Abstract
Objective: This study was performed to analyse the carbohydrate quantity of the non-standardised breakfast meal test consumed as part of a screening test for gestational diabetes.
Design: A prospective descriptive design was utilised.
Setting: Screening for gestational diabetes was performed in the High-Risk Antenatal Clinic at Tygerberg Academic Hospital, Cape Town, South Africa.
Subjects: Fifty pregnant women who met the local selection criteria for diabetes screening.
Outcome measures: The contents of the patient-provided breakfast meal tests were evaluated individually for total carbohydrate amount and compared with the 75 grams of carbohydrate provided by the oral glucose tolerance test (OGTT).
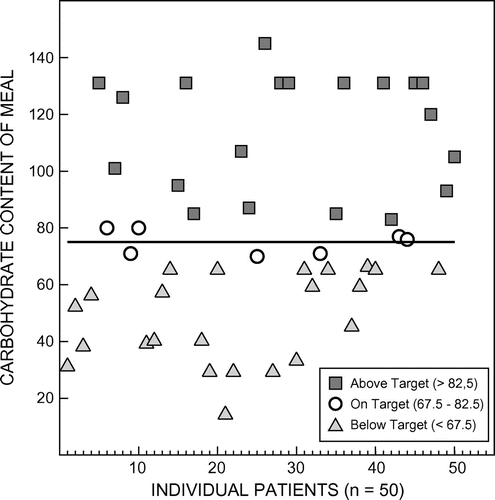
Results: The median carbohydrate amount was 71 g but the range (55–145 g) was wide. Only seven meals (14%) fell within 10% of the 75 g carbohydrate target.
Conclusion: The patient-provided breakfast meal showed wide variation in carbohydrate amount. If a meal test is to be used instead of the formal OGTT a carefully measured, prepared, palatable, readily available product would need to be sourced and provided.
Layman’s summary: It is necessary to screen for the development of diabetes during pregnancy. The standard test with 75 g of glucose is unpalatable and is sometimes replaced by a meal test. However, when this meal test is provided by the women themselves without standardisation, the sugar and starch quantities are too variable. Careful consideration needs to be given to an alternative screening test if it is to be reliable.
Strong lay message: Non-standardised screening meal tests for gestational diabetes should not be used.
Keywords:
Introduction
The incidence of gestational diabetes mellitus (GDM) is rising due to several factors. In developed countries populations are becoming older while in developed and developing countries rising levels of obesity, high-calorie diets and decreased physical activity are having profoundly negative effects. Widely influential international studies, institutes and study groups have pronounced on the changing diagnostic thresholds for gestational diabetes.Citation1–4 In current literature both universal and selective screening are still debated, as is the capacity of obstetric services to carry the ever-increasing burden of patients with gestational diabetes mellitus.Citation5–8
One of the central issues that has received less attention is the screening and/or diagnostic test. In a review on screening for gestational diabetes, Petrović recommends combining the screening and diagnostic test using a 75 g oral glucose tolerance test (OGTT).Citation9 In this regard the Western Cape Province of South Africa operates according to a provincial guideline on diabetes mellitus in pregnancy which advocates a selective rather than a universal approach to screening.Citation10 The local application of this policy has recently been described.Citation6 The guideline provides two options for testing. The first is a 75 g OGTT with fasting and two-hour blood glucose sampling. However, this standardised glucose load may be substituted by a non-standardised glycaemic load (the patient’s breakfast) brought to the clinic and consumed after the fasting glucose test. The two-hour post-prandial blood glucose measurement is still performed. Although perceived as a gold standard, the value of the formal OGTT is debatable because it is a relatively expensive and time-consuming test that is regarded as generally unpalatable and has poor reproducibility.Citation11 The local ‘breakfast’ test is more palatable and self-administered, but the reproducibility and carbohydrate quantity are uncertain.
The current study was performed to interrogate the carbohydrate quantity of the non-standardised breakfast meal test consumed as part of the screening test for gestational diabetes after a fasting blood glucose value had been determined and before determination of a two-hour value.
Methods
A prospective descriptive design was utilised. The Western Cape Province guideline for Diabetes in Pregnancy specifies the group of women at high risk for developing gestational diabetes mellitus (Table ). During this study 50 women who met the selection criteria for diabetes screening in the High-Risk Clinic at Tygerberg Academic Hospital were approached and gave written informed consent. These study participants constituted the first 50 eligible women when the principal author was available in the clinic. After recruitment of each individual the contents of their ‘patient-provided breakfast’ meal, taken after the fasting glucose value and before the two-hour glucose value/measurement, was carefully recorded. (No specific instructions, other than ‘bring your usual breakfast along’, were given to the patients.) The recorded information included the type of food, brand name if applicable, portion size and volume of any liquid consumed. Food purchased at the local hospital shop was weighed using a calibrated (one gram) kitchen scale. Estimations of portion size were used for food brought from home. These data were entered onto an Excel® (Microsoft Corp, Redmond, WA, USA) spreadsheet and analysed independently by the study dietitian (LvW). As carbohydrate (quantity and type) is the main determining factor of the post-prandial glycaemic response, the ‘patient-provided breakfast’ meals were analysed at least for total carbohydrate quantity.Citation12–14 Food composition tables and food labels (when available) were used to calculate the total quantity of carbohydrate in each meal.Citation15 This was compared with the 75 g carbohydrate quantity of the OGTT. Data were analysed using Microsoft Excel® 2013 and are expressed as median, ranges or n (%). The study was approved by the Ethics Committee for Human Research of the Faculty of Medicine and Health Sciences, Stellenbosch University (S13/10/205).
Table 1: Risk factors to identify screening candidates for gestational diabetes
Results
The 50 patients were collected over a three-week period in July/August 2014 in the High-Risk Clinic of Tygerberg Academic Hospital. All patients approached to participate in the study consented to the analysis. The descriptive data of patients in the study are given in Table . Data are given as median (range) unless otherwise specified.
Table 2: Descriptive baseline data
The type of food chosen by the study group consisted mainly of food and beverages purchased at the hospital shop (38 patients; 76%) and 12 patients (24%) brought food with them from home. The median carbohydrate quantity of the 50 breakfast meals analysed was 71 grams with a range from 15 to 145 grams. Specific contents of certain patient-provided breakfast meals are shown in Table in order to demonstrate the variable carbohydrate amounts. Only seven (14%) of the meals were within 10% of the 75 g (carbohydrate) target, while 23 (46%) meals were below target and 20 (40%) meals were above target. The variations of carbohydrate quantity in relation to the 75 g (OGTT) ideal are shown in Figure .
Table 3: Examples of certain specific patient-provided breakfast meal tests
Discussion
The goal of this study was to analyse the carbohydrate amount of the non-standardised breakfast meal test, in order to formulate a reproducible, standardised, palatable and reasonably equivalent option to test against a formal 75 g OGTT in a subsequent study. Although the median carbohydrate quantity of the 50 breakfast meals analysed was 71 g, the range was very wide (15–145 g) and only seven meals (14%) fell within 10% of the 75 g target.
Screening for gestational diabetes is standard practice and is performed according to well-established guidelines in high-income settings. In low-resource settings with time, financial and laboratory constraints, guidelines are often absent, lack uniformity and their applicability has been questioned (Utz)Citation16. Nonetheless, the World Health Organization (WHO) has recognised these discrepancies and sought to provide a basis for universal guidelines.Citation17 Palatability aside, the reproducibility of the 75 g OGTT in pregnant women has long been questioned, with a recent study from South Africa indicating the greater reliability of fasting glucose.Citation18 Several studies have investigated more palatable and reproducible alternatives to the OGTT. A small Canadian study compared a standardised meal with the 75 g OGTT in non-pregnant subjects and found that two-hour glucose values were more consistent after the standardised meal test.Citation19 A group in Japan developed a standardised cookie consisting of 75 g carbohydrate and 25 g fat, while a North American team utilised muffins and coffee or tea but again, in both studies, the subjects were not pregnant.Citation20,21 In a powered study, Racusin et al. utilised 10 commercially produced candy twists (50 g) using a crossover design, first in non-pregnant and then pregnant subjects. They found the candy twists to be a reliable alternative to a 50 g glucose test but with fewer false-positive screens.Citation22 The protein, fat and carbohydrate quantity of certain meal tests is given in Table .
Table 4: Comparison of the macronutrient distribution of certain meal testsTable Footnote*
The effectiveness and feasibility of using a solid meal instead of an OGGT has been demonstrated in several studies.Citation20,21 The study by Wolever et al. (1998) showed that a solid meal was more consistent for diagnosing GDM based on the two-hour value than the OGGT.Citation19
In a ‘designed’ breakfast with 75 g of carbohydrate, the fat should provide no more than 44% and the protein no more than 15–30% of the total energy to ensure minimal effect on the glycaemic response.Citation23,25 Various studies have documented that if fat is added in small to moderate amounts the effect is insignificant.Citation23–25 Altering the protein:fat ratio of a meal (30% protein and 15% fat compared with 15% protein and 34% fat of the total energy tested in a randomised cross-over study) did not affect the post-prandial glucose levels when the carbohydrate amount was consistent.Citation25 The type of carbohydrate itself would be ‘similar’ to that used in other solid meal tests (as alternatives to the OGGT), namely a mixture of polysaccharides and disaccharides providing a total of 75 g carbohydrate.
Conclusion
The results of this study showed that the patient-provided breakfast meal currently utilised at this facility is not standardised. If a meal test is to be used instead of the formal OGTT a carefully measured, prepared, readily available product would need to be sourced and provided.
References
- HAPO Study Cooperative Research Group. The hyperglycemia and adverse pregnancy outcome (HAPO) study. Int J Gynaecol Obstet. 2002;78(1):69–77.
- Crowther CA, Hiller JE, Moss JR, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. New Engl J Med. 2005;352(24):2477–86.10.1056/NEJMoa042973
- Walker J. NICE guidance on diabetes in pregnancy: management of diabetes and its complications from preconception to the postnatal period. NICE clinical guideline 63. London, March 2008. Diabetic Med. 2008;25(9):1025–7.10.1111/dme.2008.25.issue-9
- Metzger BE. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676–82.
- Miailhe G, Kayem G, Girard G, et al. Selective rather than universal screening for gestational diabetes mellitus? Eur J Obstet Gynecol Reprod Biol. 2015;191:95–100.10.1016/j.ejogrb.2015.05.003
- Hall D, du Toit M, Mason D, et al. Diabetes mellitus in pregnancy, still changing. JEMDSA. 2015;08(12):1–7.
- Ekeroma AJ, Chandran GS, McCowan L, et al. Impact of using the international association of diabetes and pregnancy study groups criteria in South Auckland: prevalence, interventions and outcomes. Aust New Zealand J Obstet Gynaecol. 2015;55(1):34–41.10.1111/ajo.2015.55.issue-1
- Buckley BS, Harreiter J, Damm P, et al. Gestational diabetes mellitus in Europe: prevalence, current screening practice and barriers to screening. A review. Diabet Med. 2012;29(7):844–54.10.1111/j.1464-5491.2011.03541.x
- Petrović O. How should we screen for gestational diabetes? Curr Opin Obstet Gynecol. 2014;26(2):54–60.10.1097/GCO.0000000000000049
- Maternal Guideline Reference Group and the Subcommittee of the Society for Maternal and Foetal Medicine in South Africa. Diabetes in pregnancy, provincial guideline of the Western Cape, for the management of diabetes and its complications from pre-conception to the postnatal period. Western Cape: Provincial Government; 2010.
- Mooy JM, Grootenhuis PA, de Vries H, et al. Intra-individual variation of glucose, specific insulin and proinsulin concentrations measured by two oral glucose tolerance tests in a general Caucasian population: the Hoorn study. Diabetologia. 1996;39(3):298–305.10.1007/BF00418345
- Sheard NF, Clark NG, Brand-Miller JC, et al. Dietary carbohydrate (amount and type) in the prevention and management of diabetes: a statement by the American diabetes association. Diabetes Care. 2004 September;27(9):2266–71.10.2337/diacare.27.9.2266
- Wolever TM, Bolognesi C. Prediction of glucose and insulin responses of normal subjects after consuming mixed meals varying in energy, protein, fat, carbohydrate and glycemic index. J Nutr. 1996 November;126(11):2807–12.
- Nuttall FQ, Mooradian AD, DeMarais R, et al. Tihe glycemic effect of different meals approximately isocaloric and similar in protein, carbohydrate, and fat content as calculated using the ADA exchange lists. Diabetes Care. 1983 Sep–Oct;6(5):432–5.10.2337/diacare.6.5.432
- Wolmarans P, Danster N. Condensed food composition tables for South Africa. Medical Research Council; 2010.
- Utz B, Kolsteren P, De Brouwere V. Screening for gestational diabetes mellitus: are guidelines from high-income settings applicable to poorer countries? Clin Diabetes. 2015;33(3):152–8.10.2337/diaclin.33.3.152
- World Health Organization. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy. 2013.
- Bonongwe P, Lindow SW, Coetzee EJ. Reproducibility of a 75G oral glucose tolerance test in pregnant women. J Perinat Med. 2015;43(3):333–8.
- Wolever TM, Chiasson JL, Csima A, et al. Variation of postprandial plasma glucose, palatability, and symptoms associated with a standardized mixed test meal versus 75 g oral glucose. Diabetes Care. 1998 March;21(3):336–40.10.2337/diacare.21.3.336
- Harano Y, Miyawaki T, Nabiki J, et al. Development of cookie test for the simultaneous determination of glucose intolerance, hyperinsulinemia, insulin resistance and postprandial dyslipidemia. Endocr J. 2006;53(2):173–80.10.1507/endocrj.53.173
- Traub ML, Jain A, Maslow BS, et al. The ‘muffin test’–an alternative to the oral glucose tolerance test for detecting impaired glucose tolerance. Menopause. 2012 January;19(1):62–6.10.1097/gme.0b013e318221bfc9
- Racusin DA, Antony K, Showalter L, et al. Candy twists as an alternative to the glucola beverage in gestational diabetes mellitus screening. Am J Obstet Gynecol. 2015;212:522.e1–5.
- Owen B, Wolever TM. Effect of fat on glycaemic responses in normal subjects: a dose-response study. Nutr Res. 2003;23(10):1341–7.10.1016/S0271-5317(03)00149-0
- Normand S, Khalfallah Y, Louche-Pelissier C, et al. Influence of dietary fat on postprandial glucose metabolism (exogenous and endogenous) using intrinsically C-enriched durum wheat. Br J Nutr. 2001;86(01):3–11.10.1079/BJN2001359
- Papakonstantinou E, Triantafillidou D, Panagiotakos D, et al. A high protein low fat meal does not influence glucose and insulin responses in obese individuals with or without type 2 diabetes. J Human Nutr Dietetics. 2010;23(2):183–9.10.1111/jhn.2010.23.issue-2