ABSTRACT
Background
Among B-cell lymphoma, multiple myeloma (MM) is an incurable malignancy. miR-140-3p was known to be an inhibitor in malignant tumors. However, the function of miR-140-3p in MM remains unclear.
Methods
qRT-PCR was performed to determine the expressions of miR-140-3p and BZW2 mRNA. The protein level of BZW2 was determined by the western blot. Cell viability or cell apoptosis was detected by the MTT assay or flow cytometry, respectively. Binding between miR-140-3p and BZW2 was validated using the dual luciferase assay. Xenograft model was applied to verify the results of in vitro study.
Results
The level of miR-140-3p was significantly downregulated in MM. Overpexression of miR-140-3p impaired the proliferation of MM cell lines and induced apoptosis in MM cells. miR-140-3p was validated to target BZW2 and inhibit the expression of BZW2. BZW2 was involved in the regulation of miR-140-3p on MM cell vitality and apoptosis. In vivo study revealed that miR-140-3p impeded tumorigenesis of MM cell line in nude mice.
Conclusion
Our present study revealed that miR-140-3p served as a suppressor in MM by negatively regulating BZW2. Thus, miR-140-3p could act as a new target for treating MM.
KEYWORDS:
Introduction
Multiple myeloma (MM) is one of the malignancies and originates from plasma cells in bone marrow. It is currently classified by the WHO as a type of B-cell lymphoma [Citation1]. Although autologous stem cell transplantation, immunomodulatory drug (IMiD), proteasome inhibitors, monoclonal antibodies and other treatments have greatly extended the overall survival of the patients with MM, it still cannot be completely cured [Citation2]. Currently, the prognosis of patients with MM is still poor [Citation3]. Therefore, it is urgent to further understand the pathological mechanisms of MM for exploring new therapies.
microRNAs (miRNAs) were proved to be critical regulators in MM. For instance, miR-342 and miR-363 were reported to suppress the progression of MM by tarting RUNX2 [Citation4]. It was also demonstrated that miR-26a attenuated CD38 to serve as a tumor suppressor in MM [Citation5]. Moreover, Wu et al. confirmed that miR-145-3p enhanced the bortezomib sensitivity of MM cell via inhibition of HDAC4 [Citation6]. miR-140-3p was validated as a suppressive regulator in multiple types of malignancies. In colorectal cancer, overexpression of miR-140-3p led to a promotion of apoptosis in tumor cells via targeting PD-L1 [Citation7]. In bladder cancer, miR-140-3p attenuated the malignant phenotypes of tumor cells by repressing FOXQ1 [Citation8]. Additionally, miR-140-3p was verified to suppress proliferation of breast cancer cell via directly binding with TRIM28 [Citation9]. A study revealed that the expression of miR-140-3p was dysregulated in MM [Citation10]. However, the functional role of miR-140-3p in MM needs further investigation.
Basic leucine zipper and W2 domain 2 (BZW2) are transcription factors from basic region leucine zipper (bZIP) superfamily [Citation11]. Previous studies have illustrated that BZW2 was involved in the progression of multiple cancers. In hepatocellular carcinoma, elevated BZW2 induced drug resistance via activating PI3K/Akt signaling [Citation11]. In lung adenocarcinoma, BZW2 was also proved to be a marker of prognosis in patients [Citation12]. Moreover, in colorectal cancer, it was demonstrated that BZW2 promoted malignant phenotypes of cancer cells by facilitating the activation of ERK/MAPK signaling [Citation13]. However, no evidence was shown to illustrate the role of BZW2 in the progression of MM. Our previous bioinformatic analysis found miR-140-3p might directly bind with BZW2. Hence, we speculated that BZW2 mediated functional effect of miR-140-3p in MM.
In the current work, we demonstrated that miR-140-3p was decreased in MM tissue and MM cell lines. miR-140-3p abrogated the growth of MM. Mechanically, miR-140-3p exhibited suppressive effect via targeting BZW2. Our study might provide novel understanding of the pathogenesis of MM.
Materials and methods
Culture of NCI-H929 cells
Human MM cell lines (NCI-H929, MM16, U266, INA-6 and OPM2) and human nucleus pulposus cells (nPCs) were bought from American type culture collection. Cells were cultured in RPMI-1640 (Thermo Fisher Scientific, Waltham, MA, USA) medium with 10% FBS (Thermo Fisher Scientific) at 37°C with 5% CO2.
Western blot
Total protein extraction of tissue or cell was prepared by RIPA solution, equal amount of the sample was added to 10% SDS-PAGE, and proteins were separated and then transferred into PVDF membranes. Next, the unspecific sites in the membranes were blocked by 5% non-fat milk. The protein bands were then incubated with rabbit anti-BZW2 (Abcam, ab254771), cleaved caspase 3 (Abcam, ab32042), cleaved PARP (Abcam, ab32064) mouse anti-β-actin (Abcam, ab170325) separately. Furthermore, non-specific binding primary antibodies in the membranes were washed by PBS. The secondary antibodies, goat anti-rabbit IgG (Abcam, ab205718) or goat anti-mouse IgG (Abcam, ab205719) were incubated with membranes for 1 h. The protein band was visualized by a Western Lightning system.
qRT-PCR
Total RNA was extracted and purified by Trizol reagent (Invitrogen, Waltham, MA, USA), and reversed into cDNA using RT reagent kit (TaKaRa, Shiga, Japan). The cDNA used for qRT-PCR was carried out and mixed with SYBR Master Mixture (TaKaRa) according to the manufacturer's instructions. Then, qRT-PCR was performed using the SYBR Premix Ex Taq II kit (Takara) as follows: 2 min at 94°C, followed by 35 cycles (30 s at 94°C and 45 s at 55°C). The target gene expression was presented using the 2-ΔΔΔCT method. The forward primer sequence of BZW2 was 5′-TTTCTGGACTCTACAGGCTCAA-3′ and the reverse primer sequence was 5′-ACCATCATCTATGCGCGTTCC-3′. The primer sequence of miR-140-3p was 5′-TACCACAGGGTAGAACCACGG-3′.
Cell proliferation assay
The proliferation of NCI-H929 cells was examined by the MTT kit (Beyotime, Shanghai, China). Cells (5 × 103 per well) were seeded in a 96-well plate overnight. After being transfected with miR-140-3p mimics or pcDNA-BZW2 for 36 h, cells were incubated with 100 μL of MTT reagent (5 mg/mL) at dark for 4 h and the observation was measured at 570 nm using a MicroplateReader.
Cell apoptosis assay
Apoptosis Detected Kit (Thermo Fisher Scientific) was prepared for apoptosis assay. NCI-H929 cells were diluted to 106 cells/mL after 48 h culture, washed with PBS and then resuspended in binding buffer. Then, the cell suspension was mixed with 5 μL V-FITC and 10 μl PI for 15 min, the apoptosis rate was calculated using a flow cytometer.
Cell transfection
NCI-H929 or U266 cells were seeded to a 6-well plate for 12−24 h, then transfected with miR-140-3p mimics (Genepharma), NC mimics (Genepharma), pcDNA3,1-BZW2 (Genepharma) or pcDNA3.1 (Genepharma) using Lipofectamine 3000 reagent (Invitrogen) for 36 h according to the manufacture's protocol. The effect of plasmid transfection was detected by qRT-PCR.
For BZW2 knockdown, NCI-H929 cells were transfected with NC siRNA or BZW2 siRNA (Genepharma) for 36 h using Lipofectamine 3000 reagent (Invitrogen) according to the manufacturer's instruction.
Dual luciferase reporter gene assay
Starbase was performed for bioinformatic analysis of BZW2 and miR-140-3p. The full length sequences of wild and mutant type of BZW2 3′UTR were cloned and inserted into pGL3-basic luciferase reporter gene plasmid (Genechem, Shanghai, China) to generate BZW2 overexpression plasmid pGL3-WT-BZW2 and pGL3-MUT-BZW2. Then, cells were co-transfected with miR-140-3p mimics/inhibitor and WT-/MUT-BZW2 for 36 h. Luciferase Assay kit (Promega) was used to perform luciferase activity analysis.
Tumor xenograft
NCI-H929 cells were harvested and resuspended at a density of 5 × 107 cells/mL. The BALB/C mice (6-weeks-old) were divided into two groups (four mice each group), and intraperitoneally injected 200 μL of cell suspension. The tumor volume was calculated at days 5, 10, 15, 20, 25 and 30. After 30 days, the mice were anesthetized with pentobarbital sodium and killed, and the tumor was removed for examining the BZW2 expression. Animal experiments were approved.
Ethics statement
MM tissue (MM group, 15 cases) and adjacent tissue (normal group, 15 cases) were obtained from patients undergoing surgery for tumor excision. This study was approved.
Statistical analysis
All data were obtained from three or more experiments and expressed as mean ± SD. The GraphPad Prism 8.0 was used for statistical analysis, data difference was identified by student t-test between two groups or one-way ANOVA among multiple groups.
Results
miR-140-3p was decreased, whereas BZW2 was upregulated in MM
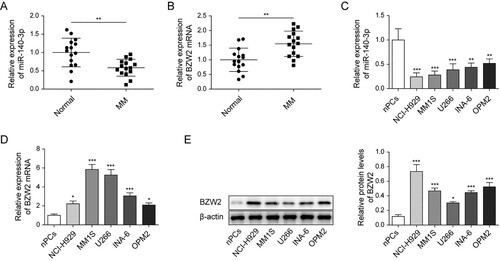
The ratio of CD138 + positive cells was significantly upregulated in MM tissues (Figure S1). Then, we investigated the expression of miR-140-3p and BZW2 in MM. Results revealed that miR-140-3p was substantiallly decreased in MM tissue (A). On contrary, the level of BZW2 mRNA was elevated in MM tissue compared to that in adjacent normal tissue (B). Additionally, the expression of miR-140-3p was significantly upregulated in MM cell lines than in human normal plasma cell line (C). Consistently, the expressions of BZW2 were notably upregulated in MM cell lines (D and 1E). Collectively, the results illustrated that miR-140-3p was downregulated, whereas BZW2 was upregulated in MM.
Figure 1. miR-140-3p was decreased, whereas BZW2 was upregulated in MM. The expression of miR-140-3p (A) and BZW2 mRNA (B) in MM tissue and adjacent normal tissue was detected by qRT-PCR. The expression of miR-140-3p (C) and BZW2 mRNA (D) in MM cell lines and normal plasma cell line was determined by qRT-PCR. (E) The protein level of BZW2 in MM cell lines and normal plasma cell line was determined by western blots. *P < 0.05; **P < 0.01; ***P < 0001.
miR-140-3p impaired proliferation while enhanced apoptosis in MM cell lines
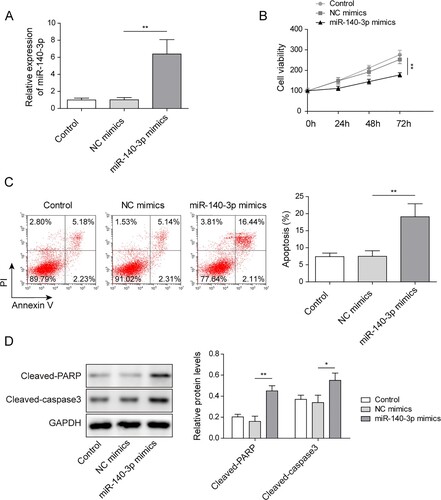
To investigate the role of miR-140-3p in MM cell lines, miR-140-3p was increased after being transfected with miR-140-3p mimics in NCI-H929 cell line (A). Interestingly, we verified miR-140-3p mimics substantially abrogated cell viability of NCI-H929 (B). Additionally, flow cytometry illustrated that miR-140-3p mimics notably elevated apoptotic rate in CI-H929 cell line (C). Furthermore, the upregulation of miR-140-3p notably increased the levels of cleaved caspase 3 and cleaved PARP (D). The data in U266 cells were consistent with those in NCI-H929 cells (Figure S2A−D). Taken together, our results validated miR-140-3p impaired cell viability, whereas enhanced apoptosis in MM cell lines.
Figure 2. miR-140-3p suppressed proliferation and induced apoptosis in MM cell lines. NCI-H929 cell line was transfected with miR-140-3p mimics or NC mimics. (A) qRT-PCR was used to determine the expression of miR-140-3p. (B) Cell viability was tested by MTT assay. (C) Flow cytometry was measured to analyze cell apoptosis. (D) The levels of cleaved caspase 3 and cleaved PARP in MM cells were detected by western blot. **P < 0.01.
BZW2 was targeted by miR-140-3p
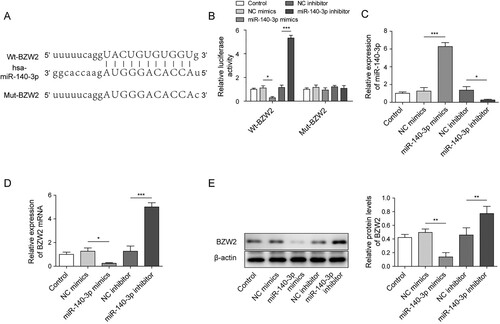
StarBase 2.0 was used and indicated a miR-140-3p potential binding site in 3′UTR of BZW2 mRNA (A). Dual luciferase assay was applied to verify the binding of miR-140-3p and BZW2. As shown in B, when co-transfected miR-140-3p mimics with reporter containing wild-type binding sequence (WT-BZW2), the relative luciferase activity was significantly dropped. Moreover, when co-transfected miR-140-3p inhibitor with WT-BZW2, the relative luciferase activity was notably elevated. However, when co-transfected mimics/inhibitor with mutated binding sequence (MUT-BZW2), the relative luciferase activity did not significantly change (B). The efficiency of miR-140-3p mimics/inhibitor was validated (C). As we expected, the expression of BZW2 was negatively regulated by miR-140-3p mimics and raised by miR-140-3p inhibitor at both mRNA and protein level (D and E). Collectively, the results suggested that BZW2 was targeted by miR-140-3p.
Figure 3. BZW2 was targeted by miR-140-3p. (A) The binding site between miR-140-3p and BZW2 was predicted by starBase 2.0. (B) The direct binding was verified by dual luciferase assay. The expression of miR-140-3p (C) and BZW2 mRNA (D) in the NCI-H929 cell line was determined by qRT-PCR. (E) The protein level of BZW2 in the NCI-H929 cell line was determined by western blots. *P < 0.05; **P < 0.01; ***P < 0001.
BZW2-mediated regulative work of miR-140-3p in MM cell line
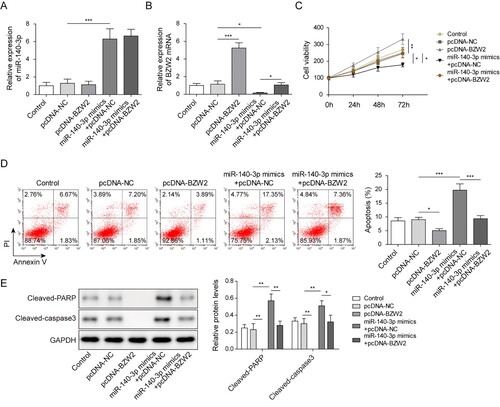
To investigate whether miR-140-3p regulated viability and apoptosis via BZW2, we overexpressed BZW2 in the presence of miR-140-3p mimics or in the absence of miR-140-3p mimics. It was showed that the overexpression of BZW2 failed to affect the expression of miR-140-3p (A). However, pcDNA-BZW2 successfully increased the BZW2 expression when solely transfected into cell and recovered the expression of BZW2 repressed by miR-140-3p mimics (B). The MTT assay demonstrated that the overexpression of BZW2 promoted the viability of MM cell and restored the cell viability repressed by miR-140-3p (C). Moreover, the overexpression of BZW2 suppressed the apoptotic rate of MM cells induced by miR-140-3p (D). MiR-140-3p-induced upregulation of cleaved caspase 3 and cleaved PARP was significantly reversed by the overexpression of BZW2 (E). On the contrary, knockdown of BZW2 notably inhibited the viability of MM cells via inducing the apoptosis (Figure S3A−D). Collectively, our results illustrated that overexpression of BZW2 reversed the effect of miR-140-3p on MM cells.
Figure 4. BZW2 was involved in the regulative work of miR-140-3p in the MM cell line. NCI-H929 cell line was transfected with BZW2 overexpressing plasmid (pcDNA-BZW2) or negative control (pcDNA-NC) alone or co-transfected with miR-140-3p mimics or NC mimics. The expression of miR-140-3p (A) and BZW2 mRNA (B) in NCI-H929 was determined by qRT-PCR. (C) MTT assay was used to detect cell viability. (D) Cell apoptosis was determined by flow cytometry. (E) The levels of cleaved caspase 3 and cleaved PARP in MM cells were detected by western blot. *, P < 0.05; **, P < 0.01; ***, P < 0001.
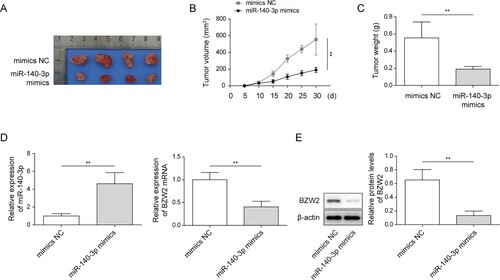
miR-140-3p suppressed tumorigenesis in vivo
To verify our in vitro data, we confirmed whether miR-140-3p regulated tumorigenesis of MM cell line in vivo. As shown in A–C, miR-140-3p-transfected MM cell line illustrated weak capacity of tumorigenesis, indicated by small volume and less tumor weight. We also verified the elevated level of miR-140-3p in tumor tissue (D). Moreover, the level of BZW2 mRNA and protein in xenograft tumor tissue were substantially dropped by miR-140-3p mimics (D and E). The above-mentioned results indicated that miR-140-3p repressed tumor growth in vivo.
Figure 5. miR-140-3p-suppressed tumorigenesis in vivo. NCI-H929 cell line was transfected with miR-140-3p mimics or NC mimics. Then, the cells were injected into nude mice for xenograft. (A) The image of NCI-H929 cell line-generated tumor tissues. (B) The calculation of the volume of tumor tissue. (C) Weight of the tumor tissue. (D) The expression of miR-140-3p and BZW2 in xenograft tumor tissue was determined by qRT-PCR. (E) The protein level of BZW2 in xenograft tumor tissue was determined by western blots. *P < 0.05; **P < 0.01; ***P <0001.
Discussion
MM was an incurable malignancy with short survival rate. In 2020, there are approximately 12,830 new estimated cases of MM and 32,270 MM-related deaths [Citation14]. Hence, to further reveal the pathology of MM is of great importance. In current study, our data confirmed that miR-140-3p was decreased in MM tissue and cell line. Functional study indicated that miR-140-3p inhibited the growth of MM cell. Mechanically, miR-140-3p exhibited suppressive effect via targeting BZW2.
Several studies have revealed that miR-140-3p was a tumor suppressive miRNA in many cancers. In current work, we found miR-140-3p abrogated proliferation and induced apoptosis in MM cells. Moreover, xenograft assay also validated miR-140-3p repressed tumorigenesis in vivo. Our results were consistent with previous studies. As shown by Jiang et al, overexpression of miR-140-3p led to a suppression of proliferation and an elevation of apoptosis in colorectal cancer [Citation7]. Additionally, miR-140-3p was proved to impair proliferation in cervical cancer [Citation15]. By inhibiting JAK1, miR-140-3p was also proved to retard growth of non-small cell lung cancer [Citation16]. These studies and our present work revealed a suppressive role of miR-140-3p in cancers. On contrary, some studies suggested a contradictive conclusion. For instance, in ovarian cancer, miR-140-3p was shown to impair cytotoxicity of nature killer cell via MAPK1 [Citation17]. miR-140-3p was also validated to emerge oncogenic role in chordoma. Inhibition of miR-140-3p sensitized tumor cell to chemotherapy via inducing PTEN [Citation18]. Therefore, miR-140-3p may play different functions in different cancers.
Our present study revealed that BZW2 was upregulated in MM. Mechanically, we also found a novel regulative work between miR-140-3p and BZW2. Our data revealed that BZW2 was suppressed by miR-140-3p. These findings were consistent with previous studies of BZW2 in cancer. As shown by Gao et al, BZW2 knockdown impaired the growth of bladder cancer cells [Citation19]. Moreover, BZW2 was also proved to promote osteosarcoma cell growth by inactivation of Akt/mTOR signaling [Citation20]. Besides the regulative work on cell growth, BZW2 was also demonstrated to play critical role in other malignant phenotypes of tumor. For instance, BZW2 facilitated invasion and migration in hepatoma cells [Citation21]. Our further work should investigate whether miR-140-3p attenuates BZW to attenuate invasion and migration in MM cells. On the contrary, previous study found that miR-140-3p could target TRIM28 in breast cancer [Citation9]. Thus miR-140-3p might regulate the expression of BZW2 through TRIM28 or other proteins in MM. Further study will focus on this point. Indeed, the expression of mRNA and protein of BZW2 in different MM cell lines was not consistent. The different experimental conditions might result in this phenomenon.
In conclusion, we revealed that miR-140-3p was decreased in MM tissue and cell line. Overexpression of miR-140-3p inhibited the growth of MM cells via targeting and attenuating the expression of oncogenic gene BZW2.
| Abbreviation list | ||
| MM | = | multiple myeloma |
| miRNA | = | microRNA |
| FOXQ1 | = | forkhead box Q1 |
| PD-L1 | = | Programmed cell death 1 ligand 1 |
| TRIM28 | = | tripartite motif containing 28 |
| BZW2 | = | basic leucine zipper and W2 domains 2 |
| ERK | = | extracellular regulated MAP kinase |
| PI3 K | = | phosphatidylinositol 3-kinase |
| MAPK | = | mitogen-activated protein kinase |
| qRT-PCR | = | Reverse transcription followed by quantitative real-time PCR |
| WB | = | western blots |
Supplemental Material
Download TIFF Image (939.3 KB)Supplemental Material
Download TIFF Image (927.5 KB)Supplemental Material
Download TIFF Image (129.3 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364(11):1046–1060.
- Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111(5):2516–2520.
- Rajkumar SV. Multiple myeloma: every year a new standard? Hematol Oncol. 2019;37(Suppl. 1):62–65.
- Gowda PS, Wildman BJ, Trotter TN, et al. Runx2 suppression by miR-342 and miR-363 inhibits multiple myeloma progression. Mol Cancer Res. 2018;16(7):1138–1148.
- Hu Y, Liu H, Fang C, et al. Targeting of CD38 by the tumor suppressor miR-26a serves as a novel potential therapeutic agent in multiple myeloma. Cancer Res. 2020;80(10):2031–2044.
- Wu H, Liu C, Yang Q, et al. MIR145-3p promotes autophagy and enhances bortezomib sensitivity in multiple myeloma by targeting HDAC4. Autophagy. 2020;16(4):683–697.
- Jiang W, Li T, Wang J, et al. miR-140-3p suppresses cell growth and induces apoptosis in colorectal cancer by targeting PD-L1. Onco Targets Ther. 2019;12:10275–10285.
- Wang Y, Chen J, Wang X, et al. miR-140-3p inhibits bladder cancer cell proliferation and invasion by targeting FOXQ1. Aging (Albany NY). 2020;12(20):20366–20379.
- Zhou Y, Wang B, Wang Y, et al. miR-140-3p inhibits breast cancer proliferation and migration by directly regulating the expression of tripartite motif 28. Oncol Lett. 2019;17(4):3835–3841.
- Lionetti M, Biasiolo M, Agnelli L, et al. Identification of microRNA expression patterns and definition of a microRNA/mRNA regulatory network in distinct molecular groups of multiple myeloma. Blood. 2009;114(25):e20–e26.
- Jin X, Liao M, Zhang L, et al. Role of the novel gene BZW2 in the development of hepatocellular carcinoma. J Cell Physiol. 2019;234(9):16592–16600.
- Wang S, Bai W, Huang J, et al. Prognostic significance of BZW2 expression in lung adenocarcinoma patients. Int J Clin Exp Pathol. 2019;12(12):4289–4296.
- Huang L, Chen S, Fan H, et al. BZW2 promotes the malignant progression of colorectal cancer via activating the ERK/MAPK pathway. J Cell Physiol. 2020;235(5):4834–4842.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30.
- Ma J, Zhang F, Sun P. miR-140-3p impedes the proliferation of human cervical cancer cells by targeting RRM2 to induce cell-cycle arrest and early apoptosis. Bioorg Med Chem. 2020;28(3):115283.
- Hu C, Zou Y, Jing LL. miR-140-3p inhibits progression of non-small cell lung cancer by targeting Janus kinase 1. J Biosci. 2020;45(48):1–11.
- Wang J, Zhu M, Zhou X, et al. MiR-140-3p inhibits natural killer cytotoxicity to human ovarian cancer via targeting MAPK1. J Biosci. 2020;45(66):1–10.
- Zhao K, Li X, Chen X, et al. Inhibition of miR-140-3p or miR-155-5p by antagomir treatment sensitize chordoma cells to chemotherapy drug treatment by increasing PTEN expression. Eur J Pharmacol. 2019;854:298–306.
- Gao H, Yu G, Zhang X, et al. BZW2 gene knockdown induces cell growth inhibition, G1 arrest and apoptosis in muscle-invasive bladder cancers: a microarray pathway analysis. J Cell Mol Med. 2019;23(6):3905–3915.
- Cheng DD, Li SJ, Zhu B, et al. Downregulation of BZW2 inhibits osteosarcoma cell growth by inactivating the Akt/mTOR signaling pathway. Oncol Rep. 2017;38(4):2116–2122.
- Liu J, Yang T, Zhang Y, et al. Promotion of BZW2 by LINC00174 through miR-4500 inhibition enhances proliferation and apoptosis evasion in laryngeal papilloma. Cancer Cell Int. 2020;20:471.
