ABSTRACT
Acute myeloid leukemia (AML) is a malignant disease of the hematopoietic system. Residual leukemic cells after treatment are associated with relapse. Thus, detecting minimal residual disease (MRD) is significant. Major techniques for MRD assessment include multiparameter flow cytometry (MFC), polymerase chain reaction (PCR), and next-generation sequencing (NGS). At a molecular level, AML is the consequence of collaboration of several gene alterations. Some of these gene alterations can also be used as MRD markers to evaluate the level of residual leukemic cells by PCR and NGS. However, when as MRD markers, different gene alterations have different clinical values. This paper aims to summarize the characteristics of various MRD markers, so as to better predict the clinical outcome of AML patients and guide the treatment.
Abbreviations
| AML | = | (Acute myeloid leukemia) |
| CBF-AML | = | (Core binding factor acute myeloid leukemia) |
| NPM1 | = | (Nucleophosmin 1) |
| WT1 | = | (Wilms tumor 1) |
| EVI1 | = | (Ecotropic virus integration site-1) |
| FLT3 | = | (FMS-like tyrosine kinase 3) |
| CR | = | (Complete remission) |
| CRi | = | (CR with incomplete hematologic recovery) |
| CRp | = | (CR with incomplete platelet recovery) |
| MRD | = | (Measurable residual disease/Minimal residual disease) |
| LSCs | = | (Leukemia Stem Cells) |
| FCM | = | (Flow cytometry) |
| MFC | = | (Multiparameter flow cytometry) |
| LAIPs | = | (Leukemia-associated immunophenotypes) |
| PCR | = | (Polymerase chain reaction) |
| RT-qPCR/RQ-PCR | = | (Real time quantitative PCR) |
| ddPCR | = | (Digital droplet PCR) |
| FACS-FISH | = | (Fluorescence-activated cell sorting-Fluorescence in situ hybridization) |
| NGS | = | (Next generation sequencing) |
| WGS | = | (Whole Genome Sequencing) |
| WES | = | (Whole Exome Sequencing) |
| VAF | = | (Variant allele frequency) |
| BM | = | (Bone marrow) |
| PB | = | (Peripheral blood) |
| OS | = | (Overall survival) |
| CIR | = | (Cumulative incidence of relapse) |
| CCR | = | (Continuous complete remission) |
| DFS | = | (Disease-free survival) |
| RFS | = | (Relapse-free survival) |
| LFS | = | (Leukemia-free survival) |
| EOT | = | (End of treatment) |
| MSDT | = | (Matched sibling donor transplantation) |
| allo-HSCT | = | (Allogeneic hematopoietic stem cell transplantation) |
| GO | = | (Gemtuzumab ozogamicin) |
| HMA | = | (Hypomethylating agents) |
| CHIP | = | (Clonal hematopoiesis of indeterminate potential) |
1. Introduction
AML is a malignant disease of myeloid hematopoietic stem/progenitor cells. With classical ‘7 + 3’ regimens (regimens with 3 days of an anthracycline and 7 days of cytarabine) as induction therapy for AML patients, 60%–80% of younger adults and 40%–60% of older adults can achieve CR. However, even if CR or CRi is achieved, relapse is more likely in patients with detectable residual disease. MRD represents the presence of detectable leukemic cells with a sensitivity of 1:104–1:106, however, sensitivity in morphology-based assessments is 1:20 [Citation1]. LSCs can drive relapse, hence the presence of residual LSCs following remission increases the possibility of AML relapse. Thus, it is increasingly important to identify residual disease far below the morphology-based 5% blast threshold [Citation1,Citation2]. As well as relating to prognosis and relapse, MRD is also a significant indicator in the clinical practice of AML [Citation3,Citation4]. Therefore, it is extremely significant to assess MRD in AML patients.
2. Techniques for MRD assessment
The major techniques for MRD assessment include MFC, PCR, and NGS. MFC techniques based on antigen expression patterns mainly distinguish malignant cells from normal hematopoietic cells by LAIPs which refer to the aberrant expression of markers or marker combinations on the leukemic cells which are either absent or rarely found in normal or recovering BM or normal blood [Citation5–7]. Different from MFC, NGS and PCR are mainly aimed at analyzing various gene alterations in AML.
2.1 PCR
PCR methods, mainly including RT-qPCR and ddPCR, can be used to evaluate MRD. RT-qPCR can amplify leukemia- associated genetic abnormalities with a sensitivity of 10 −3–10 −5 [Citation8]. Markers such as NPM1 and several fusion genes, such as CBFB-MYH11, RUNX1-RUN1T1 and PML-RARA can be applied in this technology. Some studies though suggest that certain markers like FLT3, NRAS, KRAS and IDH1/2, are not ideal for several reasons which will be described later on [Citation1,Citation7–9]. The main advantage of RT-qPCR is that it is a widely standardized method for the described targets. However, compared to MFC, RT-qPCR is only applicable in less than 40% of patients [Citation1,Citation2,Citation8].
ddPCR is a method based on water–oil emulsion droplet technology and implementing PCR data with Poisson statistics, which allows quantification of the number of target molecules in a sample [Citation10]. ddPCR can absolutely quantify the target gene without the need for a standard curve and has higher precision, which is the most significant advantage compared to RT-qPCR [Citation10,Citation11]. The sensitivity of ddPCR can reach 1:100,000 and its absolute precision depends on the mean number of molecules per partition, although the cost is the method’s main limitation [Citation2]. There are also various other parameters that need to be standardized both in RT-qPCR and ddPCR, including sample processing, sample type, heparin vs. EDTA tubes for sample collecting and analytical cutoffs.
2.2 NGS
MRD detection by NGS is based on measuring all mutations [Citation12]. NGS, via allowing parallel and repeated sequencing of millions of small DNA fragments can evaluate a few gene or an entire genome [Citation13]. Main NGS technologies include WGS, WES, and Targeted-gene sequencing [Citation14]. Since most clinically relevant mutations are known for myeloid malignant, targeted sequencing is a common detection method at present [Citation12]. NGS is widely used in myeloid malignancies in clinical practice by introducing a variety of commercially available panels which can cover between 25 and more than 50 relevant genes/hotspots, such as the Illumina TruSight Myeloid panel, the Human Myeloid Neoplasms QIASeq DNA Panel and so on. Among most AML patients, more than one recurrent somatic mutation can be detected by using comprehensive myeloid gene panels [Citation12,Citation15]. RTq-PCR/ddPCR belong to single-gene methods for MRD assessment and are subject to false negative results due to clonal evolution because AML patients can relapse with different mutations from those in diagnosis. In contrast, NGS can assess multiple genes simultaneously and allow the tracking of distinct mutational patterns, therefore false negative results caused by clonal evolution can be avoided [Citation15,Citation16]. Nevertheless, the sensitivity of NGS could be limited by the sequencing error rate. This limitation can be overcome by error-corrected sequencing, one of NGS techniques in hemato-oncology, which is based on barcoding the individual DNA molecules used for NGS library preparation, as this technique circumvents the standard NGS sensitivity of 1% VAF [Citation2,Citation7,Citation8,Citation12,Citation15]. VAF (mutant sequencing reads / total sequencing reads) depends on allele coverage, thus for the purpose of detecting the often rather low VAFs of mutations at early relapse, high levels of coverage are necessary [Citation15]. Moreover, factors that need to be standardized for NGS include the sequencing platform, the library preparation protocol, the choice of clinically relevant genes to be targeted, depth of coverage required and data analysis pipeline and variant calling algorithms [Citation7]. In general, NGS is relatively easy to perform, sensitive and applicable to specific subgroups. However, in addition to the limited sensitivity and specificity, and statistical and computational challenges, the disadvantage of NGS-based MRD is the inability to distinguish between residual leukemia and clonal hematopoiesis [Citation8,Citation17].
3. Markers in MRD assessment
The occurrence of AML is the result of many factors. At the molecular level, AML is the consequence of collaboration between at least three broad classes of gene alterations [Citation18]. On one hand, these gene alterations work together to cause AML, on the other hand, some of them could be used as markers to assess MRD in order to better monitor the progress of the disease and provide a reference for the selection of treatment options.
3.1 Class I gene alterations
Class I gene alterations, including FLT3, KIT and RAS-associated signaling pathway, could activate signal transduction pathways and enhance proliferation with survival advantages of hematopoietic stem cells [Citation18,Citation19].
3.1.1 FLT3
FLT3 mutations, including FLT3-ITD and FLT3-TKD, occur in approximately 30% of patients with AML [Citation20–23]. FLT3-ITD mutations are relevant for disease classification due to high incidence, occurring in 20%–35% of normal karyotype AML patients and representing poor prognosis [Citation20,Citation22,Citation24]. Some researchers hold the view that FLT3 is not an applicable MRD marker because of its unstable expression and insensitive technology [Citation22,Citation23,Citation25–28]. On the contrary, there are some supportive viewpoints:(1) FLT3-ITD is associated with the leukemic clone rather than pre-leukemic clone [Citation29]. (2) Each patient’s FLT3-ITD mutation is of unique length, which can serve as a longitudinal signature [Citation29,Citation30]. (3) When using FLT3 inhibitors, FLT3-ITD MRD can indicate the prognosis, evaluate the efficacy and serve as a valuable surrogate endpoint [Citation20,Citation29–33]. For FLT3-positive AML patients treated with gilteritinib, the OS of these MRD-positive (defined as an FLT3-ITD VAF > 10 −4) patients was significantly lower than in those with a VAF ≤ 10 −4, which demonstrated that gilteritinib can induce deep molecular responses [Citation30]. (4) FLT3-ITD can also be a good marker in patients receiving allo-HSCT: higher allele ratio and MRD positive before or after transplantation are associated with increased relapse rate [Citation24,Citation34,Citation35].
3.1.2 KIT
c-KIT is common in CBF-AML (about ∼20%), which is associated with a higher incidence of relapse [Citation32,Citation36]. KITmut is rarely used as a marker of MRD, but it is associated with MRD. Especially mutations at exon 17 have inferior prognostic impact in patients with RUNX1-RUNX1T1, rather than in patients with CBFB-MYH11, and are related to the presence of MRD. At EOT, KITmut is associated with higher RUNX1-RUNX1T1 transcript levels, higher levels of reduction and the lower proportion of achievement of MRDneg [Citation32,Citation36–39].
3.1.3 RAS-associated signaling pathway
RAS mutations are accounted for 15%–40% in AML, which have no impact on survival or remission and can be lost as the disease progresses [Citation40,Citation41]. Ishida et al. support that RAS pathway mutations, which are highly unstable at relapse, should be chosen as a target with caution [Citation42]. However, when monitoring MRD by NGS [Citation36,Citation43], NRAS mutation is associated with moderate to high positive predictive value, and KRAS mutation is related to high negative predictive value and can be used to evaluate the efficiency of NGS panel.
3.2 Class II gene alterations
Class II gene alterations including PML-RARA, RUNX1-RUNX1T1, CBFB-MYH11, NPM1, etc., can affect a master transcription factor or a protein involved in hematopoietic differentiation [Citation18,Citation19].
3.2.1 RUNX1-RUNX1T1 and CBFB-MYH11 in CBF-AML
AML with chromosomal translocation t (8;21) or chromosomal rearrangement inv (16;16), corresponding to the RUNX1-RUNX1T1 or CBFB-MYH11 fusion genes are known as CBF-AML, which accounts for about 15% of adult AML [Citation44]. CBF-AML belongs to a favorable risk category as per 2017 ELN risk stratification, however about 40% of these patients can relapse [Citation45]. Thus, it is significant to identify patients who may relapse, in which RUNX1-RUNX1T1 and CBFB-MYH11 play an important role as MRD markers.
MFC, PCR and FISH can be applied in detecting residual leukemia cells carrying RUNX1-RUNX1T1 and CBFB-MYH11 fusion genes. Researchers found that the concordance rate was 67% between FCM and RT-qPCR [Citation46], 63.7% between FACS-FISH and RT–PCR, and 64.3% between FACS-FISH and FC [Citation47]. Furthermore, various MRD techniques are complementary. The combined use of these different MRD techniques can predict the prognosis and relapse more accurately [Citation46–48]. For example, Ouyang et al. [Citation48] carried on research about CBF-AML and found that RT-qPCR levels between 0.1% and 10% failed to differentiate the risk of relapse, however, MFC positivity could identify individuals with higher relapse risk after induction therapy in this group of patients.
3.2.1.1 RUNX1-RUNX1T1
RUNX1-RUNX1T1 as an MRD marker is with less controversy. At various stages of leukemia treatment, its transcript levels, log reduction and conversion from MRDneg to MRDpos hold critical significance (shown in (A)).
Figure 1. Factors that can predict a better outcome at different time points for RUNX1-RUNX1T1 (A), CBFB-MYH11 (B) and NPM1 (C), respectively.
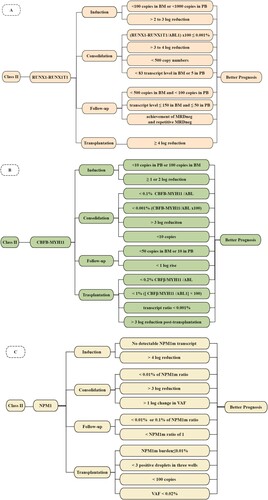
After induction therapy, presence of <100 copies in BM and <1000 copies in PB were prognostic, which can identify 47% of patients with a CIR of 7% (representing 15% of all relapsing patients) and identify 78% of patients with a CIR of 15% (compared with 50% in the remaining 22%), respectively [Citation49]. Moreover, a cut-off level of about 2–3 log reduction was with prognostic value. Patients whose copies were beyond this threshold, including pediatric patients, were associated with lower CIR and superior OS [Citation37,Citation49,Citation50].
After consolidation therapy, compared with PB-MRDneg, PB-MRDpos (with a threshold of 0.001%) was significantly associated with both a higher risk of relapse (4-year CIR, 50.9% vs. 23.6%) and a shorter survival rate (4-year OS, 63.6% vs. 96.0%) [Citation51]. On the other hand, for BM-MRD, 3 or 4 log reduction and a cut-off level of 500 copy number were significant factors [Citation49,Citation52]: for patients with >4 log reduction and copy number <500, CIR was 13% and 28%; but for those with <4 log reduction and copy number >500, CIR was 49% and 100%, respectively [Citation49]; failing to achieve a 3-log reduction could also discriminate high-risk patients (CIR:46.9% vs. 22.9%) [Citation52]. At the EOT, MRDneg in both BM and PB, or MRD levels less than 83 RUNX1-RUNX1T1 transcript level in BM and 5 in PB, or ≥3 log reduction predicted lower CIR [Citation37,Citation44].
During follow-up period, >500 RUNX1-RUNX1T1 copies in BM and >100 copies in PB could discriminate patients with a higher risk of relapse and shorter survival [Citation49]. Research showed a lower cut-off value: MRD transcript level ≤150 in BM and ≤50 in PB. There were two reasons, differences in material that was used and in sensitivity of the MRD assays. Further, they also reported that in both BM and PB, achievement of MRDneg and repetitive MRDneg can predict a lower rate of relapse. For patients converted from MRDneg to MRDpos, the rate of relapse was 24% in BM and 64% in PB [Citation37]. Nonetheless, there was no analysis between the above patients and those who had not converted from MRD negative to MRD positive.
RUNX1-RUNX1T1 as an MRD marker is also commonly applied to monitor transplant patients. From Qin YZ’s study, more than 12 months after allo-HSCT, <4 log reduction can accurately predict relapse [Citation53]. However, some researchers believe that PCR-MRD following allo-HSCT was more predictive of relapse rather than MRD before transplantation, for the reason that in their research before allo-HSCT there was no direct correlation between MRD level and relapse. One of the limitations of this single-center study was the small numbers of patients [Citation54].
Further, treatment regimens have an impact on MRD for RUNX1-RUNX1T1. There were greater MRD log reductions in transcripts of patients given GO [Citation49]. Dasatinib treatment was associated with lower RUNX1-RUNX1T1 transcript levels and stronger MRD reduction, as well as with achievement of MRDneg at EOT [Citation37]. Compared to the standard dose group (60 mg/m2/d), MRD log reduction was faster and deeper, and the percentage of patients that achieved a 3 log reduction was higher in the high dose daunorubicin group (90 mg/m2/d) [Citation55].
Besides, it could also indicate treatment strategies. Research showed that CBF-AML patients with low levels of RT–PCR (between 0.01 and 0.05) at the conclusion of induction/consolidation chemotherapy, benefited most from maintenance HMA, particularly those who had a reduction in the RT–PCR within the first 2 cycles of HMA therapy [Citation56]. Its MRD could also direct IFN-α treatment for AML patients with t(8;21) who were MRD positive after allo-HSCT [Citation57].
3.2.1.2 CBFB-MYH11
The role and significance of CBFB-MYH11 as MRD marker is similar to RUNX1-RUNX1T1 (shown in )).
After induction therapy, PB transcript levels, PB log reduction(<1 vs. ≥1) and BM copy number (>100) were significant. In terms of PB transcript levels, which was the most prognostic factor, CIR and survival rate from CR were 21% and 89% for patients with a transcript level of <10 copies, whereas they were 56% and 45% for patients with a level of 10–500 copies, 100% and 67% for patients with a level of >500 copies [Citation49]. While BM log reduction levels had little to do with RFS [Citation49,Citation58]. For pediatric patients, similarly with RUNX1-RUNX1T1, <2 log reduction in CBFB-MYH11 transcript level, after the first and second cycle of ICE, was associated with higher CIR [Citation50].
After the first course of consolidation, MRD > 0.1% was predictive of worst 2-year CCR (74% if < 0.1% vs. 40% if > 0.1%). After the second course, MRD > 0.2% for patients receiving chemotherapy/autologous-HSCT indicated a higher CIR, lower DFS and OS [Citation59]. Moreover, at this time point, > log reduction had a stronger prognostic impact on CCR (83% vs. 28%) and 2-year OS (100% vs. 67%) [Citation60]. At the end of consolidation therapy, from Yin JA et al, the most prognostic factor for relapse risk was a PB copy number of 10 (<10 copies, CIR 36% vs. >10 copies, CIR 78%) [Citation49]. Furthermore, PB-MRD level was also related to CCR:85% if MRD < 0.001%, compared to 13% if MRD ≥ 0.001% [Citation60]. In BM samples, the 2-year RFS measured from the end of consolidation was 79% for patients with PCRneg, compared 54% for patients who never achieved PCRneg during consolidation [Citation58].
During follow-up, the persistence of inv(16) has been described in patients in long-term remission [Citation58]. CBFB-MYH11 copies > 50 in BM and > 10 in PB were associated with a higher rate of relapse and shorter survival from CR [Citation49]. In another study, the follow-up time period was defined as more than 4 weeks after completion of the last consolidation cycle. It was reported that among 14 patients who relapsed, none of them achieved PCRneg in BM during follow-up, and 13 of them had persistently higher copy ratios. For patients who converted from PCRneg to PCRpos with CBFB-MYH11 copy ratios greater than 10, the median time to relapse was 6 months, for those with CBFB-MYH11 copy ratios of less than 10, the median time was 21 months and for ≥ 1 log rise in PCR levels imminent relapse was predicted [Citation61].
Before transplant, CBFB-MYH11 expression levels can be used as a marker to identify high-risk patients: patients with preMRD > 0.2% before HLA- MSDT can predict a higher CIR, lower LFS and OS [Citation62]. At the time of allo-HSCT, MRD by RT–PCR ≥ 1% was associated with inferior OS and LFS, but it was not related to increased relapse incidence. On day + 100 after allo-HSCT, although patients who were MRDpos (transcript ratio > 0.001%) had a higher incidence of relapse (27.6% vs. 9.7%), there was no statistical significance [Citation63]. However, another study, in which a cut-off value of 0.2% was used in CBFB/MYH11-positive AML, reported that a post-transplant MRD level higher than 0.2% can predict a higher CIR and lower LFS [Citation62]. In Tang’s research, the 3-year CIR and LFS were 8.6% vs. 43.2% and 71.7% vs. 44.5% for patients with a 3-log MRD reduction at each of the first 3 months after transplantation and without a 3-log MRD reduction at least once in the first 3 months, respectively [Citation54].
3.2.2 NPM1
AML with NPM1 mutation occurs in approximately 30% of adult AML patients and 60% of adult AML patients with normal karyotype, which is the most common subtype and represents a favorable outcome [Citation64,Citation65]. NPM1 mutation as a molecular characteristic for AML is an ideal marker to assess MRD (shown in )).
After double induction therapy, compared with patients with refractory diseases, NPM1 transcript level, which can be considered as a considerable prognostic marker for remission duration and OS, was significantly lower for patients who achieved CR (61 vs.1542 NPM1mut/104 ABL copies). Also, for patients who achieved RNA-based RQ-PCR negative, which was defined as no detectable NPM1 m transcript, the 4-year CIR was 6.5% in contrast to 55.3% [Citation66]. MRD log reduction is also a significant predictor with a cut-off value of 4 log reduction being confirmed [Citation67,Citation68]. Besides, among patients with a > 4 log reduction in PB-MRD, the risk of relapse was similar between patients with a positive PB- or BM-MRD and patients with both MRD negative [Citation68].
Research established two cut-off levels of absolute NPM1 m ratio of 0.01 and of 3 log reduction, which displayed low sensitivities of 32% and 22%, but high specificities of 92% and 100%. In multivariate analysis with age and ELN risk stratification after consolidation, the NPM1 m ratio and kinetics exceeding the established cut-off levels were the only prognostic variables for CIR and OS [Citation69]. From other studies, thresholds of 1 log change in VAF, 3 log reduction or 0.01% of NPM1 m/ABL ratio also had statistical significance [Citation70–72].
During follow up, in Schnittge’s study [Citation73], 1227 samples were divided into different sub cohorts, and follow-up period was divided into four time intervals (interval 1/2/3/4 for MRD assessments between day18–60, 61–120, 121–365, > 365 after begin of therapy, respectively). The first cohort was evaluated during and after intensive first-line therapy. The second cohort was evaluated during second-line chemotherapy after relapse or persistence of leukemia. The NPM1/ABL1 mutation thresholds of 0.01% in the first cohort and 0.1% in the second cohort can be successfully applied to distinguish patients with longer and shorter survival. When the molecular prediction of relapse was analyzed, based on their data, they reported that sampling intervals of 42 days would allow one to early predict 75% of all relapses. In another study, researchers observed that sensitivity, specificity and both positive and negative predictive value of a cut-off NPM1 m ratio of 1 were 100% – within 100 days, for patients with values below this cut-off, none of them relapsed. In contrast, all patients with values higher than this cut-off relapsed with a median time of 58 days [Citation69].
Shayegi thought that at lower MRD levels, the allogeneic graft is able to repress leukemia, but after crossing a certain MRD burden, the graft-versus-leukemia reaction is no longer sufficient for preventing relapse [Citation74]. Thus, among patients who received transplantation, NPM1 MRD is a critical marker. A MRD cut-off value of 0.01% before transplantation has prognostic value [Citation72,Citation73]. Bill defined MRDneg by ddPCR as NPM1 m burden ≤ 0.01% or < 3 positive droplets in three wells. They observed that pre-transplant MRDpos can predict for higher 2-year CIR and lower OS compared to pre-transplant MRDneg (CIR:64.7 vs. 6.0%; OS:38.8 vs. 71.7%, respectively). In their research, they found a phenomenon that at the time of allo-HSCT, MRDpos patients were less often in CR1 and more frequently in CR2. This could indicate that patients who have experienced relapse before, might be more difficult to get into a deep molecular response in a later CR [Citation75]. A threshold of 0.02% also had prognostic significance both before and after transplantation in Delsing’s study, but their limitation was the small number of samples [Citation76]. However, according to Kayser [Citation77], pre-transplant NPM1 MRD level > 1% (less than 100 copies of mutated NPM1/104 ABL1 copies) by RT-qPCR was an independent prognostic factor for poor survival. After allo-HSCT, an effect of NPM1 m level on survival could be shown for samples analyzed between 61–120 and 121–365 days `[Citation73].
As mentioned above, risk stratification for AML patients with NPM1 can be done according to its specific MRD thresholds. But some scholars hold the opposite opinion [Citation78]. They concluded that the binary presence or absence of an NPM1 mutation, combined with MRD levels following induction therapy, should continue to be used in therapeutic management rather than stratification according to the NPM1mut level. One reason was that the prognostic impact of the NPM1mut level is largely, albeit not entirely, due to the concomitant FLT3-ITD mutations. In fact, the level of NPM1 MRD is closely related to therapy. For patients with a <4 PB-MRD log reduction between NPM1 m base line level and NPM1 m MRD at CR/CRp or for patients with MRDpos (>0.01%) after consolidation therapy, DFS and OS were significantly improved by allo-HSCT. This benefit was not observed in patients with a >4 log reduction in PB-MRD or MRDneg [Citation68,Citation72]. However, between the NPM1 positive and negative cohorts with azacitidine therapy, there was no OS difference, which suggested a limited therapeutic impact of HMA in NPM1 positive AML [Citation79]. Both after induction therapy and at EOT, compared to control arm, an NPM1 m MRDneg (<0.1%) was more frequently observed in patients treated with GO [Citation80].
3.2.3 WT1
The expression of WT1 can be detected in about 75%–100% of adult AML patients and is associated with different molecular factors like FLT3, NPM1, PML-RARA, fusion genes in CBF-AML and so on [Citation81,Citation82]. There is a lot of support and opposition to the application of WT1 in MRD detection. On one hand, due to lack of sensitivity and specificity, WT1 is not suggested to be used as an MRD marker unless there is no other MRD marker, especially if NGS and/or digital PCR are not routinely available [Citation1,Citation7–9,Citation83]. Moreover, the prognostic effect of WT1 levels could be misunderstood depending on the distribution of the collaborative mutations [Citation82]. For patients who undergo transplantation, the accuracy and reproducibility of molecular monitoring of WT1 transcripts are affected by the limited regulation of engraftment after allo-HSCT and hematopoietic recovery after intensive chemotherapy [Citation84]. On the other hand, the supporting views are as follows: firstly, WT1 is helpful to improve the reliability of MRD-based prognostic stratification, when there are other MRD markers are present like FLT3-ITD or NMP1 [Citation81,Citation85], or when combining with FCM [Citation86]. Secondly, the expression of WT1 can indicate therapy: those who not achieve MRD negativity after 6 cycles of HMA are considered as candidates for an alternative therapy [Citation87]. Thirdly, WT1 mRNA level can reflect tumor burden [Citation88]. Many researches confirmed that the MRD level of WT1 and its kinetics could distinguish individuals with higher risk to a poor prognosis; like a 2 log reduction after induction and consolidation therapy [Citation81,Citation89,Citation90], or 250 copies/104 ABL before and after transplantation [Citation84,Citation91,Citation92].
3.2.4 TP53
TP53 mutations account for <10% of de novo AML, 20%–37% of secondary AML/therapy-related AML, and are associated with a complex karyotype [Citation93–95]. TP53 mutations are a poor prognostic factor, irrespective of the molecular MRD status [Citation17]. Residual TP53 mutations not only can contribute to chemoresistance but also should be included as part of the mutation clearance assessment at CR [Citation16,Citation93,Citation96]. At the time of allo-SCT, TP53 clearance can predict better outcomes in patients who had frontline hypomethylating agent therapy [Citation97].
3.2.5 EVI1
EVI1 is a transcription factor locating on chromosome 3 (3q26.2) [Citation98]. Aberrant expression of EVI1 is present in 8%–10% of AML cases [Citation99]. The overexpression of EVI1 and EVI1 + has a harmful impact on outcome [Citation98–100], and a leukemic clone with EVI1 upregulation is persistent in BM follow-up [Citation101]. About 20% of AML with EVI1 overexpression are associated with a normal karyotype and two-thirds of these patients do not have any conventional molecular markers for MRD [Citation102]. The combined MDS-EVI1/EVI1 gene may serve as an alternative MRD marker in AML, especially in samples where other specific markers are lacking [Citation103], but there are some limitations for the usage of EVI1in MRD monitoring. First, quantification of EVI1 expression is complex, because EVI1 has various splice variants and 5 alternative mRNA 5’-ends [Citation104,Citation105]. Second, the magnitude of reduction in EVI1 expression levels between diagnosis and follow-up is not sufficient to allow sensitive detection of MRD. Besides, since high EVI1 overexpression is associated with adverse cytogenetic abnormalities, the distinction between the pejorative influence of EVI1 overexpression by itself and EVI1 overexpression as part of a larger set of unfavorable abnormalities remains challenging [Citation106].
3.3 Class III gene alterations
Class III are those alterations that promote epigenetic modifications of chromatin in a large area and affect further transcription factors or components of the transcriptional co-activation complexes [Citation18,Citation19].
3.3.1 IDH1 and IDH2
IDH1 and IDH2 mutations are accounted for about 9%–23% of AML, and often coexist with other genes, like NPM1, FLT3 and so on [Citation107,Citation108]. Some studies have confirmed that IDH mutations are a reliable and effective marker for MRD assessment [Citation107,Citation109,Citation110]. PCR is more sensitive and convenient than NGS for detecting IDH mutations [Citation107,Citation111,Citation112]. In the context of using IDH inhibitors, mutation clearance and kinetics by NGS are associated with clinical response rate [Citation113,Citation114]. However, the predictive/prognostic role of IDH mutations which may be influenced either by the clinical context or by concomitant mutations including FLT3, NPM1 and DNMT3A [Citation108,Citation115], and the possibility of being lost as disease progresses [Citation1,Citation7–9], hinder its application in MRD detection. Some researchers though think that IDH1/2 may be useful when used in combination with a second MRD marker [Citation1].
3.3.2 DTA mutations
DTA mutations, including DNMT3A, TET2 and ASXL1, are associated with CHIP and often initiate mutations that remain in subclinical states for long periods. DTA mutations are not associated with an increased risk of relapse [Citation116,Citation117], but they often persist after induction therapy with a lower probability of clearance (especially DNMT3A and TET2) [Citation16,Citation118].
After transplantation, as late relapse can occur after the re-evolution of persistent pre-leukemic clones, monitoring CHIP lesions in MRD evaluation can have potential benefits on post-graft response and long-term relapse [Citation119]. Although using DNMT3A as MRD marker does not predict the outcome, in the following contexts, DNMT3A monitoring may be beneficial in MRD assessment: after allo-HSCT, monitoring DNMT3A by ddPCR can enhance routine qPCR-based MRD techniques in tracing leukemia dynamics [Citation120]; DNMT3A can only be used as a biomarker for those patients in whom DNMT3A mutation is lost after therapy [Citation121].
However, there are some negative opinions about the role of DTA mutations in MRD detection. Studies have shown that removal of DTA mutations or CHIP-related mutations that might be considered as pre-leukemic cell identification markers [Citation93], can improve the ability of mutation clearance or MRD to predict the outcomes [Citation116,Citation122]. Meanwhile, DTA mutations are age-related and it were not correlated with an increased relapse rate [Citation116].
3.3.3 MLL-PTD
MLL rearrangements (MLLr) account for 3%–4% of patients with AML and can give rise to 82 known genetic fusions, in which the following 8 are the most common: PTD, AF4, AF6, AF9, AF10, ENL, ELL and EPS15 [Citation123,Citation124]. Monitoring MLL expression and its kinetics by PCR, including PTD, AF9 and other fusions, is conducive to identify high risk of relapse and to direct therapies or intervention [Citation125–128]. In 80% of patients exhibiting MLL-PTD, the expression of the transcript was high enough to be used as an MRD follow-up tool [Citation129]. MLL-PTD also may be useful when used in combination with a second MRD marker and the expression of MLL gene is more specific and sensitive than WT1 or FCM-MRD [Citation125]. However, occasional loss or gain of MLL-PTD at time of relapse, hinder its use in MRD monitoring [Citation1,Citation11,Citation129].
4. Characteristic of MRD markers
As mentioned above, an applicable MRD marker needs at least one of the following conditions (shown as supporting points in ): ①Expression level should be enough to be detected and be stable or persist; ②Have a proportional or inverse relationship between its expression and disease burden; ③Can enhance, complement, or evaluate other MRD technologies; ④Helpful for risk stratification; ⑤Can indicate next steps of therapy; ⑥Could be served as a valuable surrogate endpoint; ⑦Have unique characteristics of gene structure, which can serve as a longitudinal signature; ⑧May be useful when used in combination with a second MRD marker.
Table 1. Part of molecular alterations that are more controversial whether they can be used as a marker of MRD. 1 point for supporting opinions and −1 point for opposing opinions.
However, there are some limitations about some molecular alterations on MRD monitoring, like (shown as opposing points in ): ①Lack of sensitivity or specificity; ②Expression level is age-related; ③Interference with the ability of mutation clearance or MRD to predict the outcomes; ④The prognostic effect of some molecular alterations could be affected by collaborative mutations or cytogenetic abnormalities and clinical context; ⑤Expression is unstable or complex; ⑥Insensitive technology; ⑦The prognostic significance of some mutations is not clear.
5. Conclusion
MRD plays a critical role in prognosis and treatment for AML. Different subtypes of AML can choose different MRD detection methods. FCM is suitable for most AML patients (more than 85%) [Citation2], but it still has some limitations. The combined application of different MRD detection techniques can improve the detection ability of MRD. Besides, it is very important to select applicable MRD markers according to different molecular characteristic of AML, but every molecular alteration has advantages and limitations, which is the dispute whether it can be used as a good MRD marker. Thus, we need further study and research in order to better guide clinical treatment and improve prognosis.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Schuurhuis GJ, et al. Minimal/measurable residual disease in AML: a consensus document from the European LeukemiaNet MRD working party. Blood. 2018;131(12):1275–1291.
- Gomez-Arteaga A, Guzman ML. Minimal residual disease in acute myeloid leukemia. Adv Exp Med Biol. 2018;1100:111–125.
- Moors I, et al. Clinical implications of measurable residual disease in AML: review of current evidence. Crit Rev Oncol Hematol. 2019;133:142–148.
- Ossenkoppele G, et al. Can we incorporate MRD assessment into clinical practice in AML? Best Pract Res Clin Haematol. 2019;32(2):186–191.
- Feller N, et al. Defining consensus leukemia-associated immunophenotypes for detection of minimal residual disease in acute myeloid leukemia in a multicenter setting. Blood Cancer J. 2013;3(8):e129–e129.
- Rossi G, et al. Leukemia-associated immunophenotypes subdivided in “categories of specificity” improve the sensitivity of minimal residual disease in predicting relapse in acute myeloid leukemia. Cytometry B Clin Cytom. 2020;98(3):216–225.
- Cruz NM, et al. Minimal residual disease in acute myelogenous leukemia. Int J Lab Hematol. 2017;39(Suppl 1):53–60.
- Ravandi F, Walter RB, Freeman SD. Evaluating measurable residual disease in acute myeloid leukemia. Blood Adv. 2018;2(11):1356–1366.
- Luskin MR, Stone RM. Can minimal residual disease determination in acute myeloid leukemia Be used in clinical practice? J Oncol Pract. 2017;13(8):471–480.
- Cilloni D, et al. Digital PCR in myeloid malignancies: ready to replace quantitative PCR? Int J Mol Sci. 2019;20(9):2249. DOI:https://doi.org/10.3390/ijms20092249
- Waterhouse M, et al. Droplet digital PCR for the simultaneous analysis of minimal residual disease and hematopoietic chimerism after allogeneic cell transplantation. Clin Chem Lab Med. 2019;57(5):641–647.
- Levine RL, Valk PJM. Next-generation sequencing in the diagnosis and minimal residual disease assessment of acute myeloid leukemia. Haematologica. 2019;104(5):868–871.
- Behjati S, Tarpey PS. What is next generation sequencing? Arch Dis Child Educ Pract Ed. 2013;98(6):236–238.
- Voso MT, et al. MRD in AML: the role of new techniques. Front Oncol. 2019;9:655. DOI:https://doi.org/10.3389/fonc.2019.00655
- Flach J, et al. Clinical potential of introducing next-generation sequencing in patients at relapse of acute myeloid leukemia. Hematol Oncol. 2020;38(4):425–431.
- Yang F, Anekpuritanang T, Press RD. Clinical utility of next-generation sequencing in acute myeloid leukemia. Mol Diagn Ther. 2020;24(1):1–13.
- Valk PJM. Molecular measurable residual disease detection in AML. HemaSphere. 2020;4:S2: 115-117. Educational Updates in Hematology Book, doi:https://doi.org/10.1097/HS9.0000000000000444.
- Pourrajab F, et al. Genetic characterization and risk stratification of acute myeloid leukemia. Cancer Manag Res. 2020;12:2231–2253.
- Chiaretti S, et al. Genomic characterization of acute leukemias. Med Princ Pract. 2014;23(6):487–506.
- Abdelhamid E, et al. Minimal residual disease monitoring based on FLT3 internal tandem duplication in adult acute myeloid leukemia. Leuk Res. 2012;36(3):316–323.
- Perl AE, et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N Engl J Med. 2019;381(18):1728–1740.
- Chou WC, et al. Sensitive measurement of quantity dynamics of FLT3 internal tandem duplication at early time points provides prognostic information. Ann Oncol. 2011;22(3):696–704.
- Levis, M., FLT3 mutations in acute myeloid leukemia: what is the best approach in 2013? Hematol Am Soc Hematol Educ Program, 2013. 2013: p. 220–226.
- Gaballa S, et al. Relapse risk and survival in patients with FLT3 mutated acute myeloid leukemia undergoing stem cell transplantation. Am J Hematol. 2017;92(4):331–337.
- Candoni A, et al. Predictive value of pretransplantation molecular minimal residual disease assessment by WT1 gene expression in FLT3-positive acute myeloid leukemia. Exp Hematol. 2017;49:25–33.
- Grunwald MR, et al. Improved FLT3 internal tandem duplication PCR assay predicts outcome after allogeneic transplant for acute myeloid leukemia. Biol Blood Marrow Transplant. 2014;20(12):1989–1995.
- Palmisano M, et al. NPM1 mutations are more stable than FLT3 mutations during the course of disease in patients with acute myeloid leukemia. Haematologica. 2007;92(9):1268–1269.
- Levis M, et al. FLT3 inhibitors added to induction therapy induce deeper remissions. Blood. 2020;135(1):75–78.
- Ambinder AJ, Levis M. Potential targeting of FLT3 acute myeloid leukemia. Haematologica. 2021;106(3):671–681.
- Levis MJ, et al. A next-generation sequencing-based assay for minimal residual disease assessment in AML patients with FLT3-ITD mutations. Blood Adv. 2018;2(8):825–831.
- Gebru MT, et al. Glucocorticoids enhance the antileukemic activity of FLT3 inhibitors in FLT3-mutant acute myeloid leukemia. Blood. 2020;136(9):1067–1079.
- Alnagar AA, et al. Outcome of core binding factor acute myeloid leukemia by receptor tyrosine kinase mutation. Clin Lymphoma Myeloma Leuk. 2020;20(7):459–467.
- Burchert A, et al. Sorafenib maintenance after allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia with FLT3-internal tandem duplication mutation (SORMAIN). J Clin Oncol. 2020;38(26):2993–3002.
- Schlenk RF, et al. Differential impact of allelic ratio and insertion site in FLT3-ITD-positive AML with respect to allogeneic transplantation. Blood. 2014;124(23):3441–3449.
- Helbig G, et al. Pre-transplant FLT3/ITD status predicts outcome in FLT3-mutated acute myeloid leukemia following allogeneic stem cell transplantation. Ann Hematol. 2020;99(8):1845–1853.
- Balagopal V, et al. Measurable residual disease monitoring for patients with acute myeloid leukemia following hematopoietic cell transplantation using error corrected hybrid capture next generation sequencing. PLoS One. 2019;14(10):e0224097. DOI:https://doi.org/10.1371/journal.pone.0224097
- Rücker FG, et al. Measurable residual disease monitoring in acute myeloid leukemia with t(8;21)(q22;q22.1): results from the AML study group. Blood. 2019;134(19):1608–1618.
- Ishikawa Y, et al. Prospective evaluation of prognostic impact of KIT mutations on acute myeloid leukemia with RUNX1-RUNX1T1 and CBFB-MYH11. Blood Adv. 2020;4(1):66–75.
- Marková J, et al. Monitoring of minimal residual disease in patients with core binding factor acute myeloid leukemia and the impact of C-KIT, FLT3, and JAK2 mutations on clinical outcome. Leuk Lymphoma. 2009;50(9):1448–1460.
- Liu X, et al. RAS mutations in acute myeloid leukaemia patients: a review and meta-analysis. Clin Chim Acta. 2019;489:254–260.
- Casey G, et al. N-ras mutation in acute myeloid leukemia: incidence, prognostic significance and value as a marker of minimal residual disease. Pathology. 1993;25(1):57–62.
- Ishida H, et al. Panel-based next-generation sequencing facilitates the characterization of childhood acute myeloid leukemia in clinical settings. Biomed Rep. 2020;13(5):1–1.
- Thol F, et al. Measurable residual disease monitoring by NGS before allogeneic hematopoietic cell transplantation in AML. Blood. 2018;132(16):1703–1713.
- Puckrin R, et al. Measurable residual disease monitoring provides insufficient lead-time to prevent morphologic relapse in the majority of patients with core-binding factor acute myeloid leukemia. Haematologica. 2020;106(1):56–63.
- Duployez N, et al. Prognosis and monitoring of core-binding factor acute myeloid leukemia: current and emerging factors. Expert Rev Hematol. 2015;8(1):43–56.
- Perea G, et al. Prognostic value of minimal residual disease (MRD) in acute myeloid leukemia (AML) with favorable cytogenetics [t(8;21) and inv(16)]. Leukemia. 2006;20(1):87–94.
- Wang L, et al. High prognostic value of minimal residual disease detected by flow-cytometry-enhanced fluorescence in situ hybridization in core-binding factor acute myeloid leukemia (CBF-AML). Ann Hematol. 2014;93(10):1685–1694.
- Ouyang J, et al. Comparison of multiparameter flow cytometry immunophenotypic analysis and quantitative RT-PCR for the detection of minimal residual disease of core binding factor acute myeloid leukemia. Am J Clin Pathol. 2016;145(6):769–777.
- Yin JA, et al. Minimal residual disease monitoring by quantitative RT-PCR in core binding factor AML allows risk stratification and predicts relapse: results of the United Kingdom MRC AML-15 trial. Blood. 2012;120(14):2826–2835.
- Pigazzi M, et al. Minimal residual disease monitored after induction therapy by RQ-PCR can contribute to tailor treatment of patients with t(8;21) RUNX1-RUNX1T1 rearrangement. Haematologica. 2015;100(3):e99–e101.
- Willekens C, et al. Prospective long-term minimal residual disease monitoring using RQ-PCR in RUNX1-RUNX1T1-positive acute myeloid leukemia: results of the French CBF-2006 trial. Haematologica. 2016;101(3):328–335.
- Zhu HH, et al. MRD-directed risk stratification treatment may improve outcomes of t(8;21) AML in the first complete remission: results from the AML05 multicenter trial. Blood. 2013;121(20):4056–4062.
- Qin YZ, et al. The dynamics of RUNX1-RUNX1T1 transcript levels after allogeneic hematopoietic stem cell transplantation predict relapse in patients with t(8;21) acute myeloid leukemia. J Hematol Oncol. 2017;10(1):44. DOI:https://doi.org/10.1186/s13045-017-0414-2
- Tang FF, et al. Monitoring of post-transplant CBFB-MYH11 as minimal residual disease, rather than KIT mutations, can predict relapse after allogeneic haematopoietic cell transplantation in adults with inv(16) acute myeloid leukaemia. Br J Haematol. 2018;180(3):448–451.
- Prebet T, et al. Anthracycline dose intensification improves molecular response and outcome of patients treated for core binding factor acute myeloid leukemia. Haematologica. 2014;99(10):e185–e187.
- Ragon BK, et al. Minimal residual disease eradication with epigenetic therapy in core binding factor acute myeloid leukemia. Am J Hematol. 2017;92(9):845–850.
- Mo XD, et al. Interferon-α Is effective for treatment of minimal residual disease in patients with t(8;21) acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation: results of a Prospective registry study. Oncologist. 2018;23(11):1349–1357.
- Corbacioglu A, et al. Prognostic impact of minimal residual disease in CBFB-MYH11-positive acute myeloid leukemia. J Clin Oncol. 2010;28(23):3724–3729.
- Qin YZ, et al. Allogeneic stem cell transplant may improve the outcome of adult patients with inv(16) acute myeloid leukemia in first complete remission with poor molecular responses to chemotherapy. Leuk Lymphoma. 2015;56(11):3116–3123.
- Guièze R, et al. Prognostic value of minimal residual disease by real-time quantitative PCR in acute myeloid leukemia with CBFB-MYH11 rearrangement: the French experience. Leukemia. 2010;24(7):1386–1388.
- Lane S, et al. A > or=1 log rise in RQ-PCR transcript levels defines molecular relapse in core binding factor acute myeloid leukemia and predicts subsequent morphologic relapse. Leuk Lymphoma. 2008;49(3):517–523.
- Xiaosu Z, et al. Classifying AML patients with inv(16) into high-risk and low-risk relapsed patients based on peritransplantation minimal residual disease determined by CBFβ/MYH11 gene expression. Ann Hematol. 2019;98(1):73–81.
- Yalniz FF, et al. Significance of minimal residual disease monitoring by real-time quantitative polymerase chain reaction in core binding factor acute myeloid leukemia for transplantation outcomes. Cancer. 2020;126(10):2183–2192.
- Gao MG, et al. The predictive value of minimal residual disease when facing the inconsistent results detected by real-time quantitative PCR and flow cytometry in NPM1-mutated acute myeloid leukemia. Ann Hematol. 2020;99(1):73–82.
- Zhou Y, et al. Pattern associated leukemia immunophenotypes and measurable disease detection in acute myeloid leukemia or myelodysplastic syndrome with mutated NPM1. Cytometry B Clin Cytom. 2019;96(1):67–72.
- Krönke J, et al. Monitoring of minimal residual disease in NPM1-mutated acute myeloid leukemia: a study from the German-Austrian acute myeloid leukemia study group. J Clin Oncol. 2011;29(19):2709–2716.
- Heiblig M, et al. Prognostic value of genetic alterations in elderly patients with acute myeloid leukemia: a single institution experience. Cancers (Basel). 2019;11(4):570. DOI:https://doi.org/10.3390/cancers11040570
- Balsat M, et al. Postinduction minimal residual disease predicts outcome and benefit from allogeneic stem cell transplantation in acute myeloid leukemia with NPM1 mutation: a study by the Acute Leukemia French Association group. J Clin Oncol. 2017;35(2):185–193.
- Hubmann M, et al. Molecular response assessment by quantitative real-time polymerase chain reaction after induction therapy in NPM1-mutated patients identifies those at high risk of relapse. Haematologica. 2014;99(8):1317–1325.
- Patkar N, et al. Clinical impact of measurable residual disease monitoring by ultradeep next generation sequencing in NPM1 mutated acute myeloid leukemia. Oncotarget. 2018;9(93):36613–36624.
- Jo SY, et al. Correlation of NPM1 type A mutation burden with clinical status and outcomes in acute myeloid leukemia patients With mutated NPM1 type A. Ann Lab Med. 2016;36(5):399–404.
- Lussana F, et al. Molecular detection of minimal residual disease before allogeneic stem cell transplantation predicts a high incidence of early relapse in adult patients with NPM1 positive acute myeloid leukemia. Cancers (Basel). 2019;11(10):1455. DOI:https://doi.org/10.3390/cancers11101455
- Schnittger S, et al. Minimal residual disease levels assessed by NPM1 mutation-specific RQ-PCR provide important prognostic information in AML. Blood. 2009;114(11):2220–2231.
- Shayegi N, et al. The level of residual disease based on mutant NPM1 is an independent prognostic factor for relapse and survival in AML. Blood. 2013;122(1):83–92.
- Bill M, et al. Digital droplet PCR-based absolute quantification of pre-transplant NPM1 mutation burden predicts relapse in acute myeloid leukemia patients. Ann Hematol. 2018;97(10):1757–1765.
- Malmberg D, et al E. Minimal residual disease assessed with deep sequencing of NPM1 mutations predicts relapse after allogeneic stem cell transplant in AML. Leuk Lymphoma. 2019;60(2):409–417.
- Kayser S, et al. Pretransplant NPM1 MRD levels predict outcome after allogeneic hematopoietic stem cell transplantation in patients with acute myeloid leukemia. Blood Cancer J. 2016;6(7):e449–e449.
- Linch DC, et al. Analysis of the clinical impact of NPM1 mutant allele burden in a large cohort of younger adult patients with acute myeloid leukaemia. Br J Haematol. 2020;188(6):852–859.
- Prata PH, et al. NPM1 mutation is not associated with prolonged complete remission in acute myeloid leukemia patients treated with hypomethylating agents. Haematologica. 2018;103(10):e455–e457.
- Lambert J, et al. MRD assessed by WT1 and NPM1 transcript levels identifies distinct outcomes in AML patients and is influenced by gemtuzumab ozogamicin. Oncotarget. 2014;5(15):6280–6288.
- Marjanovic I, et al. Use of Wilms Tumor 1 gene expression as a reliable marker for prognosis and minimal residual disease monitoring in acute myeloid leukemia With normal karyotype patients. Clin Lymphoma Myeloma Leuk. 2017;17(5):312–319.
- Hidaka D, et al. Wilms tumor 1 expression at diagnosis correlates with genetic abnormalities and polymorphism but is not independently prognostic in acute myelogenous leukemia: a hokkaido leukemia net study. Clin Lymphoma Myeloma Leuk. 2018;18(11):e469–e479.
- Šálek C, et al. WT1 expression in peripheral blood at diagnosis and during the course of early consolidation treatment correlates with survival in patients with intermediate and poor-risk acute myeloid leukemia. Clin Lymphoma Myeloma Leuk. 2020;20(12):e998–e1009.
- Cho BS, et al. WT1 measurable residual disease assay in patients with acute myeloid leukemia who underwent allogeneic hematopoietic stem cell transplantation: optimal time points, thresholds, and candidates. Biol Blood Marrow Transplant. 2019;25(10):1925–1932.
- Xu J, et al. Clinical features and prognosis of normal karyotype acute myeloid leukemia pediatric patients with WT1 mutations: an analysis based on TCGA database. Hematology. 2020;25(1):79–84.
- Marani C, et al. Integrating post induction WT1 quantification and flow-cytometry results improves minimal residual disease stratification in acute myeloid leukemia. Leuk Res. 2013;37(12):1606–1611.
- Rautenberg C, et al. Wilm's tumor 1-guided preemptive treatment with hypomethylating agents for molecular relapse of AML and MDS after allogeneic transplantation. Bone Marrow Transplant. 2021;56(2):442–450.
- Ido K, et al. The proportional relationship between pretransplant WT1 mRNA levels and risk of mortality after allogeneic hematopoietic cell transplantation in acute myeloid leukemia not in remission. Transplantation. 2019;103(10):2201–2210.
- Andersson C, et al. Reduction in WT1 gene expression during early treatment predicts the outcome in patients with acute myeloid leukemia. Diagn Mol Pathol. 2012;21(4):225–233.
- Mossallam GI, Abdel Hamid TM, Mahmoud HK. Prognostic significance of WT1 expression at diagnosis and end of induction in Egyptian adult acute myeloid leukemia patients. Hematology. 2013;18(2):69–73.
- Cho BS, et al. Haploidentical vs matched unrelated donor transplantation for acute myeloid leukemia in remission: a prospective comparative study. Am J Hematol. 2021;96(1):98–109.
- Park S, et al. Comparison of myeloablative (CyTBI, BuCy) versus reduced-Intensity (FluBu2TBI400) peripheral blood stem cell transplantation in acute myeloid leukemia patients with pretransplant low WT1 expression. Biol Blood Marrow Transplant. 2020;26(11):2018–2026.
- Yu J, et al. Clinical implications of recurrent gene mutations in acute myeloid leukemia. Exp Hematol Oncol. 2020;9:4. DOI:https://doi.org/10.1186/s40164-020-00161-7
- DiNardo C, Lachowiez C. Acute myeloid leukemia: from mutation profiling to treatment decisions. Curr Hematol Malig Rep. 2019;14(5):386–394.
- Yu G, et al. Gene mutation profile and risk stratification in AML1–ETO–positive acute myeloid leukemia based on next–generation sequencing. Oncol Rep. 2019;42(6):2333–2344.
- Yan B, et al. Low-frequency TP53 hotspot mutation contributes to chemoresistance through clonal expansion in acute myeloid leukemia. Leukemia. 2020;34(7):1816–1827.
- Chan O, et al. Impact of TP53 gene mutation clearance and conditioning Intensity on outcome in MDS or AML patients prior to allogeneic stem cell transplantation. Blood. 2019;134(Supplement_1):149–149.
- Wu X, et al. Prognostic significance of the EVI1 gene expression in patients with acute myeloid leukemia: a meta-analysis. Ann Hematol. 2019;98(11):2485–2496.
- Marjanovic I, et al. Expression pattern and prognostic significance of EVI1 gene in adult acute myeloid leukemia patients with normal karyotype. Indian J Hematol Blood Transfus. 2020;36(2):292–299.
- Paubelle E, et al. Efficacy of all-trans-retinoic acid in high-risk acute myeloid leukemia with overexpression of EVI1. Oncol Ther. 2019;7(2):121–130.
- Ding L, et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature. 2012;481(7382):506–510.
- Gröschel S, et al. High EVI1 expression predicts outcome in younger adult patients with acute myeloid leukemia and is associated with distinct cytogenetic abnormalities. J Clin Oncol. 2010;28(12):2101–2107.
- Weisser M, et al. Feasibility of using the combined MDS-EVI1/EVI1 gene expression as an alternative molecular marker in acute myeloid leukemia: a report of four cases. Cancer Genet Cytogenet. 2007;177(1):64–69.
- Wieser R. The oncogene and developmental regulator EVI1: expression, biochemical properties, and biological functions. Gene. 2007;396(2):346–357.
- Aytekin M, et al. Regulation of the expression of the oncogene EVI1 through the use of alternative mRNA 5'-ends. Gene. 2005;356:160–168.
- Smol T, et al. Quantification of EVI1 transcript levels in acute myeloid leukemia by RT-qPCR analysis: a study by the ALFA group. Leuk Res. 2015;39(12):1443–1447.
- Petrova L, et al. IDH1 and IDH2 mutations in patients with acute myeloid leukemia: suitable targets for minimal residual disease monitoring? Clin Biochem. 2018;61:34–39.
- Grassi S, et al. Digital droplet PCR is a specific and sensitive tool for detecting IDH2 mutations in acute myeloid LeuKemia patients. Cancers (Basel). 2020;12(7):1738. DOI:https://doi.org/10.3390/cancers12071738
- Jeziskova I, et al. Quantitative detection of IDH2 mutation for minimal residual disease monitoring in patients with acute myeloid leukemia and its comparison with mutations in NPM1 gene. Leuk Lymphoma. 2013;54(4):867–870.
- Debarri H, et al. IDH1/2 but not DNMT3A mutations are suitable targets for minimal residual disease monitoring in acute myeloid leukemia patients: a study by the acute Leukemia French association. Oncotarget. 2015;6(39):42345–42353.
- Petiti J, et al. Highly sensitive detection of IDH2 mutations in acute myeloid leukemia. J Clin Med. 2020;9(1):271. DOI:https://doi.org/10.3390/jcm9010271
- Abdelhamid E, et al. Minimal residual disease assessment of IDH1/2 mutations in acute myeloid leukemia by LNA-RQ-PCR. Tunis Med. 2016;94(3):190–197.
- Stein EM, et al. Molecular remission and response patterns in patients with mutant-IDH2 acute myeloid leukemia treated with enasidenib. Blood. 2019;133(7):676–687.
- Roboz GJ, et al. Ivosidenib induces deep durable remissions in patients with newly diagnosed IDH1-mutant acute myeloid leukemia. Blood. 2020;135(7):463–471.
- Ok CY, et al. Persistent IDH1/2 mutations in remission can predict relapse in patients with acute myeloid leukemia. Haematologica. 2019;104(2):305–311.
- Jongen-Lavrencic M, et al. Molecular minimal residual disease in acute myeloid leukemia. N Engl J Med. 2018;378(13):1189–1199.
- Genovese G, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. 2014;371(26):2477–2487.
- Getta BM, et al. Multicolor flow cytometry and multigene next-generation sequencing Are complementary and Highly predictive for relapse in acute myeloid leukemia after allogeneic transplantation. Biol Blood Marrow Transplant. 2017;23(7):1064–1071.
- Hirsch P, et al. Genetic hierarchy and temporal variegation in the clonal history of acute myeloid leukaemia. Nat Commun. 2016;7:12475. DOI:https://doi.org/10.1038/ncomms12475
- Brambati C, et al. Droplet digital polymerase chain reaction for DNMT3A and IDH1/2 mutations to improve early detection of acute myeloid leukemia relapse after allogeneic hematopoietic stem cell transplantation. Haematologica. 2016;101(4):e157–e161.
- Jeziskova I, et al. Distribution of mutations in DNMT3A gene and the suitability of mutations in R882 codon for MRD monitoring in patients with AML. Int J Hematol. 2015;102(5):553–557.
- Morita K, et al. Clearance of somatic mutations at remission and the risk of relapse in acute myeloid leukemia. J Clin Oncol. 2018;36(18):1788–1797.
- Burillo-Sanz S, et al. MLL-rearranged acute myeloid leukemia: influence of the genetic partner in allo-HSCT response and prognostic factor of MLL 3’ region mRNA expression. Eur J Haematol. 2018;100(5):436–443.
- Burmeister T, et al. Evidence-based RT-PCR methods for the detection of the 8 most common MLL aberrations in acute leukemias. Leuk Res. 2015;39(2):242–247.
- Liu J, et al. Monitoring mixed lineage leukemia expression may help identify patients with mixed lineage leukemia–rearranged acute leukemia who are at high risk of relapse after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2014;20(7):929–936.
- Huang S, et al. Prognostic significance of mixed-lineage leukemia (MLL) gene detected by real-time fluorescence quantitative PCR assay in acute myeloid leukemia. Med Sci Monit. 2016;22:3009–3017.
- Abildgaard L, et al. A novel RT-qPCR assay for quantification of the MLL-MLLT3 fusion transcript in acute myeloid leukaemia. Eur J Haematol. 2013;91(5):394–398.
- Huang S, et al. [Prognostic significance of detecting MLL-AF9 fusion gene expression in patients with acute myeloid leukemia by real-time fluorescence quantitative PCR]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2013;21(6):1435–1440.
- Ommen HB, et al. Relapse kinetics in acute myeloid leukaemias with MLL translocations or partial tandem duplications within the MLL gene. Br J Haematol. 2014;165(5):618–628.
