugation of the molecular structure. DSPPO1 has a double spirobifluorene core structure and two diphenylphosphine oxide units. The phosphine oxide linker has a tetrahedral geometrical structure and does not extend the conjugation of the double spirobifluorene core. Therefore, the high triplet energy of the double spirobifluorene core is observed in the DSPPO1. The triplet energy of DSPPO1 was higher than common red, green and blue host materials for PHOLEDs. Hence, DSPPO1 can be used as a triplet exciton blocking material for PHOLEDs. The photophysical analysis data of DSPPO1 are presented in .
Table 1. Analysis data of DSPPO1.
4. Conclusions
In summary, a new thermally stable electron transport material, DSPPO1, was synthesized successfully as an electron transport layer for PHOLEDs. DSPPO1 showed good thermal stability due to a high glass transition temperature of 156°C and a high triplet energy of 2.77 eV. Additionally, a deep HOMO level of −6.51 eV for hole blocking and a LUMO level of −2.70 eV for electron injection were obtained. Therefore, DSPPO1 can be suitable as the hole and triplet exciton blocking type electron transport materials for PHOLEDs, and device performances of the DSPPO1 devices would be characterized as electron transport materials for PHOLEDs later.
Funding
This work was supported by GRRC program of Gyeonggi Province [GRRC Dankook2014–B01] and high efficiency and long lifetime in green phosphorescent organic light-emitting diodes using host and hole transport materials funded by MOTIE.
Additional information
Mr. Yong Joo Cho received his B.S. degree in 2010 and his M.S. degree in 2012. He is now a Ph.D. candidate at the Department of Polymer Science and Engineering of Dankook University. His major interest is the synthesis of organic electronic materials for organic light-emitting diodes.
Prof. Jun Yeob Lee received his Ph.D. degree from Seoul National University in Korea in 1998. After his postdoc at Rensselaer Polytechnic Institute (1998–1999), he joined Samsung SDI and developed an active matrix organic light-emitting diode in six years. He has since been working as Assistant Professor at the Department of Polymer Science and Engineering of Dankook University. His main research areas are the synthesis of organic electronic materials and the development of a novel device structure for organic electronic devices.
References
- C. Adachi, M.A. Baldo, M.E. Thompson and S.R. Forrest, J. Appl. Phys. 90, 5048 (2001). doi: 10.1063/1.1409582
- L. Xiao, S.-J. Su, Y. Agata, H. Lan and J. Kido, Adv. Mater. 21, 1271 (2009). doi: 10.1002/adma.200802034
- J.Y. Lee, J. Inf. Disp. 15, 139 (2014). doi: 10.1080/15980316.2014.937364
- S.O. Jeon, S.E. Jang, H.S. Son and J.Y. Lee, Adv. Mater. 23, 1436 (2011). doi: 10.1002/adma.201004372
- S.E. Jang, C.W. Joo, S.O. Jeon, K.S. Yook and J.Y. Lee, Org. Electron. 11, 1059 (2010). doi: 10.1016/j.orgel.2010.03.005
- S.E. Jang, K.S. Yook and J.Y. Lee, Org. Electron, 11, 1154 (2010). doi: 10.1016/j.orgel.2010.04.004
- T. Spehr, R. Rudzich, T. Fuhrmann and J. Salbeck, Org. Electron. 4, 61 (2003). doi: 10.1016/j.orgel.2003.08.002
- C. Hosokawa, H. Higashi, H. Nakamura and T. Kusumoto, Appl. Phys. Lett. 67, 2455 (1995). doi: 10.1063/1.115295
- Z. Jiang, H. Yao, Z. Zhang, C. Yang, Z. Liu, Y. Tao, J. Qin and D. Ma, Org. Lett. 11, 2607 (2009). doi: 10.1021/ol9008816
- T.P.I. Saragi, T. Spehr, A. Siebert, T. Fuhrmann-Lieker and J. Salbeck, Chem. Rev. 107, 1011 (2007). doi: 10.1021/cr0501341
- X. Cheng, G.H. Hou, J.H. Xie and Q.L. Zhou, Org. Lett. 14, 2381 (2004). doi: 10.1021/ol0492546
- J.Y. Shen, C.Y. Lee, T.H. Huang, J.T. Lin, Y.T. Tao, C.H. Chien and C. Tsai, J. Mater. Chem. 15, 2455 (2005). doi: 10.1039/b501819f
- J. Salbeck, F. Weissoertel and J. Bauer, Macromol. Symp. 125, 121 (1997). doi: 10.1002/masy.19981250110
- R. Pudzich, T. Fuhrmann-Lieker and J. Salbeck, Adv. Polym. Sci, 199, 83 (2006).
- T. Kowada, T. Kuwabara and K. Ohe, J. Org. Chem. 75, 906 (2010). doi: 10.1021/jo902482n
- D. Thirion, C. Poriel, F. Barrire, R. Mtivier, O. Jeannin and J. Rault-Berthelot, Org. Lett. 11, 4974 (2009). doi: 10.1021/ol901750x
- C. Poriel, J.-J. Liang, J. Rault-Berthelot, F. Barrière, N. Cocherel, A.M.Z. Slawin, D. Horhant, M. Virboul, G. Alcaraz, N. Audebrand, L. Vignau, N. Huby, G. Wantz and L. Hirsch, Chem. Eur. J. 13, 10055 (2007). doi: 10.1002/chem.200701036
- D. Horhant, J.J. Liang, M. Virboul, C. Poriel, G. Alcaraz and J. Rault-Berthelot, J. Org. Lett. 8, 257 (2006). doi: 10.1021/ol0526064
- Y.J. Cho, O.Y. Kim and J.Y. Lee, Org. Electron. 13, 351 (2012). doi: 10.1016/j.orgel.2011.11.028
- Y.J. Cho and J.Y. Lee, Org. Electron. 13, 1044 (2012). doi: 10.1016/j.orgel.2012.03.006
- Y.J. Cho and J.Y. Lee, Thin Solid Films. 522, 415 (2012). doi: 10.1016/j.tsf.2012.08.034
- M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, J.A. Montgomery, T. VrevenJr., K.N. Kudin, J.C. Burant, J.M. Millam, S.S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G.A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J.E. Knox, H.P. Hratchian, J.B. Cross, C. Adamo, J. Jaramillo, R. Gomperts, R.E. Stratmann, O. Yazyev, A.J. Austin, R. Cammi, C. Pomelli, J.W. Ochterski, P.Y. Ayala, K. Morokuma, G.A. Voth, P. Salvador, J.J. Dannenberg, V.G. Zakrzewski, S. Dapprich, A.D. Daniels, M.C. Strain, O. Farkas, D.K. Malick, A.D. Rabuck, K. Raghavachari, J.B. Foresman, J.V. Ortiz, Q. Cui, A.G. Baboul, S. Clifford, J. Cioslowski, B.B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R.L. Martin, D.J. Fox, T. Keith, M.A. Al-Laham, C.Y. Peng, A. Nanayakkara, M. Challacombe, P.M.W. Gill, B. Johnson, W. Chen, M.W. Wong, C. Gonzalez and J.A. Pople, Gaussian03, Revision B05 (Gaussian, Inc., Pittsburgh, PA, 2003).
- M. Sauer, J. Hofkens and J. Enderlein, Basic Principles of Fluorescence Spectroscopy, in Handbook of Fluorescence Spectroscopy and Imaging: From Single Molecules to Ensembles, edited by M. Sauer, J. Hofkens and J. Enderlein (Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2011), pp. 15–17.