ABSTRACT
Objectives
The fact that a lower warfarin maintenance dose is required by Asian populations is well-known. Currently, the American College of Chest Physicians recommends commencing warfarin at a dose between 5 and 10 mg for venous thromboembolism (VTE). However, the optimal initiating dose in Asians is unknown. This study aimed to evaluate the efficacy of a 3 mg versus a 5 mg of warfarin initiating dose and a corresponding nomogram in patients with VTE.
Methods
Eligible patients were randomized to receive 3 mg or 5 mg per day warfarin for the first 2 days of treatment. The subsequent dose was adjusted according to the warfarin nomogram. The primary outcome was the number of patients who achieved an INR 2.0–3.0 within 8 days.
Results
Fifty-six patients were enrolled. There was no significant difference in baseline characteristics between the groups. Seventeen (60.7%) patients in the 3-mg group and 22 (78.6%) patients in the 5-mg group achieved a therapeutic INR within 8 days (p = 0.146). However, there were significantly more patients in the 5-mg group who achieved the target INR on day 5 (53.6% vs 25.0%, p = 0.029). Furthermore, VKORC1-1639G > A was associated with an increased likelihood to achieve the target INR within 5 days (OR 3.81, 95%CI 1.19–12.16, p = 0.021).
Conclusions
The efficacy of a 3 mg warfarin starting dose with subsequent dose adjustment was similar to that of 5 mg on day 8 after warfarin initiation. However, a 5 mg initiating dose resulted in more patients who achieved therapeutic INR on day 5.
Introduction
Despite the introduction of direct oral anticoagulants as the alternative initial treatment for venous thromboembolic disease, warfarin remains the frequently prescribed anticoagulant in the vast majority of the world, particularly in the area where the accessibility to direct oral anticoagulants is limited. Furthermore, warfarin is the mainstay of oral anticoagulant therapy in patients with severe renal impairment. In addition to venous thromboembolism (VTE), warfarin is used to prevent systemic thromboembolic complication in patients with atrial fibrillation and mechanical heart valves. For VTE treatment, warfarin is initiated with concomitant heparin administration until a therapeutic prothrombin time (PT)-international normalized ratio (INR) is achieved, and then heparin is discontinued. Presently, an initiating dose between 5 and 10 mg of warfarin for the first 1–2 days is recommended by the American College of Chest Physicians (ACCP) in patients commencing oral anticoagulation based on prior studies comparing the efficacy of a 5 mg versus 10 mg warfarin starting dose in western populations [Citation1–4].
Nonetheless, a diversity of anticoagulant responses to warfarin has been observed among various populations. Several factors, including ethnicity and genetic variants of cytochrome P4502C9 (CYP2C9) and vitamin K epoxide reductase complex subunit 1(VKORC1) genes, have been demonstrated to play a role in the interethnic disparity of warfarin response and maintenance dose requirements [Citation1, Citation5–7]. Prior studies demonstrated that Asian patients required a significantly lower maintenance dose of warfarin than other ethnicities [Citation8–10]. Furthermore, the prevalence of a warfarin-sensitive haplotype of the VKORC1 gene was more frequently found in Asian populations [Citation11–14]. The −1639G > A polymorphism, a common variant of VKORC1 (c.−1639G > A, rs9923231), is significantly associated with warfarin sensitivity and mainly explains the difference in average dose requirements between different ancestral populations [Citation15]. The patients who are −1639G/A heterozygotes or −1639A/A homozygotes will require lower warfarin doses than those with −1639G/G homozygotes [Citation15]. Consequently, the Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline recommends calculating warfarin maintenance dosing based on published pharmacogenetic algorithms incorporating the genotypes of VKORC1 and CYP2C9 [Citation15].
Currently, the optimal starting dose in Asians has not been elucidated. In addition, the results from previous studies conducted in western populations may not be entirely applicable to patients in Asian countries. Moreover, a lower initiating dose (3 mg) is commonly prescribed in Thailand. Therefore, we conducted a study to investigate the efficacy of a lower (3 mg) initiating dose compared with the current standard (5 mg) initiating dose with subsequent dose adjustment according to a corresponding dosing schedule in patients with VTE. (Thai Clinical Trial Registry TCTR20180328002)
Methods
Adult patients diagnosed with venous thromboembolic disease at Siriraj Hospital were screened within 48 hours after the diagnosis of VTE for eligibility in the study. The patients were eligible for the study if they were 18 years of age or older, were diagnosed with symptomatic proximal deep vein thrombosis (DVT) of the extremity or DVT at other sites (for example, cerebral venous sinus thrombosis, and/or pulmonary embolism (PE)) and planned to receive warfarin with a target international normalized ratio (INR) of 2.0–3.0. The exclusion criteria were an absolute contraindication to anticoagulant therapy (e.g. active bleeding), high risk of bleeding (e.g. recent eye, brain or spinal cord surgery within 3 months), history of concurrent congestive heart failure, history of liver cirrhosis (Child Pugh class B or C), concomitant use of aspirin, prolonged baseline prothrombin time (PT) and/or activated partial thromboplastin time (aPTT) above the upper normal limits, platelet counts of less than 50×109/L, serum albumin less than 2.0 g/dL, pregnancy, and patients who were unable to perform oral feeding. The study protocol was reviewed and approved by the Siriraj Institutional Review Board. Written informed consent was obtained from each participant in accordance with the Declaration of Helsinki prior to the enrollment in the study.
Study protocol
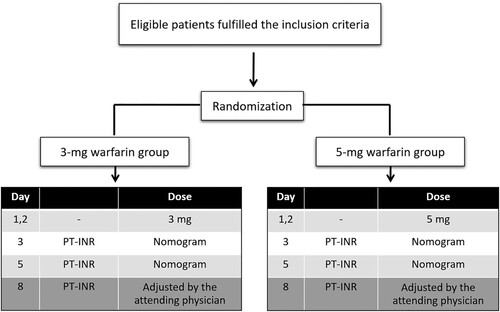
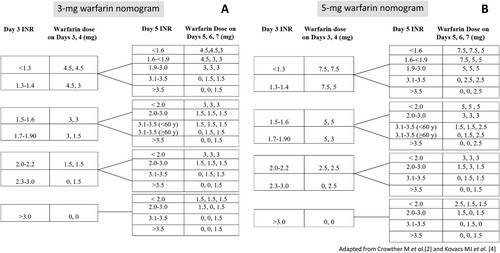
The eligible patients were randomized by blocked randomization with block size of 4 stratifying for age to receive a warfarin initiating dose of 3 mg or 5 mg per day for the first 2 consecutive days (day 1 and day 2) ( and ). The first day of warfarin treatment was designated day 1. Subsequent warfarin dosing was adjusted according to the INR and the warfarin dosing protocol (). The choice of concomitant heparin treatment was made according to the attending physician’s discretion. Blood samples were collected from venipuncture and were sent for baseline PT, INR, aPTT, complete blood count and a liver function test prior to the initiation of warfarin anticoagulant therapy. In addition, blood for INR testing was collected on days 3, 5 and 8 of warfarin treatment. Furthermore, blood samples were drawn and sent for the evaluation of the single nucleotide polymorphisms (SNPs) of the CYP2C9 and VKORC1 genes to determine the prevalence of the genetic variables and the impact on the warfarin response among the participants. The outcomes assessors of the INR and the SNPs of CYP2C9 and VKORC1 genes were blinded to the treatment allocation. The enrolled inpatients or outpatients were followed on days 3, 5, and 8 of warfarin treatment.
Figure 3. Warfarin dosing protocol for patients receiving a 3- mg initiating dose (A) or a 5- mg initiating dose (B).
The primary outcome was the number of patients who achieved the target INR of 2.0–3.0 within 8 days after the initiation of warfarin anticoagulation. Secondary outcomes were the incidence of early bleeding complications within 8 days of warfarin anticoagulant therapy, the incidence of excessive anticoagulation (INR > 5.0) within 8 days of warfarin anticoagulant therapy and the genotype characteristics of theCYP2C9 and VKORC1 genes.
Laboratory measurements
PT-INR
Blood samples were collected in sodium citrate (0.109 M, 3.2%) tubes and were centrifuged within 120 min at 300 g for 15 min at 4 °C. The supernatant was collected. The PT-INR was determined by an automated coagulation analyzer (Sysmex CA1500 and all reagents, Siemens), according to the manufacturer’s protocols.
CYP2C9 and VKORC1 genotyping
Blood samples were collected in EDTA tubes. Whole blood DNA was extracted by a salting-out method. DNA fragments of interest were amplified using a polymerase chain reaction (PCR). DNA fragments containing polymorphic regions of CYP2C9, namely, CYP2C9*2 (c.430C > T, p.R144C, rs1799853) and CYP2C9*3 (c.1075A > C, p.I359 L, rs1057910), were amplified using forward and reverse primers according to Abbas et al. [Citation16] and Xie et al. [Citation17], respectively. Newly designed primers were used for amplification of the regions containing VKORC1_−1639 (c.−1639G > A, G3673A, rs9923231), VKORC1_Exon2 (c.173 + 1000C > T/C6484 T/1173C > T, rs9934438) and VKORC1_3'UTR (c.492 + 134G > A/G9041A/3730G > A, rs7294) [Citation18] as shown in .
Table 1. Primer sequences for molecular genotyping of CYP2C9 and VKORC1alleles.
All of the PCRs were performed on the Applied Biosystems Veriti™ Thermal Cycler (Applied Biosystems, CA, USA) in a total volume of 12.5 μL containing 50 ng of genomic DNA,1.0 mM MgCl2, 200 μM dNTP, 0.4 μM of each primer, 0.25U IMMOLASE™ DNA Polymerase (Bioline USA Inc., USA) and 1X ImmoBuffer (Bioline). The cycling conditions included denaturation at 95 °C for 10 min, followed by 35 cycles at 95 °C for 30 s, 56 °C (for VKORC1) or 58 °C (for CYP2C9) for 30 s, 72 °C for 30 s, and a final extension at 72 °C for 7 min. The purification of PCR products for sequencing included incubation of the PCR products with USB ExoSAP-IT® For PCR Product Cleanup (Affymetrix, Inc., USA) at 37 °C for 30 min, followed by heat inactivation at 80 °C for 15 min. The PCR products were sequenced using an ABI Prism® BigDye Terminator Cycle Sequencing Kit, version 3.1 (Applied Biosystems, CA, USA) on an Applied Biosystems 3130xl Genetic Analyzer.
Statistical analysis
The sample size was calculated with a type I error of 5% and the power of test of 80% based on previous data of the comparison of proportion of patients who achieved therapeutic INR with a different dose of warfarin [Citation2, Citation19] and the hypothesis that a 5-mg initiating dose may result in more number of patients who achieve the target INR (2.0–3.0) within 8 days. Therefore, a total sample size of 56 with 28 patients per group was obtained. Subject characteristics were described using descriptive statistics, including means, standard deviation, median, minimum and maximum, frequencies and percentage. The normality of the distribution of the variables was examined with the Kolmogorov–Smirnov or the Shapiro–Wilk tests. For continuous variables, Student’s t-tests or Mann–Whitney U test were used to compare groups. For dichotomous variables, Chi-square or Fisher’s exact tests were used for comparisons. For all tests performed, a two-tailed p-value <0.05 was considered to have statistical significance. Statistical analyses were performed with SPSS 18.0 (SPSS, Chicago, IL, USA).
Results
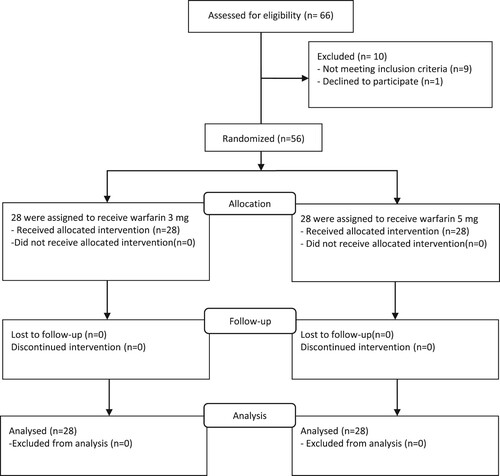
Fifty-six patients were enrolled in the study. The patients were randomized to receive a 3 mg or 5 mg warfarin initiating dose with a total of 28 patients in each group. The mean age was 46.5 ± 17.3 and 47.3 ± 15.6 years in the 3-mg and 5-mg groups, respectively. DVT was the most common type of VTE in both groups. There were no statistically significant differences of baseline demographic and clinical characteristics between the groups, including mean age, gender, body weight, type of VTE, comorbidities, baseline laboratory values (including hemoglobin and hematocrit (Hct), platelet count, serum albumin, serum total and direct bilirubin, serum aspartate aminotransferase, serum alanine aminotransferase, PT, INR, aPTT), concomitant drug use or type of heparin treatment ().
Table 2. Baseline characteristics.
Primary outcome
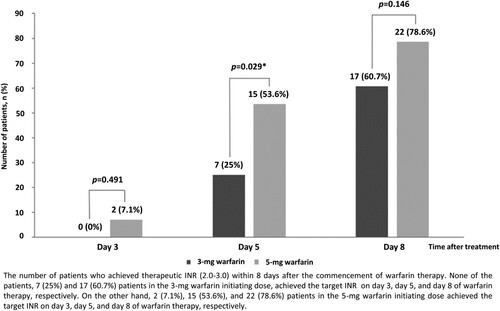
Seventeen (60.7%) and 22 (78.6%) patients in the 3-mg and 5-mg groups, respectively, achieved a therapeutic INR (2.0–3.0) within 8 days after the commencement of warfarin therapy (p = 0.146). The number of patients who attained the target INR within 5 days of warfarin treatment was significantly higher in the 5-mg group than in the 3-mg group (53.6% vs. 25.0%, p = 0.029) ().
Secondary outcome
One bleeding occurred in a patient diagnosed with left popliteal vein thrombosis in the 3-mg group on day 5 of warfarin and enoxaparin (1 mg/kg every 12 hours) treatment. The patient returned for a scheduled follow-up on day 5 when the physical examination revealed ecchymoses on the left leg. The INR was 2.17. She denied preceding trauma but complained of worsening pain in the left leg since she underwent diagnostic ultrasonography four days ago. The hemoglobin decreased from 11.5 g/dl at baseline to 10.4 g/dl. The sonographic findings of the left leg were suggestive of intramuscular hematoma at upper to mid-calf. Warfarin and enoxaparin were temporarily discontinued. Intravenous vitamin K, fresh frozen plasma, and 1 unit of the packed red cell was given to the patient with subsequent improvement of symptoms.
In addition, one patient in the 3-mg group had excessive anticoagulation (INR 5.24) without bleeding on day 5 of warfarin treatment. The patient had concomitant use of warfarin and over-the-counter non-steroidal anti-inflammatory drugs (NSAIDs). His INR subsequently decreased to the therapeutic level after discontinuation of the NSAIDs ().
Table 3. Secondary outcome.
Characteristics of the CYP2C9 and VKORC1 genes
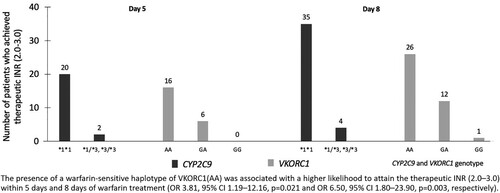
Molecular analysis of the SNPs of the CYP2C9 gene in patients in both groups revealed wild type alleles (*1/*1) and variant alleles (*1/*3 and *3/*3) in 92.9% and 7.1% of patients, respectively. However, the *1/*2 variant of the CYP2C9 gene was not detected in this study. The patterns of the SNPs of the CYP2C9 gene were similar in both groups, with no statistically significant difference between the groups (). Genotyping of the VKORC1 gene demonstrated the warfarin-sensitive haplotype (AA), the heterozygous variants (GA) and the homozygous wild-type (GG) in 55.4%, 41.1% and 3.6% of the patients, respectively. Similar to the results of the CYP2C9 genotype, the distribution of the VKORC1 genotype was not significantly different between the groups (). Significantly more patients carrying a warfarin-sensitive haplotype of the VKORC1 gene (AA) achieved the target INR than those who carried a VKORC1 GA haplotype. Furthermore, the presence of VKORC1AA haplotype was associated with a higher likelihood to attain the therapeutic INR (2.0–3.0) within 5 days (OR 3.81, 95%CI 1.19–12.16, p = 0.021) and within 8 days of warfarin treatment (OR 6.50, 95% CI 1.80–23.90, p = 0.003) (). Nevertheless, the number of patients who possessed a GG haplotype of VKORC1 was too small to statistically compare between the groups.
Discussion
Currently, the initiating dose of warfarin recommended by the ACCP is between 5 and 10 mg for the first 1–2 days after commencement of oral anticoagulant treatment; the chosen dose for patients diagnosed with VTE is based on previous randomized controlled studies [Citation1–4]. The efficacy of a 5 mg initiating dose was demonstrated by Crowther et al. in which 53 patients requiring long-term anticoagulant therapy were randomized to receive 5 mg per day or 10 mg per day warfarin for the first 2 days of the initial oral anticoagulation. The subsequent warfarin dose was adjusted according to the INR result and the standard dosing nomogram [Citation2]. The primary outcome was the proportion of patients who achieved an INR 2.0–3.0 on days 3–4 or days 4–5 of the treatment [Citation2]. In that study, a significantly larger percentage of patients who received a 5 mg initiating dose attained the primary endpoint than those who received a 10 mg initiating dose (66% vs. 24%, p < 0.003) [Citation2]. In contrast, Kovacs et al. demonstrated the superior efficacy of a 10 mg initiating dose of warfarin in another randomized controlled study that included 201 patients diagnosed with DVT only [Citation4]. All patients were treated on an outpatient basis and were randomized to receive warfarin at a 10 mg or 5 mg loading dose on two successive days. The primary outcome was the time required to achieve the therapeutic INR of greater than 1.9. Interestingly, the time to achieve the therapeutic INR was significantly shorter in the 10 mg initiating group (4.2 vs. 5.6 days, p < 0.001) [Citation4]. Consequently, warfarin at a starting dose between 5 and 10 mg per day given on the first 1 or 2 days of initial oral anticoagulant therapy was recommended by the ACCP [Citation1] and is generally accepted in current medical practice.
However, several factors play a role in warfarin response, resulting in the variation of warfarin dose requirements. Prior studies have shown that Asian patients require a lower maintenance dose of warfarin when compared with those from other ethnic origins [Citation5–7]. In addition, the varied patterns of CYP2C9 and VKORC1 polymorphisms among different populations contribute to the interethnic variability of warfarin dose requirement [Citation8–10]. Hence, the results from previous studies conducted in western populations may not be entirely applicable to patients from other ethnicities, particularly Asian populations. Presently, the optimal warfarin initiating dose in Asian patients is unknown. Nevertheless, a lower warfarin starting dose of 3 mg is widely prescribed in Thailand with uncertain comparison of the efficacy with the previously recommended 5 and 10 mg starting doses.
A prior retrospective analysis of the efficacy of a lower (3 mg) warfarin initiating dose in 164 patients who required long-term oral anticoagulant therapy at a tertiary care hospital revealed that only 47 (29%) patients achieved the target INR of 2.0–3.0 between day 3 and day 5 of warfarin treatment [Citation19]. When compared with the results previously reported by Crowther et al. [Citation2], the rate of therapeutic anticoagulant achievement with a 3 mg initiating dose appeared to be rather less efficacious [Citation19]. Nonetheless, due to the retrospective nature of the study along with several limitations, including incomplete chart documentation, non-uniform warfarin dose adjustment and a variable frequency of INR testing, the efficacy of the 3 mg starting dose might not have been accurately estimated [Citation19]. Thus, the results of the current randomized study will be useful to determine the valid efficacy of a lower warfarin starting dose.
To the best of our knowledge, this is the first randomized controlled study aimed to compare between a 3 and 5 mg warfarin initiating dose, which carried out in Southeast Asian population. The current findings demonstrated a comparable efficacy between both warfarin dosages determined by the number of patients who achieved the target INR within 8 days of the initiation of warfarin therapy. However, there was a statistically significant difference in the number of patients who attained the therapeutic INR within 5 days of warfarin treatment, with considerably more patients in the 5-mg group than those in the 3-mg group. Thus, a 5 mg initiating dose appeared to result in a more rapid achievement of therapeutic INR. Nonetheless, the anticoagulation effect observed in both groups was a result of not only the initial dose of warfarin but also the following dose adjustment based on the warfarin dosing protocol in each group.
Despite the more rapid accomplishment of the target INR in patients receiving a 5 mg starting dose, the effect of the initial dose and subsequent dose adjustment in this group seemed to diminish on day 8 when the efficacy of the 3 mg initiating dose was similar to that observed in the 5-mg group. This finding might be because more than half of the patients in the 5-mg group had already reached a therapeutic INR within 5 days of warfarin treatment, and hence, an additionally smaller proportion of patients would be left to reach the primary end point on day 8. Accordingly, a larger number of patients in the 3-mg group who did not reach the target INR within 5 days of treatment would subsequently attain therapeutic anticoagulation on day 8. As a result, a similar number of patients in both groups then achieved the primary outcome on day 8 with a non-significant difference in the efficacy between the groups.
When compared with a previous study utilizing a 5 mg loading dose of warfarin for the first 2 days of treatment [Citation2], the proportion of patients who received a 5 mg starting dose and achieved the target INR (2.0–3.0) within 5 days of warfarin treatment in this study was rather comparable to that in the previously reported results [Citation2] (53.6% in this study vs. 66%). However, the warfarin dosing nomograms used in prior studies required a daily INR testing after the first two 5-mg initiating doses [Citation2–4], unlike the warfarin dosing protocol in this study which required the INR measurement on day 3 and day 5 of warfarin treatment. Moreover, the warfarin doses on days 5, 6, and 7 in our study were adjusted according to the corresponding dosing protocol based on the initial INR response on day 3 of warfarin therapy in a similar fashion to the previous 10-mg warfarin dosing nomogram, but with less intensity of the dose-escalation [Citation4].
In addition, the efficacy of the 3 mg loading dose as determined by the number of patients who attained the target INR within 5 days of the treatment in the current study was similar to previous findings in a retrospective study (25% in this study vs. 29% [Citation19]), suggesting that a lower starting dose may be suboptimal to induce therapeutic anticoagulation within 5 days.
There was one bleeding event that was not associated with supratherapeutic INR and one incidence of excessive anticoagulation without bleeding in a patient with concomitant use of NSAIDs and warfarin. Thus, both 3 and 5 mg warfarin starting doses appeared to be safe for the commencement of oral anticoagulation.
Generally, the CYP2C9 *1/*1 and warfarin-sensitive haplotype of VKORC1 (AA) are found more frequently among those patients of Asian ethnicity [Citation20]. In addition, the prevalence of CYP2C9 and the VKORC1 SNPs formerly reported in Thai patients and blood donors are consistent with the current results with a previously reported prevalence of CYP2C9 *1/*1, CYP2C9 *1/*3, VKORC1(AA), VKORC1 (GA), and VKORC1(GG) of 88.7%−95.0%, 5.0%−11.3%, 49.5%−63.6%, 33%−46.4% and 2.1%−5.7%, respectively [Citation21–24]. In this study, patients who carried a warfarin-sensitive haplotype of the VKORC1 gene (AA) were significantly more likely to achieve the target INR earlier during the treatment than those who had an intermediately sensitive haplotype (GA). However, the CYP2C9 gene variant did not affect the number of patients who reached the primary outcome. Thus, the VKORC1 SNPs rather than the CYP2C9 SNPs are probably the principal genetic factors modifying the anticoagulant response to warfarin in Thai patients.
Additionally, because there was no statistically significant difference in the prevalence of the CYP2C9 and VKORC1gene variants between patients in the 3-mg and 5-mg initiating dose groups, the relatively superior efficacy of the 5 mg starting dose on the induction of therapeutic anticoagulation within 5 days of treatment was most likely a result of the effect of the initiating dose and the subsequent dose adjustment according to the corresponding dosing schedule.
Furthermore, because we intended to explore the genetic landscape of the enrolled participants and determine the correlation between the CYP2C9 and VKORC1 genotypes and the clinical outcome, therefore, we did not use the genotypes to guide the warfarin dosing but rather to investigate whether the genetic variables would be the independent factors affecting the outcome of the study.
There were limitations to the current study. First, the sample size was small. Second, the majority of participants were middle-aged patients, and few elderly patients were enrolled. Therefore, the results might not be entirely applicable to elderly populations. Third, a significant difference of the CYP2C9 and VKORC1 SNPs between the groups might not be well-demonstrated because of the limited sample size. Finally, because the subsequent dose of warfarin was adjusted according to the INR and the warfarin dosing protocol, the efficacy of the initiating dose of warfarin might differ from that found in our study when the alternative warfarin dosing schedules are utilized. Also, the warfarin dosing protocol used in this study has not been previously validated. Thus, further study with a larger sample size would be beneficial to verify the current findings.
Conclusion
The efficacy of a 3 mg warfarin initiating dose was comparable to that of a 5 mg initiating dose on day 8 after the commencement of warfarin anticoagulation. However, the 5 mg initiating dose and subsequent dosing adjustment according to the warfarin dosing protocol resulted in a significantly higher number of patients who achieved a therapeutic INR within 5 days without an increased risk of bleeding. Thus, a 5 mg initiating dose with a corresponding dosing nomogram may be the optimal starting dose in young to middle-aged Thai patients with VTE.
Declarations
Code availability
Not applicable.
Ethics approval and consent to participate
This study was approved by the Ethics Committee for Research in Human Subjects at the Siriraj Institutional Review Board. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (Siriraj Institutional Review Board, Faculty of Medicine Siriraj Hospital, Mahidol University, Thailand) and with the Helsinki Declaration of 1975, as revised in 2008. Informed consent was obtained from all patients for being included in the study.
Consent for publication
This manuscript has been approved by all authors. A copy of the consent document is available for review from the Editor-in-Chief of Hematology.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
All authors contributed to the study's conception and design. WR, KM, and BS collected the data. YL, WT, and CL performed molecular genetic studies and approved the results. BS and WR drafted the manuscript. Bundarika Suwanawiboon revised the final manuscript. All authors read and approved the final manuscript.
Acknowledgments
This research was supported by the Siriraj Research Fund of Faculty of Medicine Siriraj Hospital, Mahidol University. We would like to thank Ms. Kemajira Karaketklang for the statistical analyses.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ansell J, Hirsh J, Hylek E, et al. Pharmacology and management of the vitamin K antagonists: American college of chest physicians evidence-based clinical practice guidelines (8th Edition). Chest. 2008;133:160S–198S. doi:10.1378/chest.08-0670.
- Crowther MA, Ginsberg JB, Kearon C, et al. A randomized trial comparing 5-mg and 10-mg warfarin loading doses. Arch Intern Med. 1999;159:46–48. doi:10.1001/archinte.159.1.46.
- Harrison L, Johnston M, Massicotte MP, et al. Comparison of 5-mg and 10-mg loading doses in initiation of warfarin therapy. Ann Intern Med. 1997;126:133–136. doi:10.7326/0003-4819-126-2-199701150-00006.
- Kovacs MJ, Rodger M, Anderson DR, et al. Comparison of 10-mg and 5-mg warfarin initiation nomograms together with low-molecular-weight heparin for outpatient treatment of acute venous thromboembolism. A randomized, double-blind, controlled trial. Ann Intern Med. 2003;138:714–719. doi:10.7326/0003-4819-138-9-200305060-00007.
- Geisen C, Watzka M, Sittinger K, et al. VKORC1 haplotypes and their impact on the inter-individual and inter-ethnical variability of oral anticoagulation. Thromb Haemost. 2005;94:773–779.
- D'Andrea G, D'Ambrosio RL, Di Perna P, et al. A polymorphism in the VKORC1 gene is associated with an interindividual variability in the dose-anticoagulant effect of warfarin. Blood. 2005;105:645–649.
- Rieder MJ, Reiner AP, Gage BF, et al. Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N Engl J Med. 2005;352:2285–2293. doi:10.1056/NEJMoa044503.
- Herman D, Peternel P, Stegnar M, et al. The influence of sequence variations in factor VII, gamma-glutamyl carboxylase and vitamin K epoxide reductase complex genes on warfarin dose requirement. Thromb Haemost. 2006;95:782–787. doi:10.1160/TH05-10-0678.
- Marsh S, King CR, Porche-Sorbet RM, et al. Population variation in VKORC1 haplotype structure. J Thromb Haemost. 2006;4:473–474. doi:10.1111/j.1538-7836.2006.01759.x.
- Dang MT, Hambleton J, Kayser SR. The influence of ethnicity on warfarin dosage requirement. Ann Pharmacother. 2005;39:1008–1012. doi:10.1345/aph.1E566.
- Zhao F, Loke C, Rankin SC, et al. Novel CYP2C9 genetic variants in Asian subjects and their influence on maintenance warfarin dose. Clin Pharmacol Ther. 2004;76:210–219. doi:10.1016/j.clpt.2004.05.005.
- Takahashi H, Wilkinson GR, Caraco Y, et al. Population differences in S-warfarin metabolism between CYP2C9 genotype-matched Caucasian and Japanese patients. Clin Pharmacol Ther. 2003;73:253–263. doi:10.1067/mcp.2003.26a.
- Lee SC, Ng SS, Oldenburg J, et al. Interethnic variability of warfarin maintenance requirement is explained by VKORC1 genotype in an Asian population. Clin Pharmacol Ther. 2006;79:197–205. doi:10.1016/j.clpt.2005.11.006.
- Takahashi H, Wilkinson GR, Nutescu EA, et al. Different contributions of polymorphisms in VKORC1 and CYP2C9 to intra- and inter-population differences in maintenance dose of warfarin in Japanese, Caucasians and African-Americans. Pharmacogenet Genomics. 2006;16:101–110. doi:10.1097/01.fpc.0000184955.08453.a8.
- Johnson JA, Caudle KE, Gong L, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for pharmacogenetics-guided warfarin dosing: 2017 update. Clin Pharmacol Ther. 2017;102:397–404. doi:10.1002/cpt.668.
- Abbas SSU, Bhatti S, Naqi A, et al. Interethnic diversity of CYP2C9 alleles on pharmacogenetics in healthy Sindhi and Baluchi population samples: correlation to global allelic frequencies scientific reports. 2012;1:520. doi:10.4172/scientificreports.520.
- Xie HG, Prasad HC, Kim RB, et al. CYP2C9 allelic variants: ethnic distribution and functional significance. Adv Drug Deliv Rev. 2002;54:1257–1270. doi:10.1016/S0169-409X(02)00076-5.
- Owen RP, Gong L, Sagreiya H, et al. VKORC1. Pharmacogenet Genomics. 2010;20:642–644. doi:10.1097/FPC.0b013e32833433b6.
- Suwanawiboon B, Kongtim P, Chinthammitr Y, et al. The efficacy of 3-mg warfarin initiating dose in adult Thai patients, who required long-term anticoagulant therapy. J Med Assoc Thai. 2011;94(Suppl 1):S225–S231.
- Moyer TP, O'Kane DJ, Baudhuin LM, et al. Warfarin sensitivity genotyping: a review of the literature and summary of patient experience. Mayo Clin Proc. 2009;84:1079–1094. doi:10.4065/mcp.2009.0278.
- Klamchuen S CA, Angchaisuksiri P, Sasanakul W. Frequency of polymorphisms associated with VKORC1 gene in Thai blood donors and patients receiving warfarin. J Hematol Transf Med. 2008;18:307–313.
- Sermsathanasawadi N, Sritongsathian C, Pongrattanaman N, et al. The influence of VKORC1 polymorphisms on warfarin doses in Thai patients with deep vein thrombosis. J Med Assoc Thai. 2015;98:549–554.
- Kuanprasert S, Dettrairat S, Palacajornsuk P, et al. Prevalence of CYP2C9 and VKORC1 mutation in patients with valvular heart disease in northern Thailand. J Med Assoc Thai. 2009;92:1597–1601.
- Sangviroon A, Panomvana D, Tassaneeyakul W, et al. Pharmacokinetic and pharmacodynamic variation associated with VKORC1 and CYP2C9 polymorphisms in Thai patients taking warfarin. Drug Metab Pharmacokinet. 2010;25:531–538. doi:10.2133/dmpk.DMPK-10-RG-059.