ABSTRACT
Introduction: Compared with the 3 + 7 regimen, the addition of gemtuzumab ozogamicin (GO) has improved survival in patients with acute myeloid leukemia (AML). We conducted a systematic review and meta-analysis to examine the overall efficacy and safety of GO in combination with conventional chemotherapy regimens in patients with AML.
Methods: We searched several databases (MEDLINE, Embase, Web of Science and Cochrane Library). Hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated for overall survival (OS) and relapse-free survival (RFS); odds ratios (ORs) with 95% CIs were calculated for the other outcomes.
Results: Ten records involving 11 randomized controlled trials (RCTs) met the inclusion criteria. GO plus induction chemotherapy significantly increased RFS (HR: 0.84, 95% CI: 0.73–0.98), decreased the incidence of relapse (OR: 0.78, 95% CI: 0.68–0.91) and resistant disease (OR: 0.72, 95% CI: 0.61–0.84), and had no significant effect on the rate of complete remission (CR) with or without incomplete platelet recovery (OR: 1.21, 95% CI: 0.94–1.55), 30-day mortality (OR: 1.25, 95% CI: 0.99–1.57). Subgroup analysis showed significant OS benefits for patients with favorable cytogenetic (HR: 0.50, 95% CI: 0.28–0.89) or given GO at induction stage (HR: 0.91, 95% CI: 0.84–1.00). Compared with other dosing schedule groups, 3 mg/m2 fractionated schedule had a greater RFS benefit (HR: 0.52, 95% CI: 0.36–0.76) and lower relapse risk (OR: 0.48, 95% CI: 0.28–0.84).
Conclusions: Adding low-dose GO to induction or both induction and post-remission chemotherapy has considerable efficacy and unequivocal safety for newly diagnosed adult AML.
Introduction
Acute myeloid leukemia (AML) is a malignant clonal disorder of bone marrow hematopoietic stem/progenitor cells characterized by abnormal proliferation of primitive cells in the bone marrow. Although AML occurs at any age, it is the most common type of acute leukemia in adults and is seen mainly in the elderly. A recent epidemiological analysis showed that the incidence of AML increases with age, with a median age at diagnosis of 68 years [Citation1].
For several decades, the incidence of AML has been on the rise. Several environmental and genetic risk factors have been shown to predispose individuals to the development of AML. Meanwhile, this uptrend is associated with the increasing incidence of therapy-related AML, as more cancer patients receive cytotoxic chemotherapy to cure their primary malignancies [Citation2]. However, the standard of care for AML has remained largely stagnant since the 1970s, with the ‘7 + 3’ regimen remaining the most common induction therapy [Citation3]. In recent years, with the continuous research in molecular biology, more and more molecular targets have been discovered and unprecedented advances have been made in the treatment of AML. Targeted therapeutics such as gemtuzumab ozogamicin (GO) have become a common addition to standard chemotherapy. GO is an antibody-coupled drug consisting of a humanized IgG4 monoclonal antibody and a calicheamicin derivative, which binds specifically to CD33-expressing leukemia cells, where the linker is hydrolyzed and then releases calicheamicin, which enters the nucleus and binds to DNA to break the double helix chain, ultimately leading to cell death [Citation4]. GO received accelerated approval by the US Food and Drug Administration (FDA) for the treatment of elderly relapsed CD33+ AML with a recommended regimen of 9 mg/m2 for two doses 14 days apart in 2000. But a confirmatory study, the SWOG S0106 trial showed that adding GO to induction chemotherapy had no greater clinical benefit and increased the early mortality and the incidence of fatal liver damage for newly diagnosed AML, thereby it was withdrawn from the market in 2010. However, afterwards a subsequent randomized studies, included the ALFA 0701 trial, indicated a survival benefit and non-increased mortality of lower dose GO. In September 2017, FDA reapproved GO for treatment of newly diagnosed (adults) and relapsed/refractory (aged ≥ 2 years) CD33+ AML [Citation5].
In light of the approvals, withdrawals and re-approvals, we conducted a meta-analysis to compare whether the addition of GO to standard chemotherapy in AML improves its efficacy and safety, as well as to determine the optimal dose and treatment phase of GO in combination.
Materials and methods
Study registration and protocol
This systematic review and meta-analysis was registered on the PROSPERO (CRD42020190386) and was reported according to the latest Preferred Reporting Items for Systematic Reviews and Meta-analysis 2020 guideline (PRISMA) [Citation6]. The PRISMA 2020 checklist is shown in supplementary materials (S1).
Data sources and searches
Randomized controlled trials published in any language up to 17 November 2021 were searched in MEDLINE, Embase, Web of Science, and Cochrane Library. Searches were performed using a combination of subject terms and free-text terms for patients, interventions, and study designs of interest. A detailed search strategy is available in supplementary material (S2).
Study selection criteria
Inclusion criteria followed the PICOS principle: (1) participants: patients had de novo or secondary untreated AML or entered complete remission (CR) after induction chemotherapy; (2) intervention and control: trials included a comparison of adding GO to conventional chemotherapy with conventional chemotherapy alone; (3) outcomes: included efficacy endpoints as overall survival (OS), relapse-free survival (RFS), CR with or without incomplete platelet recovery, relapse risk and resistant disease, and safety end points as 30-day mortality; (4) study design: clinical RCTs published in any language.
Exclusion criteria included the following: (1) research objects were pediatric patients, relapsed or refractory AML or acute promyelocytic leukemia patients; (2) trials that focused on other drugs or regimens; (3) unable to get full text and valid outcomes; (4) research type was not clearly explained or not RCTs; (5) duplicated records.
After removing duplicate studies, two authors screened the search results according to the title and abstract to evaluate the eligibility. Any inconsistencies were discussed with a third author to achieve final consensus.
Data extraction and quality assessment
According to the research need, we extracted data for each record using pre-designed tables, including references, trials name, disease, number of patients, median age, gender, treatment stage, baseline chemotherapy, dosing schedule of GO, median follow-up time and outcome indicators. We also combined relevant reports from included trials to obtain richer data. The updated Cochrane RoB2 (risk of bias 2) tool was used to assess the risk of bias in the included trials. GRADE (Grading of Recommendations Assessment, Development, and Evaluations) was used to assess the quality of evidence. All data extraction and quality assessment were completed by one author, the accuracy was verified by a second author, and the disagreements were resolved by discussion with a third author.
Statistical analysis
The statistical analyses were performed using Review Manager, version 5.3. HRs with 95% confidence intervals (CIs) were calculated for OS and RFS. Odds ratios (ORs) with 95% CIs were calculated for the other outcomes. Statistical heterogeneity was examined by χ2 test and was considered significant when I2 > 40% according to the Cochrane Collaboration. A fixed-effects model was used for pooled estimation when heterogeneity was not significant. Conversely, random-effects model was used. Subgroup analyses were performed to detect the reasons for heterogeneity and evaluate the effect of different factors, such as dosing schedule of GO, cytogenetic and treatment stage, on GO-based chemotherapy for AML. Sensitivity analysis was applied by excluding individual trials in turn to assess the stability of the results. Each step was done collectively by two authors and the disagreements were resolved by discussion with a third author.
Results
Study selection results
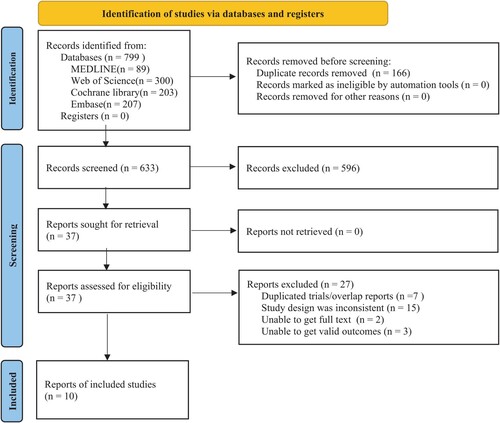
In total, 799 records related to the subject of this research were retrieved from the databases. 37 records were obtained after eliminating duplicated and ineligible records by screening titles and abstracts. Then, total 27 records were excluded due to ineligible (supplementary materials S3). We reviewed the full text and identified ten records [Citation7–16] involving 11 RCTs that met the inclusion criteria for meta-analysis. The detailed flow chart of the screening process is shown in .
Characteristics of included trials and patients
Among of the ten records, five focused on GO in the induction and post-remission phases without a second independent randomization [Citation7–10,Citation16], one performed a second independent randomization in the consolidation phase [Citation13], two studied GO in the induction phase only [Citation11,Citation12] and the remaining two involved GO in the post-remission phase [Citation14,Citation15]. All clinical trials involved in this meta-analysis were prospective randomized phase III trials, analyzing a total of 6333 patients, with 3163 patients in the GO group and 3170 patients in the no-GO group. The median follow-up time ranged from 20 to 50.9 months. Detailed characteristics of the trials and included patients are presented in .
Table 1. Characteristics of studies included in meta-analysis.
Risk of bias and quality of evidence
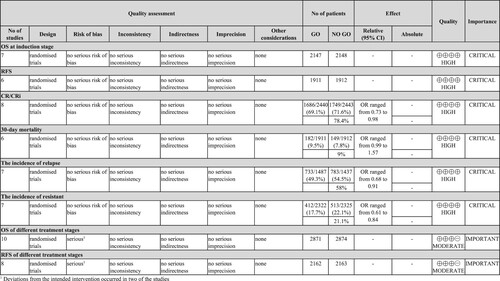
The risk of bias assessment for each outcome was performed using the cochrane ROB2 tool. Each outcome has its own risk of bias assessment figures (supplementary materials S4). All included trials were open-label randomized trials, and two of the studies [Citation14,Citation15] were classified as high risk due to large gaps in the number of patients deviating from the intended intervention. The GRADEpro procedure was applied to OS and RFS at different treatment stages and moderate quality of evidence was detected, with high quality of evidence for other outcomes. The detailed data are shown in .
Efficacy and safety at induction stage
Overall survival
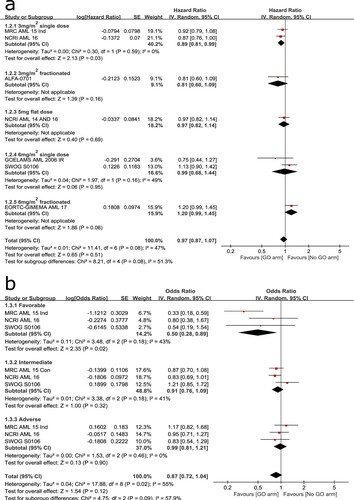
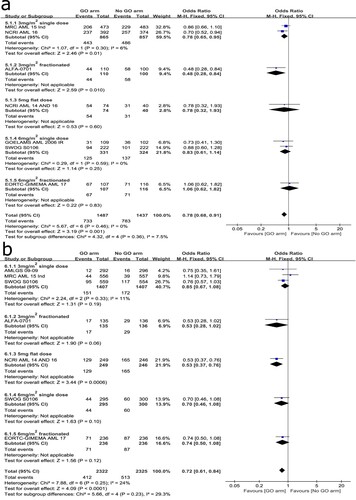
A total of seven trials were analyzed to compare OS at the induction stage. Significant heterogeneity (I2 = 47%) was detected so that it was calculated using a random-effects model. The overall result showed no statistical significance between the two arms (HR: 0.97, 95% CI: 0.87–1.07, p = 0.51). Subgroup analysis suggested that OS was significantly improved in 3 mg/m2 single-dose group (HR: 0.89, 95% CI: 0.81–0.99, p = 0.03) but not in other subgroups ((A)). Sensitivity analysis by excluding each of the trials in turn indicated the result was stable.
Three trials were involved to analyze the effect of cytogenetic on OS. A random-effects model was employed because no statistically significant heterogeneity was observed among the trials (I2 = 55%). The overall result suggested no statistical significance between the two arms (HR: 0.87, 95% CI: 0.72–1.04, p = 0.12). However, subgroup analysis indicated a significant OS benefit for patients with favorable cytogenetic (HR: 0.50, 95% CI: 0.28–0.89, p = 0.02) ((B)).
Relapse-free survival
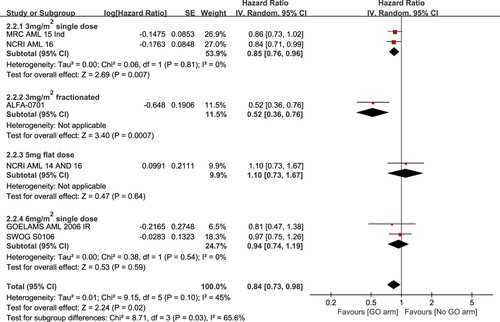
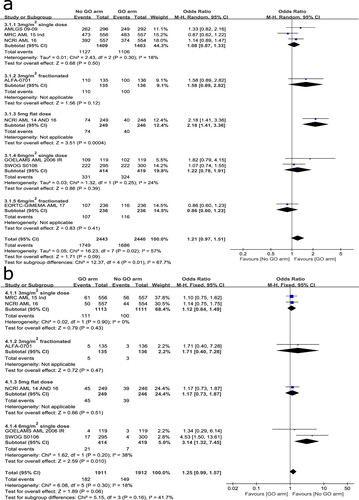
The analysis of RFS was based on six trials included. Significant heterogeneity was detected among the trials (I2 = 45%) so that it was calculated using a random-effects model. Overall analysis showed that regimens with GO added to induction chemotherapy had significantly higher RFS than those without the addition of GO (HR: 0.84, 95% CI: 0.73–0.98, p = 0.02). Subgroup analysis showed a greater RFS benefit for patients given GO at 3 mg/m2 single dose (HR: 0.85, 95% CI: 0.76–0.96, p = 0.007) and 3 mg/m2 fractionated schedule (HR: 0.52, 95% CI: 0.36–0.76, p = 0.0007) but not for those given GO at a flat of 5 mg dose (HR: 1.10, 95% CI: 0.73–1.67, p = 0.64) or 6 mg/m2 single dose (HR: 0.94, 95% CI: 0.74–1.19, p = 0.59) ().
CR with or without incomplete platelet recovery
A total of eight trials were analyzed to compare the rate of CR with or without incomplete platelet recovery at the induction stage. Significant heterogeneity was found among the trials (I2 = 57%) so that it was calculated using a random-effects model. Overall analysis showed that the addition of GO to induction chemotherapy had no significant effect on the rate of CR with or without incomplete platelet recovery (OR: 1.21, 95% CI: 0.97–1.51, p = 0.09) ((A)). Subgroup analysis suggested a significant remission rate for patients who received a flat dose of 5 mg GO plus induction chemotherapy compared with those given induction chemotherapy alone (OR: 2.18, 95% CI: 1.41–3.36, p = 0.0004). The heterogeneity may also be related to differences in baseline chemotherapy (LDAC) between trials. Sensitivity analysis by sequential exclusion of the NCRI AML14 and 16 trials [Citation12] or other trials showed good stability of the overall results.
30-day mortality
A total of six trials were involved to evaluate the 30-day mortality. No significant heterogeneity was observed among the trials (I2 = 18%) so that it was calculated using a fixed-effects model. Overall result showed no statistical differences between the two arms (OR: 1.25, 95% CI: 0.99–1.57, p = 0.06) ((B)). Subgroup analysis suggested a significant increase of 30-day mortality in 6 mg/m2 single-dose subgroup (OR: 3.14, 95% CI: 1.32–7.45, p = 0.01) but not in the other subgroups. Sensitivity analysis by excluding the SWOG S0106 trial [Citation10] or the GOELAMS AML 2006 IR trial [Citation8] showed that the overall result kept stable. Therefore, the addition of low-dose GO to induction chemotherapy could not increase 30-day mortality.
The incidence of relapse and resistant disease
A total of seven trials were involved to analyze the relapse risk. No significant heterogeneity was observed among the trials (I2 = 0%) so that a fixed-effects model was applied. Overall analysis indicated that adding GO to induction chemotherapy led to a significant decrease of relapse risk (OR: 0.78, 95% CI: 0.68–0.91, p = 0.001). Subgroup analysis showed that the greater benefit was seen in the lower dose subgroups, especially 3 mg/m2 fractionated schedule (OR: 0.48, 95% CI: 0.28–0.84, p = 0.01). Sensitivity analysis suggested that the overall result was relatively stable ((A)).
The analysis of the incidence of resistant disease was based on seven trials. No significant heterogeneity existed among the trials (I2 = 24%) so that a fixed-effect model was applied. It is clear that adding GO to induction chemotherapy was associated with a significant decrease of resistant disease (OR: 0.72, 95% CI: 0.61–0.84, P < 0.0001) ((B)). Subgroup analysis showed a significant benefit for patients given a flat dose of 5 mg GO plus induction chemotherapy compared with those given induction chemotherapy alone (OR: 0.53, 95% CI: 0.37–0.76, p = 0.0006). As the different baseline chemotherapy of the NCRI AML14 and 16 trial [Citation12], sensitivity analysis by excluding each of the trials in turn was conducted and suggested that the overall result was relatively stable.
Efficacy at different treatment stage
Ten trials included were involved to evaluate the OS of different treatment stages. It was calculated using a fixed-effects model because no significant heterogeneity was found among the trials (I2 = 34%). Overall result suggested no statistical difference between the two arms (HR: 0.98, 95% CI: 0.92–1.05, p = 0.53). However, subgroup analysis showed statistical significance in the induction stage group (HR: 0.91, 95% CI: 0.84–1.00, p = 0.04) but not in the other subgroups ((A)). Thus, GO-based chemotherapy given at the induction stage significantly increased OS, while given at thepost-remission stage had no significant effect on OS.
Figure 7. Forest plots for OS grouped by treatment stage (A) and RFS grouped by treatment stage (B).
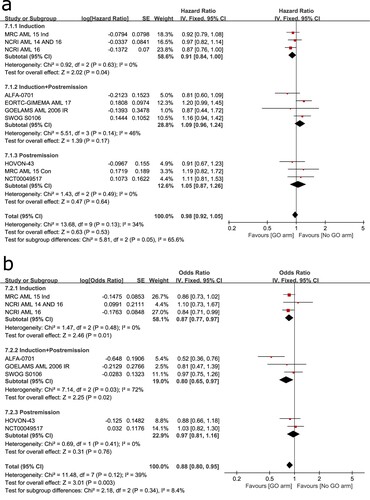
The analysis of RFS was based on eight trials included. No significant heterogeneity was observed among the trials (I2 = 39%) so that it was calculated using a fixed-effects model. Overall result showed a significant benefit for RFS in GO arm (HR: 0.88, 95% CI: 0.80–0.95, p = 0.003) ((B)). Subgroup analysis suggested that adding GO to induction chemotherapy (HR: 0.87, 95% CI: 0.77–0.97, p = 0.01) or both induction and post-remission chemotherapy (HR: 0.80, 95% CI: 0.65–0.97, p = 0.02) could significantly improve RFS but only adding to post-remission chemotherapy had no significant effect on RFS (HR: 0.97, 95% CI: 0.81–1.16, p = 0.76).
Discussion
Advances in the molecular biology of AML have facilitated the clinical development of new molecularly targeted therapies. As the first antibody–drug conjugate approved by FDA for AML, GO has undergone withdrawal and re-approval. In recent years, there are many researches aimed at this controversy so that it is necessary to integrate and re-evaluate the existing researches.
This meta-analysis presented that adding low-dose GO to conventional chemotherapy not only was associated with fewer early mortality than high-dose dose GO but also significantly improved OS and RFS, decreased the incidence of relapse and resistant disease. In particular, 3 mg/m2 fractionated schedule of GO had a greater benefit for RFS and produced a more notable reduction in relapse compared with 3 mg/m2 single-dose schedule. Thus 3 mg/m2 fractionated schedule was considered to be an optimal option in GO-based chemotherapy which was also supported by an European Medicines Agency review [Citation17]. Apart from the dosing schedule of GO, cytogenetic characteristics and treatment stage are also influencing factors for the efficacy of GO-based chemotherapy. Our meta-analysis showed that only patients with favorable cytogenetic and given GO at induction stage or both induction and post-remission stage had significant OS benefits. Because remission rate and early mortality were unaffected, the improvement in survival seemed to depend on the reduction of relapse and resistant disease, revealing that patients receiving GO-based chemotherapy had achieved more durable remission. There is also evidence that the proportion of patients with the minimal residual disease was reduced by the GO-based chemotherapy to support this conclusion [Citation18].
Results from our meta-analysis are quite reliable as it was based on several multicenter prospective randomized phase III trials with high quality and we referenced relevant records and reports to obtain up-to-date and comprehensive data. Our meta-analysis also has unavoidable limitations. It might produce some variations for treatment effectiveness, because the analysis was based on the summary data rather than individual patient data. Thus, we conducted subgroup analysis to make up for the deficiency.
A recent study showed that high-dose DA therapy eliminated human AML CD33+ CD45+ cells in peripheral blood but not in bone marrow, but the addition of GO to DA therapy almost completely eliminated human AML CD33+ CD45+ cells in peripheral blood and bone marrow. Hence, this provides a strong rationale for the use of GO as an adjuvant agent against CD33+ leukemia-initiating cells to augment existing AML chemotherapy [Citation19]. A real-life study in Lyon-University Hospital confirmed the efficacy and safety of adding GO to conventional chemotherapy in real-life setting and suggested that there was no significant difference in response and response duration between patients given GO after relapse and patients given GO as front-line therapy [Citation20]. Therefore, GO-based chemotherapy could be used as a choice of salvage treatment and a bridge to allogeneic transplant [Citation20,Citation21]. Although GO is usually used in combination with conventional chemotherapy, the addition of GO to lower intensity treatments, such as hypomethylating agents, can provide considerable efficacy for patients who are unfit for standard chemotherapy [Citation22]. The combination of GO with hypomethylating agents or other unique therapies is worth exploring.
Conclusion
In summary, adding low-dose and fractionated schedule of GO to conventional chemotherapy has a favorable benefit/risk ratio for newly diagnosed de novo or secondary adult AML. It is foreseeable that the clinical application and the prospect of GO may continue to expand and develop with more related researches being performed. In order to improve efficacy and prevent or decrease toxicity, the selection of more appropriate combinations and more suitable patients according to molecular setting, biomarker of chemotherapy and GO sensitivity still needs to be determined in a larger cohort.
Funding statement
No external funding was used in the preparation of this article.
Availability of data and materials
The data sets used and/or analysed during the present study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The need for ethics approval by an institutional board review was waived as this study does not directly involve human subjects.
Supplemental Material
Download EPS Image (7.6 MB)Supplemental Material
Download MS Word (43.7 KB)Supplemental Material
Download MS Word (18.5 KB)Supplemental Material
Download MS Word (29.9 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Shallis RM, Wang R, Davidoff A, et al. Epidemiology of acute myeloid leukemia: recent progress and enduring challenges. Blood Rev. 2019;36:70–87.
- McNerney ME, Godley LA, Le Beau MM. Therapy-related myeloid neoplasms: when genetics and environment collide. Nat Rev Cancer. 2017;17(9):513–527.
- Short NJ, Rytting ME, Cortes JE. Acute myeloid leukaemia. Lancet. 2018;392(10147):593–606.
- Appelbaum FR, Bernstein ID. Gemtuzumab ozogamicin for acute myeloid leukemia. Blood. 2017;130(22):2373–2376.
- Talati C, Sweet K. Recently approved therapies in acute myeloid leukemia: A complex treatment landscape. Leuk Res. 2018;73:58–66.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. 2021;372:n71.
- Lambert J, Pautas C, Terré C, et al. Gemtuzumab ozogamicin for de novo acute myeloid leukemia: final efficacy and safety updates from the open-label, phase III ALFA-0701 trial. Haematologica. 2019;104(1):113–119.
- Delaunay J, Recher C, Pigneux A, et al. Addition of gemtuzumab ozogamycin to chemotherapy improves event-free survival but not overall survival of AML patients with intermediate cytogenetics not eligible for allogeneic transplantation. Results of the GOELAMS AML 2006 IR study. Blood. 2011;118(21):79–79.
- Amadori S, Suciu S, Stasi R, et al. Sequential combination of gemtuzumab ozogamicin and standard chemotherapy in older patients with newly diagnosed acute myeloid leukemia: results of a randomized phase III trial by the EORTC and GIMEMA consortium (AML-17). J Clin Oncol. 2013;31(35):4424–4430.
- Petersdorf SH, Kopecky KJ, Slovak M, et al. A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood. 2013;121(24):4854–4860.
- Burnett AK, Russell NH, Hills RK, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy improves survival in older patients with acute myeloid leukemia. J Clin Oncol. 2012;30(32):3924–3931.
- Burnett AK, Hills RK, Hunter AE, et al. The addition of gemtuzumab ozogamicin to low-dose Ara-C improves remission rate but does not significantly prolong survival in older patients with acute myeloid leukaemia: results from the LRF AML14 and NCRI AML16 pick-a-winner comparison. Leukemia. 2013;27(1):75–81.
- Burnett AK, Hills RK, Milligan D, et al. Identification of patients with acute myeloblastic leukemia who benefit from the addition of gemtuzumab ozogamicin: results of the MRC AML15 trial. J Clin Oncol. 2011;29(4):369–377.
- Fernandez HF, Sun Z, Litzow MR, et al. Autologous transplantation gives encouraging results for young adults with favorable-risk acute myeloid leukemia, but is not improved with gemtuzumab ozogamicin. Blood. 2011;117(20):5306–5313.
- Löwenberg B, Beck J, Graux C, et al. Gemtuzumab ozogamicin as postremission treatment in AML at 60 years of age or more: results of a multicenter phase 3 study. Blood. 2010;115(13):2586–2591.
- Schlenk RF, Paschka P, Krzykalla J, et al. Gemtuzumab ozogamicin in NPM1-mutated acute myeloid leukemia: early results from the prospective randomized AMLSG 09-09 phase III study. J Clin Oncol. 2020;38(6):623–632.
- Ali S, Dunmore HM, Karres D, et al. The EMA review of Mylotarg (gemtuzumab ozogamicin) for the treatment of acute myeloid leukemia. Oncologist. 2019;24(5):e171–e179.
- Lambert J, Lambert J, Nibourel O, et al. Minimal residual disease assessed by WT1 expression and NPM1 mutations specific RQ-PCR assays identifies patients with distinct outcomes in the ALFA 0701 trial and is decreased by treatment with gemtuzumab ozogamicin. Blood. 2012;120(21):659.
- Zhang CC, Yan Z, Pascual B, et al. Gemtuzumab ozogamicin (GO) inclusion to induction chemotherapy eliminates leukemic initiating cells and significantly improves survival in mouse models of acute myeloid leukemia. Neoplasia. 2018;20(1):1–11.
- Laurino M, Loron S, Larcher MV, et al. Lyon-University hospital experience with gemtuzumab ozogamicin therapy in acute myeloid leukemia: a ‘real-life’ study. Mediterr J Hematol Infect Dis. 2020;12(1):e2020020.
- Debureaux PE, Labopin M, Mamez AC, et al. Fractionated gemtuzumab ozogamicin in association with high dose chemotherapy: a bridge to allogeneic stem cell transplantation in refractory and relapsed acute myeloid leukemia. Bone Marrow Transplant. 2020;55(2):452–460.
- Daver N, Kantarjian H, Ravandi F, et al. A phase II study of decitabine and gemtuzumab ozogamicin in newly diagnosed and relapsed acute myeloid leukemia and high-risk myelodysplastic syndrome. Leukemia. 2016;30(2):268–273.