ABSTRACT
Objectives
Hemophilic arthropathy is the most common complication of severe hemophilia A. This study aims to investigated joint status and related risk factors in patients with severe hemophilia A (PWSHA).
Methods
This single-center study included 31 patients. Six index joints (both elbows, knees, and ankles) were evaluated using the Hemophilia Early Arthropathy Detection with UltraSound in China (HEAD-US-C) and Hemophilia Joint Health Score (HJHS). Treatment adherence was measured using the Validated Hemophilia Regimen Treatment Adherence Scale-Prophylaxis (VERITAS-Pro). We analyzed the influence of age, treatment delay (the interval between diagnosis and the initiation of treatment), prophylaxis, and treatment adherence on joint outcomes.
Results
All patients were male (median age, 22 years). The median age at diagnosis was 1 year; that at initial treatment was 5 years. All patients experienced joint bleeding. HEAD-US-C and HJHS scores were positively correlated (R = 0.70, P < 0.0001). Median [range] HEAD-US-C and HJHS scores were 15 [0-36] and 32 [2-49], respectively. Age was positively correlated with both HEAD-US-C (P = 0.002) and HJHS scores (P < 0.0001). The difference of HEAD-US-C scores between groups with ≤1 year and >1 year treatment delay was close to significant (P = 0.055). HJHS scores were significantly different between these two groups (P = 0.03). Joint assessment scores were not significantly different between on-demand and low-dose prophylaxis groups. VERITAS-Pro scores were correlated with both HEAD-US-C and HJHS scores (P = 0.046 and P = 0.005, respectively).
Conclusions
Hemophilic arthropathy was pervasive in PWSHA. Age and poor adherence were significantly correlated with joint damage. Prompt treatment and adherence improvement may reduce severity.
1. Introduction
Hemophilia A is an X-linked congenital disease with a worldwide incidence of 1 in 10 000 births. Severe hemophilia A (SHA) is defined by a baseline activity of FVIII factors of less than 1% of normal. Spontaneous and traumatic bleeding is the hallmark of SHA, occurring in joints, muscles, mucous membranes, the intracranial cavity, and the gastrointestinal tract [Citation1].
Recurrent hemarthrosis gradually but inexorably causes synovial inflammation and hyperplasia and further impairs the structure of cartilage and bone [Citation2,Citation3]. Many patients develop hemophilic arthropathy, which is the most common complication of SHA, causing pain, joint dysfunction, and psychosocial impairment. Elbows, knees, and ankles, which are known as the index joints, are the most frequently affected joints [Citation2,Citation4,Citation5]. As joint disease has a strong impact on quality of life [Citation6], it is of great importance to recognize early reversible joint impairment and promptly optimize treatment regimens accordingly.
As bleeding episodes alone fail to reflect joint status precisely, routine joint assessment through physical examination and imaging methods is feasible and paramount for evaluating joint function as well as detecting structural impairment caused by subclinical bleeding. The other important issue is to recognize and prevent further deteriorative factors with the goal of minimizing hemarthrosis and preserving joint function.
Currently, replacement therapy is the main therapy for hemophilia A. Regular prophylactic FVIII replacement can reduce pain and bleeding episodes, which is beneficial for joint structure as well as joint function [Citation7]. Primary prophylaxis (defined as continuous FVIII administration started before clinical arthropathy, two large joint bleeding episodes, and 3 years of age [Citation1]) is the most effective regimen and is strongly recommended for joint protection [Citation1,Citation8]. However, inadequate treatment is common in the real world. Numerous patients with severe hemophilia A (PWSHA) have delayed initial treatment. Additionally, many PWSHA tend to receive on-demand treatment or intermittent prophylaxis (defined as prophylactic treatment given for a period not exceeding 45 weeks [Citation1]). Only a small portion of PWSHA can afford continuous prophylaxis, and most receive tertiary prophylaxis (defined as regular continuous treatment started after the onset of arthropathy [Citation1,Citation6]). Moreover, medication non-adherence is common, which offsets treatment outcomes. A variety of social and economic reasons may be considered for medication non-adherence, such as financial concerns, inconvenient access to hospitals, poor compliance, venous access difficulties, or lack of awareness of hemorrhage-related disability [Citation9].
In this study, we assessed joint function and structure using the Hemophilia Joint Health Score Version 2.1 (HJHS) and the Hemophilia Early Arthropathy Detection with UltraSound in China (HEAD-US-C) and surveyed treatment compliance with the Validated Hemophilia Regimen Treatment Adherence Scale-Prophylaxis (VERITAS-Pro) in 31 PWSHA. We aimed to describe the current condition of the joints in this population and to explore the potential impacts exerted by age, treatment delay (the interval between diagnosis and the initiation of treatment), prophylaxis, and adherence to treatment.
2. Materials and methods
2.1. Study design
This was a single-center, cross-sectional study conducted at Xiangya Hemophilia Diagnosis and Treatment Center, Xiangya Hospital, Central South University, Changsha, Hunan Province, China, from April 2019 to January 2020.
2.2. Subjects
All patients in this study were recruited during clinic visits to our hemophilia center and were PWSHA. The exclusion criteria include: (1) recent joint (elbow, knee, or ankle) hemarthrosis that occurred in the past 2 weeks, as this will significantly increase the joint assessment scores temporarily; (2) patient with clotting factor inhibitor, as the presence of inhibitor is supposed to result in higher joint scores; (3) children under 4 years old, as HJHS score is not validated for this group. Forty-one patients were recruited. Thirty-one of them completed all study procedures and were included in the following analyses. Written informed consent was obtained from all participants or their guardians. This study was approved by our institutional ethics committee.
2.3. Measures
2.3.1. General information questionnaire
Each subject completed a questionnaire consisting of four types of information, including demographic information, clinical characteristics, treatment regimens, and self-reported joint status. There were three treatment regimens: on-demand treatment, intermittent prophylaxis, and continuous prophylaxis. On-demand treatment was defined as replacement therapy given at the time of clinically evident bleeding. Intermittent prophylaxis referred to replacement therapy given to prevent bleeding for periods not exceeding 45 weeks in a year. Continuous prophylaxis was defined as the intent to treat for 52 weeks/year and receiving a minimum of an a priori-defined frequency of infusions for at least 45 weeks (85%) of the year under consideration [Citation10].
2.3.2. HEAD-US-C
The HEAD-US-C was adopted to assess joint structure. It is a revised version of the HEAD-US, a simplified and objective scoring system for evaluating hemophilic arthropathy, established by Martinoli et al. in 2013 [Citation11], which is recommended as the preferred imaging method by the World Federation of Hemophilia [Citation12]. The HEAD-US is sensitive for detecting abnormalities in joints [Citation13–15], comprising parameters of synovitis, cartilage, and subchondral bone. The maximum score is eight points per joint [Citation11]. To further enhance the sensitivity of the HEAD-US, joint effusion and synovial vascular hyperplasia are included by some Chinese scholars, as these are good indicators of acute joint bleeding, forming the HEAD-US-C [Citation16,Citation17]. The fluid amount is scored from 0 (no fluid) to 3 (large amount of fluid). Synovial vascular hyperplasia is scored as 0 (no hyperplasia), 1 (<3 sites of blood flow signals within the region of interest [ROI]), or 2 (≥3 sites of blood flow signals or dendritic signals within the ROI). The HEAD-US-C is well correlated with the HEAD-US, with a higher sensitivity for subclinical abnormalities in joints [Citation16]. The six index joints (both elbows, knees, and ankles) were assessed by an ultrasonics expert with the HEAD-US-C.
2.3.3. HJHS
The HJHS was used to assess joint function, which correlated strongly with HEAD-US [Citation15,Citation18]. The HJHS is a well-recognized physical examination scale used to numerically score the joint health of patients with hemophilia [Citation19,Citation20] and has been validated in a Chinese population [Citation21]. There are nine impairment items in the HJHS, including swelling, duration of swelling, muscle atrophy, crepitus of motion, extension loss, flexion loss, joint pain, strength, and gait. A higher total score indicates a worse joint health status. The HJHS assessment was performed by a single physiotherapist. All six index joints were assessed.
2.3.4. VERITAS-Pro
The VERITAS-Pro, which was developed and validated by Duncan et al. in 2010 [Citation22], was adopted to evaluate treatment adherence. It has also been validated in several other language versions [Citation23,Citation24]. The VERITAS-Pro contains six subscales, including time, dose, plan, remember, skip, and communicate. Each subscale consists of four related items. There are five options for each item, ranging from ‘Always’ to ‘Never’, corresponding to a numeric score on a five-point scale. The score range for each subscale was 4–20 points. The highest score represents the poorest adherence [Citation22]. A total of 19 participants who received prophylactic medication or their guardians were asked to complete the Chinese version of the online VERITAS-Pro questionnaire.
2.4. Statistical analysis
Data were analyzed using SPSS v. 17 (SPSS Inc., Chicago, IL, USA). The t-test and one-way analysis of variance (ANOVA) were used to compare joint scores between subgroups in terms of treatment delay, prophylaxis, and treatment adherence of patients. Two-way ANOVA was used to analyze the interaction between treatment delay and prophylaxis. The multiple linear regression analysis was used to analyze the effects of age and treatment delay on joints assessment score. Correlations between joint assessment scores (HEAD-US-C and HJHS) and other factors (age, treatment initiation age, and VERITAS-Pro) were calculated using the Pearson's correlation test. P < 0.05 was considered statistically significant.
3. Results
3.1. Subject characteristics
Demographic data, including sex, family history, occupation, and health insurance, are shown in . All participants were male. Forty-five percent of them had a positive family history of hemophilia. The median (range) age was 22 (4-41) years. Clinical data, including age at diagnosis, age at initial treatment, treatment delay, treatment regimen, and surgery for joints, are shown in . Delayed treatment was common in the participants. The medium (range) age at diagnosis was 1 year (0.08–5 years), and the medium (range) age at initial treatment was 5 years (0.08–33 years). Over half of them (55%) were diagnosed at younger than 1 year of age, but only 19% started FVIII replacement before age 1 year. The mean (median) delay was 5.62 (2.75) years. In addition, a large proportion of patients (45%) were treated on demand. Only 13% received continuous prophylaxis.
Table 1. Demographics of participants.
Table 2. Clinical characteristics of participants.
3.2. Joint status
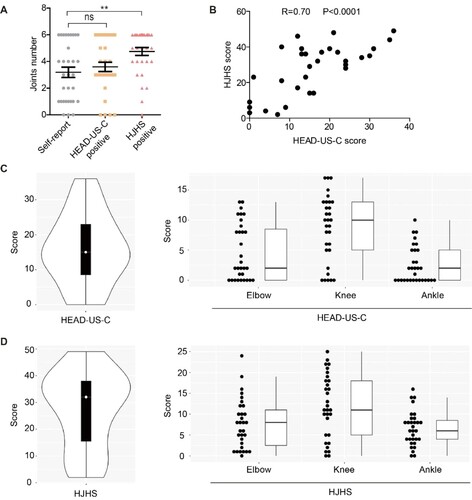
PWSHA usually have frequent bleeding, which can occur in almost any part of the body. Severe (joint, muscle, and mucous membrane bleeding) and life-threatening bleeding (intracranial, neck/throat, and gastrointestinal tract bleeding) were classified on the basis of their severity. Severe and life-threatening bleeding was present in 100% and 25.8% of patients, respectively. The total number of self-reported abnormal joints was approximately equal to that identified by the HEAD-US-C, while the number of positive HJHS was the highest ((A)). These results indicate that joint dysfunction may occur earlier than structural alteration; thus, objective assessments were vital for discovering early joint impairment. Consistent with other reports [Citation16], HEAD-US-C and HJHS scores (both are summed scores of six index joints, including bilateral elbows, knees, and ankles) were strongly correlated (r = 0.70, P < 0.0001, (B)). The median (range) scores for the HEAD-US-C and HJHS were 15 (0-36) and 32 (2-49), respectively ((C and D), left column). Distributions of joint assessment scores for elbows, knees, and ankles (all are summed scores of bilateral joints) of each participant are shown in (C and D), right column. The knee scores were the highest, suggesting the knee was the joint with the worst condition ((C and D), right column).
Figure 1. Joint assessments in our cohort. A. Numbers of abnormal index joints identified by self-report, HEAD-US-C, and HJHS. B. Correlation between HEAD-US-C and HJHS scores. C. Distribution of total HEAD-US-C scores and HEAD-US-C scores for both elbows, knees, and ankles for each participant. D. Distribution of total HJHS scores and HJHS scores for both elbows, knees, and ankles for each participant. White dots, black bars, and whisker plots represent medians, interquartile ranges, and total ranges, respectively. Dot represents HEAD-US-C or HJHS score for each patient. Abbreviations: HEAD-US-C, Hemophilia Early Arthropathy Detection with Ultrasound in China; HJHS, Hemophilia Joint Health Score.
3.3. Risk factors
3.3.1. Age
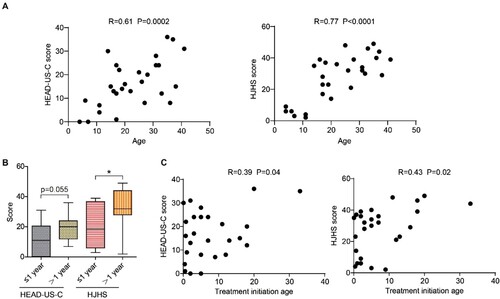
Age was positively correlated with both HEAD-US-C (r = 0.61, P = 0.0002, (A), left) and HJHS scores (r = 0.77, P < 0.0001, (A), right). This demonstrated that the damages of joints accumulated with the increasing of age.
Figure 2. The impact of age and treatment delay on joint assessments scores. A. The correlation between age and HEAD-US-C (left) and HJHS (right). B. The HEAD-US-C and HJHS scores by groups of ≤1 year delay and >1 year delay. C. The correlation between treatment initiation age and HEAD-US-C (left) and HJHS (right). Abbreviations: HEAD-US-C, Hemophilia Early Arthropathy Detection with UltraSound in China; HJHS, Hemophilia Joint Health Score.
3.3.2. Treatment delay
On the basis of the interval between diagnosis and the initiation of treatment, we divided our patients into groups of ≤1 year, >1 year, and unknown. The proportions of patients were 39%, 48%, and 13%, respectively (), suggesting that a significant proportion of our patients did not receive prompt treatment. The median (range) scores for the HEAD-US-C were 11 (0-31) (Group ≤1 year) and 20 (7-36) (Group >1 year), respectively. The median (range) scores for the HJHS were 18.5 (3-39) (Group ≤1 year) and 32 (2-49) (Group >1 year), respectively ((B)). The difference of HEAD-US-C scores between Group ≤1 year and Group >1 year was close to significant (P = 0.055). HJHS scores were significantly different between these two groups (P = 0.03) ((B)), suggesting that delayed treatment may exacerbate joint damage. To further explore if delayed treatment could increase joint assessment score, we did the correlations between treatment initiation age and joint assessment score ((C)). The correlation coefficient (r) between treatment initiation age and HEAD-US-C or HJHS score is 0.39 (P = 0.04), and 0.43 (P = 0.02), respectively ((C)). However, by further multiple linear regression analysis, the effects of age on joint assessment scores kept significant (P < 0.0005), while treatment delay was non-significant (P = 0.642).
3.3.3. Prophylaxis.
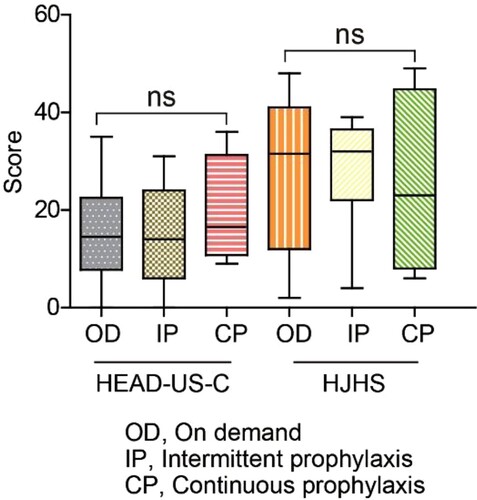
On the basis of different treatment regimens, participants were classified into three groups: on-demand (OD, n = 14), intermittent prophylaxis (IP, n = 13), and continuous prophylaxis (CP, n = 4). None of the patients received primary prophylaxis. The median (range) age was 17 (4-36) years (OD), 30 (4-41) years (IP), and 23 (6-35) years (CP), respectively. There was no statistically significant difference among the joint assessment scores of the three groups (). Besides, the two-way ANOVA showed no interaction between treatment delay and treatment method (P = 0.74).
Figure 3. The impact of prophylaxis on joint assessments scores. The HEAD-US-C and HJHS scores by group: OD, IP, and CP. Abbreviations: CP, continuous prophylaxis; HEAD-US-C, Hemophilia Early Arthropathy Detection with Ultrasound in China; HJHS, Hemophilia Joint Health Score; IP, intermittent prophylaxis; OD, on demand.
3.3.4. Treatment adherence
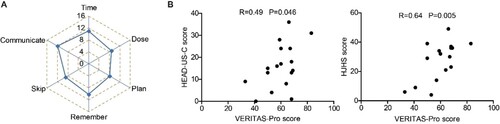
Participants who received a prophylactic regimen (n = 17) further completed the VERITAS-Pro scale. The VERITAS-Pro scale has six subscales, each with a maximum of 20 points. The mean scores of each subscale were 11.2 (time), 8.9 (dose), 8.3 (plan), 10.2 (remember), 9.0 (skip), and 12.1 (communicate) ((A)). Higher scores represented poorer adherence. VERITAS-Pro scores were positively correlated with both HEAD-US-C (r = 0.49, P = 0.046) and HJHS scores (r = 0.64, P = 0.005) ((B)), suggesting that better adherence to prophylaxis was beneficial for joint protection.
Figure 4. The impact of treatment adherence on joints assessments scores. A. The mean scores of the 6 subscales of VERITAS-Pro. B. The correlation between VERITAS-Pro score and HEAD-US-C (left) and HJHS (right). Abbreviations: HEAD-US-C, Hemophilia Early Arthropathy Detection with Ultrasound in China; HJHS, Hemophilia Joint Health Score; VERITAS-Pro, Validated Hemophilia Regimen Treatment Adherence Scale-Prophylaxis.
4. Discussion
Our data showed a well positive correlation between HEAD-US-C and HJHS ((B)), which was consistent with the literature reported a correlation between HEAD-US and HJHS [Citation15,Citation25]. It is interesting to note that the number of self-reported abnormal joints was less than that identified by the HJHS ((A)). This discrepancy can be explained by the fact that early morphological changes in joints may be asymptomatic. In addition, the HJHS identified more abnormal joints than the HEAD-US-C ((A)). This is consistent with the findings from Timmer et al. They showed that HJHS scores = 0 had a negative predictive value of 98%, while positive value of HJHS scores >3 was 95%. 33% of the joints with HJHS between 1–3 showed no abnormalities on HEAD-US [Citation14]. Prasetyo et al reported that 10 of 120 joints (8.3%) have positive HJHS while the HEAD-US score is 0[Citation18]. Besides, positive HJHS scores up to three points without abnormalities on MRI have been described in healthy young adults[Citation26]. Therefore, the number of positive HJHS joints may exceed that of HEAD-US-C. In contrast, some studies detected more abnormalities on HEAD-US than HJHS[Citation27,Citation28]. This may be caused by inter-observer differences when using the HJHS. Collectively, HEAD-US is useful to detect early joint changes, while HJHS 2.1 showed the added value of detecting relevant physical and functional changes[Citation25]. Hence, these objective measurements should be considered in addition to bleeding episodes when treatment regimens are adjusted.
Age was strongly correlated with joints scores ((A)). It was reported that the mean (SD) HEAD-US scores were 4.03 (4.59) in children (6-17 years) and 17.3 (8.96) in adults (≥18 years). Besides, the mean (SD) HJHS scores were 1.86 (3.03) in children and 17.96 (12.55) in adults [Citation29]. Though the patients in this study contained both hemophilia A (n = 62) and B (n = 11), the results indicated accumulated joint damage with age.
All of our patients received FVIII replacement therapy. However, insufficient treatment with a factor concentrate was common. On the one hand, delays in treatment were frequently seen. Only 39% of the patients had access to treatment within 1 year after the diagnosis of severe hemophilia, and 48% of the patients had exceeded 1 year of delayed treatment. Most participants were diagnosed at ages younger than 5 years. Many of them may have presented with only mild bleeding manifestation in their early age. As physical activity becomes more intensive as children grow older, the incidence of severe bleeding episodes increases. In most cases, FVIII concentrate replacement was not implemented until severe hemorrhage occurred, which explains why delayed treatment is remarkable among participants. As delay of treatment exacerbates joint impairment ((B)) and initial treatment age is positively correlated with joints assessment score ((C)), prompt treatment is important after diagnosis. In addition, due to the limited awareness and education of hemophilia A patients and their caregivers, they may not pursue proper treatment immediately, resulting in a treatment delay. However, by further multiple linear regression analysis, age weighed higher than the fact of treatment delay. Further studies with more cases are warranted to verify the effects of early treatment on joints score. Nevertheless, this collectively suggested that earlier treatment was recommended for PWSHA.
On the other hand, most participants were treated either on demand (45%) or with intermittent prophylaxis (42%). A small proportion of patients (13%) received continuous prophylaxis, which was mainly administered in a low-dose regimen and after joint impairment. The leading cause of insufficient treatment was financial burden. Although 74% of patients had health insurance (), it only covered the cost of on-demand treatment. Another possible reason is accessibility. Clotting factors are usually available in tertiary hospitals, but not in primary hospitals. However, there was no significant difference between the joint assessment scores of the on-demand and prophylactic groups (). There are several possible explanations for this. First, the sample size in Group CP was relatively small (n = 4). Second, patients in Group IP were relatively older (median [range]: 30 [4-41] years). Third, patients with a severe joint phenotype tend to utilize FVIII prophylactic infusion to mitigate clinical discomfort, while preexisting joint damage is irreversible. It has been reported that tertiary prophylaxis decreased bleeding and pain episodes but could not improve structural arthropathy deterioration [Citation13]. This finding is in agreement with the results of our study. Early initiation of prophylaxis has been proven to reduce the number of hemarthroses and joint impairment [Citation30]. The SO-FIT study showed that most children with SHA in the UK obtained prophylaxis (46.8% were primary). Unsurprisingly, the study reported good joint health in their participants, with a median HJHS score as low as 1 [Citation31]. In summary, early clotting factor replacement and primary prophylaxis are pivotal for joint health preservation.
Effective treatment is related to both high-quality management from the physician side and good compliance from the patient side. Among the six subscales in the VERITAS-Pro, adherence to ‘plan’ was the best, while ‘communicate’ was the worst. The VERITAS-Pro scores were closely related to joint health status (). It is meaningful to enhance communication between patients and healthcare providers. More and more online clinical resources are available now, such as online lectures, consultation, and medicine purchase, which are promising for improving adherence.
Our study has several strengths. First, this study considered both patient-reported outcomes and objective results from the clinician side. Second, treatment delay is a specific situation in China, especially in financially limited areas. However, some limitations of this study should be considered. The sample size was likely too small to detect the potential benefits of prophylaxis on joint structure, compared with the OD group. Besides, this was a single-center cross-sectional study without long-term observation. We will continue to follow up with these patients for further observation and validation of our results.
Acknowledgments
We are grateful to Tao Zhang for data analysis and statistical support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Srivastava A, Santagostino E, Dougall A, et al. W.F.H.G.f.t.M.o.H. panelists, a. co, WFH Guidelines for the management of hemophilia, 3rd edition. Haemophilia. 2020;26(Suppl 6):1–158.
- Rodriguez-Merchan EC, Jimenez-Yuste V, Aznar JA, et al. Joint protection in haemophilia. Haemophilia. 2011;17(Suppl 2):1–23.
- Roosendaal G, Lafeber FP. Pathogenesis of haemophilic arthropathy. Haemophilia. 2006;12(Suppl 3):117–121.
- Beeton K. Evaluation of outcome of care in patients with haemophilia. Haemophilia. 2002;8:428–434.
- Zhao H, Yang L, Long C, et al. Hemophilia care in China: review of care for 417 hemophilia patients from 11 treatment centers in Shanxi Province. Expert Rev Hematol. 2015;8:543–550. doi:10.1586/17474086.2015.1043263.
- Soucie JM, Grosse SD, Siddiqi AE, et al. The effects of joint disease, inhibitors and other complications on health-related quality of life among males with severe haemophilia A in the United States. Haemophilia. 2017;23:e287–e293.
- Miesbach W, Kittler S, Bauhofer A, et al. Long-term analysis of the benefit of prophylaxis for adult patients with severe or moderate haemophilia A. Haemophilia. 2020;26:467–477. doi:10.1111/hae.13988.
- Pasina L, Marengoni A, Ghibelli S, et al. A multicomponent intervention to optimize psychotropic Drug prescription in elderly nursing home residents: An Italian multicenter, prospective, pilot study. Drugs Aging. 2016;33:143–149. doi:10.1007/s40266-015-0336-z.
- Shapiro AD. Why is primary prophylaxis underutilized in the United States? Haemophilia. 2003;9:670–672.
- Blanchette VS, Key NS, Ljung LR, et al. Definitions in hemophilia: communication from the SSC of the ISTH. J Thromb Haemost. 2014;12:1935–1939. doi:10.1111/jth.12672.
- Martinoli C, Della Casa Alberighi O, Di Minno G, et al. Development and definition of a simplified scanning procedure and scoring method for Haemophilia Early Arthropathy Detection with Ultrasound (HEAD-US). Thromb Haemost. 2013;109:1170–1179. doi:10.1160/TH12-11-0874.
- Di Minno MND, Pasta G, Airaldi S, et al. Ultrasound for early detection of joint disease in patients with hemophilic arthropathy. J Clin Med. 2017;6(8):77. doi:10.3390/jcm6080077.
- Banchev A, Stoyanova D, Konstantinov D, et al. Beyond the bleeding rates- HEAD-US joint assessment in patients treated by late prophylaxis. Haemophilia. 2019;25:e294–e297.
- Timmer MA, Foppen W, Schutgens RE, et al. Comparing findings of routine haemophilia joint Health Score and haemophlia early arthropathy detection with ultrasound assessments in adults with haemophilia. Haemophilia. 2017;23:e141–e143.
- Måseide RJ, Berntorp E, Astermark J, et al. Haemophilia early arthropathy detection with ultrasound and haemophilia joint health score in the moderate haemophilia (MoHem) study. Haemophilia. 2021;27:e253–e259. doi:10.1111/hae.14261.
- Li J, Liu W, Guo XJ, et al. [HEAD-US-C quantitative ultrasound assessment scale in evaluation of joint damage in patients with moderate or severe hemophilia A received on-demand versus prophylaxis replacement therapy]. Zhonghua Xue Ye Xue Za Zhi. 2018;39:817–821.
- Zhang CM, Zhang JF, Xu J, et al. Musculoskeletal ultrasonography for arthropathy assessment in patients with hemophilia. Medicine (Baltimore). 2018;97:e13230), doi:10.1097/MD.0000000000013230.
- Prasetyo M, Moniqa R, Tulaar A, et al. Correlation between Hemophilia Early Arthropathy Detection with Ultrasound (HEAD-US) score and Hemophilia Joint Health Score (HJHS) in patients with hemophilic arthropathy. PLoS One. 2021;16:e0248952.
- Hilliard P, Funk S, Zourikian N, et al. Hemophilia joint health score reliability study. Haemophilia. 2006;12:518–525.
- Feldman BM, Funk SM, Bergstrom BM, et al. Validation of a new pediatric joint scoring system from the international Hemophilia prophylaxis study group: validity of the hemophilia joint health score. Arthritis Care Res (Hoboken). 2011;63:223–230. doi:10.1002/acr.20353.
- Sun J, Hilliard PE, Feldman BM, et al. Chinese hemophilia joint health score 2.1 reliability study. Haemophilia. 2014;20:435–440.
- Duncan N, Kronenberger W, Roberson C, et al. VERITAS-Pro: a new measure of adherence to prophylactic regimens in haemophilia. Haemophilia. 2010;16:247–255.
- Ferreira AA, Leite ICG, Duncan NA. Validation of the Brazilian version of the VERITAS-Pro scale to assess adherence to prophylactic regimens in hemophilia. Rev Bras Hematol Hemoter. 2018;40:18–24.
- Cuesta-Barriuso R, Torres-Ortuño A, Galindo-Piñana P, et al. Validation of the VERITAS-Pro treatment adherence scale in a Spanish sample population with hemophilia. Patient Prefer Adherence. 2017;11:653–660.
- De la Corte-Rodriguez H, Rodriguez-Merchan EC, Alvarez-Roman MT, et al. HJHS 2.1 and HEAD-US assessment in the hemophilic joints: How do their findings compare? Blood Coagul Fibrinolysis. 2020;31:387–392. doi:10.1097/MBC.0000000000000934.
- Sluiter D, Foppen W, de Kleijn P, et al. Haemophilia joint health score in healthy adults playing sports. Haemophilia. 2014;20:282–286.
- Altisent C, Martorell M, Crespo A, et al. Early prophylaxis in children with severe haemophilia A: clinical and ultrasound imaging outcomes. Haemophilia. 2016;22:218–224.
- De la Corte-Rodriguez H, Rodriguez-Merchan EC, Alvarez-Roman MT, et al. The value of HEAD-US system in detecting subclinical abnormalities in joints of patients with hemophilia. Expert Rev Hematol. 2018;11:253–261. doi:10.1080/17474086.2018.1435269.
- Kavaklı K, Özbek SS, Antmen AB, et al. Impact of the HEAD-US scoring system for observing the protective effect of prophylaxis in Hemophilia patients: A Prospective, multicenter, observational study. Turkish Journal of Hematology. 2021;38:101–110. doi:10.4274/tjh.galenos.2021.2020.0717.
- Warren BB, Thornhill D, Stein J, et al. Young adult outcomes of childhood prophylaxis for severe hemophilia A: results of the joint outcome continuation study. Blood Advances. 2020;4:2451–2459. doi:10.1182/bloodadvances.2019001311.
- Khair K, Holland M, Bladen M, et al. Study of physical function in adolescents with haemophilia: The SO-FIT study. Haemophilia. 2017;23:918–925.
