ABSTRACT
Objectives
With large patient population and complement inhibitors naïve background, the characteristics patients with paroxysmal nocturnal hemoglobinuria (PNH) in China have not been well studied, especially for different subtypes.
Methods
We retrospectively reviewed patients with complete data who visited Peking Union Medical College Hospital (PUMCH) from 2009 to 2019 and had been followed up for more than 2 years.
Results
Five hundred and twelve patients were enrolled including 56.3% males and 43.7% females. The median age at disease onset was 33 (9∼80) years. Most were aged 21∼40 years (50.6%). 52.1%, 46.3% and 1.6% of the patients had classic PNH, bone marrow failure (BMF)/PNH and subclinical PNH, respectively. Symptoms of classic PNH were associated with hemolysis, whereas bleeding was more common in BMF/PNH patients. Classic PNH had higher survival rate, larger PNH clone size, higher lactate dehydrogenase (LDH) level and lower ferritin level than BMF/PNH. Although the rate of thrombosis was similar in the classic PNH and BMF/PNH (P = 0.66), those with BMF/PNH had higher chance of renal impairment (P < 0.05). Immunosuppressive agents was more common use in BMF/PNH (P < 0.05), but glucocorticoids, iron supplements and anticoagulants were more common used in classic PNH (P < 0.05) patients. Less evolution to myeloid malignancies was observed in classic PNH than in BMF/PNH (P = 0.02). The major causes of deaths were thrombosis (29.6%), hemorrhage (18.5%) and infections (18.5%).
Conclusion
Patients with classic PNH and BMF/PNH have different clinical profiles, and we described a more hemolytic features of PNH in China which might be improved with complement inhibitors.
Paroxysmal nocturnal hemoglobinuria (PNH) originates from phosphatidylinositol glycan class A (PIGA) gene mutations, resulting in a deficiency in glycosylphosphatidylinositol (GPI)-anchored complement regulatory proteins on the surface of blood cells (including CD55 and CD59), leading to intravascular hemolysis. PNH is a life-threatening acquired disease associated with hemolytic anemia, bone marrow failure (BMF), thrombosis, and a poor quality of life [Citation1–3]. Over the past 30 years, with the popularize use of flow cytometry, the prevalence of PNH (reported by Dennis et al.) has gradually increased to 1.04/100 000 person/years [Citation4–7].
In 2005, the international working group classified PNH into three subtypes: classic PNH, PNH in the setting of BMF syndrome (BMF/PNH), and subclinical PNH (nonhemolytic PNH) [Citation8]. Reports from different countries have described the clinical features of PNH [Citation9–14]. In general, patients from Western countries have a higher percentage of classic PNH, renal failure and thrombosis complications, and thrombosis is the leading cause of death. Patients in Northeast Asia mostly develop BMF/PNH, with a small number of thrombotic cases [Citation15]. So severe infections or bleeding resulting from BMF dominate most lethal cases [Citation13].
Before the appearance of complement monoclonal antibody, treatment for classic PNH was mainly symptomatic support. Transplantation is the only cure for both classic PNH and PNH with refractory severe aplastic anemia (AA) [Citation16]. With the wide use of eculizumab and other monoclonal antibodies in most developed countries [Citation17,Citation18], the 10-year survival rate in the early twenty-first century has been significantly improved compared with that in the late twentieth century [Citation19].
With improved diagnosis and supportive care, the outlook regarding PNH in China has substantially changed. Because complement monoclonal antibodies are not currently available, knowledge of complement treatment-naive PNH in Eastern countries is critical to the understanding of PNH in the new era. Here, we reported the characteristics of 512 patients diagnosed with PNH in the past ten years from a single center in China.
Methods
Patients
This was a retrospective observational study. All the patients signed informed consent forms before enrollment. The data on successive diagnosed PNH at our hospital from January 2009 to December 2019 according to previously established criteria were analyzed. The diagnosis of PNH depends on flow cytometry. Fluorescent aerolysin (FLAER)-negative neutrophils greater than 1% was considered PNH clone positive. These patients were further stratified into classic PNH, BMF/PNH or subclinical PNH according to the criteria established by the International PNH Interest Group [Citation8]. Those who had been followed up at least every 3 months for more than 2 years were enrolled for the final analysis. The baseline demographic characteristics, clinical symptoms and signs, other clinical information and laboratory results and follow-up information were documented. To avoid data loss as much as possible, the symptoms and signs of patients based on a quality-of-life assessment questionnaire (the FACIT Weakness Scale and EORTC QLQ-C30 Questionnaire) were occasionally referenced. The study was approved by the ethics committee of Peking Union Medical College Hospital.
Data collection
Data collection included but was not limited to the following:
Baseline demographic characteristics such as sex, age, living area, present and past history, marital status and pregnancy (maternal and fetal outcomes), family history and signs.
Laboratory data such as complete blood cell (CBC) count, routine urine tests, biochemistry parameters, PNH clone size calculated by the proportion of FLAER-negative granulocytes detected by flow cytometry, coagulation and immune-related tests.
Treatment-related information, including all medical treatment and transfusion requirements.
Follow-up information, including disease comorbidities (e.g. thrombotic events, infections, bleeding, and kidney dysfunction) and disease outcomes until the end of follow-up; overall survival (OS) was calculated as the time from the first diagnosis to death.
Statistical analysis
Continuous variables that conformed to a normal distribution are represented as the mean ± SD, and analysis of variance or Student’s t-test was used to assess between-group differences. Continuous variables that conformed to a nonnormal distribution were represented as the median (minimum∼ maximum), and nonparametric Wilcoxon and Kruskal–Wallis tests were used to assess between-group differences. Categorical variables are presented as frequencies and percentages. Group difference was determined by the chi-square test or Fisher’s exact test. When OS was calculated, since patients with AA/PNH, MDS/PNH and classic PNH had different disease duration, the shortest disease duration was taken as the time for comparison. 10-year OS was estimated for the whole cohort and AA/PNH, MDS/PNH and classic PNH by Kaplan-Meier curves. Survival curves were compared using the Mantel–Cox test. P < 0.05 was considered statistically significant. Statistical analysis and mapping were performed using SPSS 26 and GraphPad Prism 8, respectively.
Results
Demographic characteristics and disease classification
The data from 512 patients, comprising 56.3% (N = 288) male and 43.7% (N = 224) female patients, were analyzed. The patients originated from 28 provinces, and 82.2% were northerners. The median age at disease start was 33 (9∼80) years. When the age distribution was examined, the patient number peaked at 21∼40 years (50.6%; N = 259), followed by 41∼60 years (25.4%; N = 130) and then ≤20 years and >60 years, which contributed 13.0% (N = 66) and 11.1% (N = 57) of patients, respectively ((A)).
Figure 1. Age distribution of all the patients and patients in different subtypes. A. Age distribution for the whole cohort. Patient number peaked at 21∼40 years (50.6%; N = 259), followed by 41∼60 years (25.4%; N = 130) and then ≤20 years and >60 years, which contributed 13.0% (N = 66) and 11.1% (N = 57) of patients, respectively. B. Age distribution for patients with different subtypes. No difference was found in age distribution for patients with classic PNH, BMF/PNH or subclinical PNH (P > 0.05).
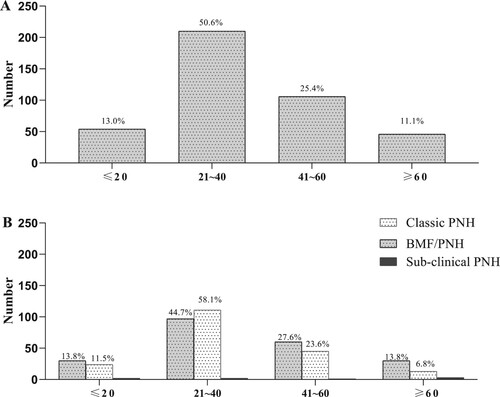
46.9% (N = 240), 51.6% (N = 264) and 1.6% (N = 8) of the patients were classified as having classic PNH, BMF/PNH and subclinical PNH, respectively. Subclinical PNH did not require therapy, and these patients were assessed mainly on telephone or electronic information contact. They had almost no hemoglobinuria and occasionally mild fatigue. Their hemoglobin (HGB) level was stable above 100 g/L, and the PNH clone size was stable within 5% (). Because subclinical PNH lacks obvious clinical symptoms and only requires regular follow-up, we next mainly described the clinical features of the whole cohort and patients with the classic PNH and BMF/PNH subtypes.
Table 1. Baseline characteristics of the patients with different disease subtypes.
No difference was found for sex ratio, age distribution for patients with classic PNH, BMF/PNH or subclinical PNH (P > 0.05). However, patients with classic PNH had a longer disease duration from diagnosis to last follow-up than BMF/PNH (96 (24,401) vs 61 (24,624), P < 0.001).
Before the diagnosis of PNH, 28.8% AA/PNH patients were diagnosed as AA, and 29.0% myelodysplastic syndrome (MDS)/PNH patients were diagnosed as MDS.
Clinical features of PNH
We defined those with ecchymosis at more than 2 places or at least twice gum/nose bleeding as ‘easy to abrasion of skin/mucous bleeding’. Easy to abrasion of skin/mucous bleeding was more common in the BMF/PNH patients than in the classic PNH patients, whereas fatigue, hemoglobinuria, palpitation, headache, abdominal pain, erectile dysfunction and backache were more common in classic PNH; dysphagia was similar in the BMF/PNH and classic PNH groups ().
Thrombosis events (TEs) occurred in 18.0% (92/512) of the patients, with 116 events during the follow-up period. TEs occurred at a median time of 44 (1,404) months after diagnosis. Seven cases had thrombosis before the diagnosis of PNH and three cases had thrombosis at the same time when the diagnosis of PNH was made. Venous events accounted for 88.8% of the total events, while arterial events accounted for 11.2%. Additionally, 77.2% (71/92) of the patients had single-site thrombosis. The most common sites for single TE were abdominal venous thrombosis (73.2%; N = 52), followed by deep venous thrombosis (DVT; 33.8%; N = 24), cerebral thrombosis (29.6%; N = 21), myocardial infarction (MI) (18.3%; N = 13) and ocular venous thrombosis (4.2%; N = 3). Furthermore, 22.8% (21/92) of patients had repeated or multiple-site thrombosis, including recurrent abdominal venous thromboses (N = 5), recurrent cerebral thrombosis (N = 2) and recurrent MIs (N = 1), abdominal venous thrombosis and DVT (N = 5), abdominal venous thrombosis and MI (N = 3), abdominal venous thrombosis, cerebral thrombosis and DVT (N = 1), cerebral thrombosis and DVT (N = 1), cerebral thrombosis combined with MI (N = 1), pulmonary embolism (PE) combined with cerebral thrombosis (N = 1) and PE combined with DVT (N = 1). The rate of thrombosis was similar for patients with classic PNH (17.3%) and those with BMF/PNH (19.1%; P = 0.66). No difference was found in the arterial/venous ratio, sites of thrombosis or ratio of single/multiple-site thrombosis between the classic PNH and BMF/PNH patients (P > 0.05).
Abnormal urine tests were identified in 40.2% of the patients, 63.8% of the tests showed positive urine RBCs, 27.6% showed positive urine protein and 11.7% showed other abnormalities. Patients with classic PNH had a higher similar chance of positive urine RBCs (68.1% vs. 58.1%; P = 0.146) and positive urine protein to those with BMF/PNH (31.9% vs. 22.1%; P = 0.127) (). An abnormal serum creatinine (Scr) level was found in 12.8% of patients. The elevation of Scr was mild, and only one patient had renal failure. No difference was found in the percentage of abnormal Scr between the classic PNH and BMF/PNH patients (10.6% vs. 15.5%; P = 0.14). According to the glomerular filtration rate (eGFR) level (mL/min/1.73 m2), of the 402 patients with available data, renal deficiency occurred in 16.9% of the patients. They were further classified according to the chronic kidney disease (CKD) stage: 46.0% were normal, 36.8% were stage CKD1, 12.4% were stage CKD2, and 4.5% were stage CKD≥3. The BMF/PNH patients (20.7%) had a higher chance of renal insufficiency (calculated by the eGFR) than the classic PNH patients (6.4%, P < 0.001).
In addition to thrombosis and renal insufficiency, hemorrhage (13.3%) was the most common complication. Glucocorticoid-related side effects (diabetes, hypertension, osteoporosis and necrosis of the femoral head) occurred in 3.9% of the patients, followed by 3.2% infections, 1.8% tumors (three kidney tumors, four breast tumors and one thyroid cancer, one cranial tumor and one lung cancer), 1.8% bile duct or urinary calculus, 1.2% immune disease (one antiphospholipid antibody syndrome, one Sjogren's syndrome, two ankylosing spondylitis, one autoimmune liver disease and one neuropsychiatric systemic lupus erythematosus (NPSLE)) and 0.6% pulmonary hypertension. The patients with BMF/PNH had more bleeding complications than those with classic PNH (17.0% vs.9.3%, P < 0.05). Other complications occurred similarly in the patients with classic PNH and BMF/PNH ().
Table 2. Complications, treatment and outcome for patients with BMF/PNH and classic PNH.
The patients with classic PNH had larger PNH clone sizes, higher levels of LDH, total bilirubin (Tbil), direct bilirubin (DBil), aspartate aminotransferase (AST), and higher blood cell counts, and lower serum ferritin and Scr levels than those with BMF/PNH. However, the two groups of patients had similar HGB levels. Surprisingly, patients with classic PNH had lower D-dimer levels. The most common symptoms and laboratory test details for the whole cohort and patients with different subtypes are shown in .
Summary of treatments
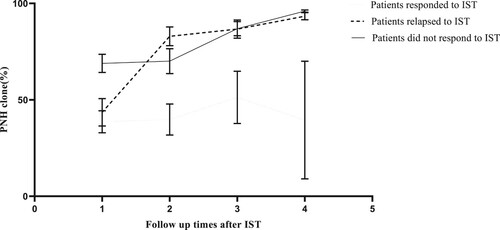
In total, 33.7% (170/504) of the patients received immunosuppressive therapies (151 patients were treated with cyclosporin A, 31 with tacrolimus, and 12 with the ATG/ALG combination), 33.7% (170/504) received androgens, 30.9% (156/504) received glucocorticoids, 15.3% (77/504) received iron supplementation therapy, 12.3% (62/504) received stimulating factors (including recombinant human erythropoietin, granulocyte colony stimulating factor and recombinant human thrombopoietin (rhTPO, Shenyang, China)), 7.5% (38/504) received anticoagulation therapy(only used for those with thrombosis, not for prophylaxis), 1.6% (8/504) received allogeneic transplantation, 1.2% (6/504) received iron chelation therapy, and 0.4% (2/504, treated at other hospitals) received chemotherapy. 59.4% (101/170) patients who received immunosuppressive therapy detected at least twice of PNH clone. Patients who responded (N = 46) to immunosuppressive therapy had smaller PNH clone size (38.64 ± 30.47%) than those who did not respond (N = 93, 66.89 ± 35.01%, P = 0.001) at diagnosis. Neither those who responded (38.64 ± 30.47% vs 39.50 ± 43.13%, P = 0.829) nor those not responded (68.89 ± 35.01% vs 96.00 ± 1.00%, P = 0.133) had change in their clone size during follow-up. However, those who relapsed to immunosuppressive therapy increased in PNH clone size during follow-up (43.53 ± 30.96% vs 93.33 ± 3.22%, P < 0.01, ).
Figure 2. Change in PNH clone size in patients with different response to immunosuppressive therapy (IST). Neither those who responded (P = 0.829) nor those not responded (P = 0.133) to immunosuppressive therapy (IST) had change in their clone size during follow-up. However, those who relapsed to IST increased in PNH clone size during follow-up (P < 0.01). PNH clone size was calculated as FLAER negative neutrophils.
Only 41.3% (38/92) of the patients with thrombosis received anticoagulants; apart from anticoagulation therapies, 25.0% of them had surgeries including splenectomy, stent implantation, craniotomy, enterotomy, and lobectomy due to severe conditions.
Therapies differed with different disease types. The use of immunosuppressive agents, androgen and stimulating factors were more common in patients with BMF/PNH (P < 0.05). Glucocorticoids and iron supplements were more commonly used for patients with classic PNH (P < 0.05). Other therapies, such as antithrombotic treatments, transfusion, iron chelation, allogeneic transplantation and chemotherapy, were compatible for patients with BMF/PNH and classic PNH (P > 0.05; ).
Clone evolution
Four hundred eighty-six patients were followed up for a median of 93 (24∼624) months since first visit in PUMCH, and only 26 patients lost at the end of follow-up. There was no difference in follow-up time between patients with BMF/PNH and classic PNH (66(24,144) vs 76(24,132), P = 0.46, ). More evolutions were observed in BMF/PNH. In total, five patients with BMF/PNH evolved into myeloid malignant tumors, including one patient with AA/PNH evolved into MDS/ refractory anemia with excess blasts 2 (RAEB2), one with MDS/PNH evolved into acute myeloid leukemia (AML), one with MDS/PNH evolved into essential thrombocytosis (ET) and one with AA/PNH and one with MDS/PNH developed into myeloid proliferation neoplasm (MPN). No such evolution was observed in classic PNH (P = 0.02).
Disease outcome
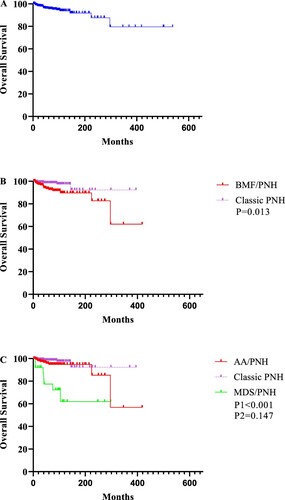
From diagnosis to the end of follow-up, 5.3% (27/512) of the patients died at a median of 41 (24, 624) months from diagnosis. The causes of death were 29.6% (8/27) thrombosis, 18.5% (5/27) hemorrhage, 18.5% (5/27) infections, 14.8% (4/27) anemia-related heart failure, 7.4% (2/27) progression to MDS or leukemia, 3.7% (1/27) tumors, and 7.4% (1/27) unknown causes. OS was lower in patients with BMF/PNH than classic PNH (10-year OS 83.7 ± 1.4% vs. 93.2 ± 3.3%; P = 0.013). However, patients with AA/PNH had a similar OS to those with classic PNH (10-year OS 94.3 ± 2.0% vs. 95.9 ± 1.8%; P = 0.147), whereas those with MDS/PNH had a lower 10-year OS (73.4 ± 10.4% vs. 95.9 ± 1.8%; P < 0.001, ).
Figure 3. Kaplan-Meier curves for the whole cohort and patients in different subtypes. A. OS for the whole cohort. B. Patients with classic PNH had better OS than those with BMF/PNH (P = 0.013). C. Detail analysis showed that OS was similar between patients with classic PNH and AA/PNH (P = 0.147), whereas OS was lower in patients with MDS/PNH than classic PNH and AA/PNH patients (P < 0.001).
Fertility of female patients
Of the 224 females studied, 139 were at child-bearing age, and 101 of them wanted to have children. However, only 14 patients were pregnant. Ten patients eventually gave birth to healthy children, and 4 patients with classic PNH had 7 abortions. Three patients had undergone regular blood transfusion during pregnancy.
Discussion
Since 1990s, flow cytometry has become the standard method for PNH diagnosis [Citation20]. All the patients included in this study were confirmed the diagnosis of PNH by flow cytometry. Our data showed the balanced sex and main northern origin characteristics. There was no epidemiological data for PNH in China so far. But early reports with relatively small samples showed that PNH were more common in North than in South China with unknown reasons [Citation21,Citation22]. More northern patients in our study may either due to higher prevalence in the north or patients coming to our center mainly from Beijing or from neighbor provinces. More than half of the patients in our cohort had classic PNH, a finding similar to that from other general hospitals [Citation13] but higher than that from hematology-specified hospitals in China [Citation14]. Patients aged at 21∼40 years accounted for more than half of the patients. Classic PNH had the same distribution as the whole cohort, indicating that PNH, particularly classic PNH, mainly occurred in younger patients, compatible with reports from others [Citation2,Citation23]. Well control of the disease, particularly control of hemolysis, will significantly recovery the working ability since most of them are at working age.
Symptoms such as fatigue, hemoglobinuria, shortness of breath, headache, abdominal pain and backache and erectile dysfunction in male patients, which were rarely reported previously in Chinese patients, were common in the classic PNH patients in this study. This finding was similar to that reported by Hubert Schrezenmeier [Citation2,Citation23]. However, easy abrasion was more common in the BMF/PNH group, and dysphagia was similar in the BMF/PNH and classic PNH groups, as expected. Laboratory tests showed similar findings in other centers, either from China or worldwide [Citation13,Citation14,Citation23,Citation24]. The patients with classic PNH had larger PNH clone sizes, higher LDH, TBil, DBil, AST, and Scr levels, higher blood cell counts, and lower serum ferritin levels than those with BMF/PNH. Notably, iron deficiency (IDA) occurred in 18.1% of the patients and was even higher in the classic PNH subtype, which is likely a clue concerning the diagnosis of PNH when searching for the reason for IDA. Patients with classic PNH had the largest clone size than those with BMF/PNH and subclinical PNH, and most of the difference in clinical features and lab tests were related to the PNH clone size, and sometimes, the value of LDH correlated with the difference as well.
TEs are common in patients from Western countries and are the major cause of death [Citation25,Citation26]. Our data showed a relatively higher rate of thrombosis (18.3%, mainly venous thrombosis) than that previously reported in China and a similar distribution of sites to Western patients [Citation15]. As verified by others, we noticed a similar rate of thrombosis between the patients with classic PNH and those with BMF/PNH (P = 0.66). Although 77.2% of the patients had single-site thrombosis, thrombosis occurred at more than one site in nearly one-fourth of the patients. Similar to patients from other Northeast Asian countries and Western countries [Citation6,Citation27–30], abdominal vein thrombosis was the most common site for thrombosis, causing symptoms such as abdominal pain, bowel obstruction, portal hypertension and related liver cirrhosis. Severe complications impair the quality of life and sometimes are lethal [Citation6]. The rate of cerebral thrombosis and MI was also high, and MIs or multisite thrombosis had worse OS. Renal damage is another important complication in less than 20% of patients, although most renal deficiencies are mild and reversible. Similar to patients from other centers [Citation14], those with larger PNH clone sizes and BMF/PNH had a higher chance of renal deficiency, probably due to the more severe hemoglobinuria in the patients with classic PNH and longer exposure to cyclosporin A in the patients with BMF/PNH.
Regarding treatment, most of the treatments are supportive or immunosuppressive agents for BMF. For patients received IST, those who responded had smaller PNH clone size at diagnosis than those who did not respond. Previous studies demonstrated that the presence of a PNH clone predicted a better hematological response in patients with SAA [Citation31]. AA patients with either an aggressive or stable evolution pattern can achieve a response [Citation32]. Our study is different because we only compared the PNH clone size at diagnosis. Those with smaller clone size may have more severe bone marrow failure and may benefit more from IST. We also noticed that although no significant change in clone size for those who responded or not responded, those who relapsed to IST increased in their clone size during follow-up. It has been reported that those with small initial PNH clones and those who responded to high-dose cyclophosphamide therapy had lower risk of developing clinically significant PNH [Citation33], which supported our findings to some extent. Most of the patients in our cohort had large PNH clone size, it may cause some bias, however.
Allogeneic transplantation was very rare at our center. We did not proceed any transplantations for the recent 5 years due to the hospital construction. On the other hand, most of the patients can live well with the present therapy and we transferred those in need to other centers which focus more on transplantations. Recently, we described the current situation of PNH transplantation in China and showed that the number of PNH transplantation increased rapidly, especially for those with haploid-identified donors [Citation34].
Thrombosis is an indicator of a lower OS, however, only 41.3% of the patients with thrombosis received anticoagulants. Since complement inhibitors are not available in China, steroids are widely used in China to control hemolysis. Therefore, glucocorticoid-related side effects occurred in 3.9% of patients, and some of them were even lethal. However, the effects are limited, and long-term medication should be avoided.
Because of PNH, very few women can give birth successfully. The rate of miscarriage or abortion was up to 50%, which was much higher than that reported in eculizumab-treated pregnant women [Citation35,Citation36], indicating the limitation of current treatments for pregnancy support.
The difference in clone size may also explain the higher rate of clone evolution in BMF/PNH than classic PNH, as shown in our previous paper, that gene mutations related to adverse clone evolution dropped when PNH clone expanded [Citation37]. It is also reported that only 2% of AA/PNH patients develop AML, whereas up to 15% of AA patients who do not have PNH clones develop AML in long-term follow-up [Citation38]. Therefore, the PIGA mutation is considered a self-rescue of BMF, preventing patients not only from hematopoiesis failure but also from evolving to high-risk MDS or AML [Citation39]. All the clone evolutions happened in patients with BMF/PNH in our study, including evolution into MDS/RAEB2, AML, ET and MPN, but the percentage was lower, compared with those with AA without PNH clones (9.8%) [Citation40]. Even though, it would be important to monitor patients over time even if they are ‘hematological stable’, to catch early progression to MDS/AML.
Similar to other reports [Citation1,Citation11,Citation13], the main causes of deaths were thrombosis, hemorrhage, infections, and anemia-related heart failure; very few deaths were from progression to MDS or leukemia, tumors and unknown reasons. Although some differences were found in OS between the patients with BMF/PNH and classic PNH, the differences may mainly originate from the patients with MDS/PNH. Our data also demonstrated that the major causes of deaths shift from infections and bleedings caused by BMF to thrombosis, like reported recently by Fu R et al. [Citation14], and require more effective treatment (like complement inhibitors) in the future.
With the rapid progress in complement treatment, patients in China may have a chance for access. Newly 2 clinical trials (crovalimab, iptacopan) for patients with classic PNH are ongoing in China, and one patient with life threatening classic PNH were treated with iptacopan through expanded compassionate use since June 2021. With large number of patients and high proportion of classic PNH, our study may serve as a kind of disease natural history control for future complement inhibitors era, especially for patients in China.
Acknowledgements
Yali Du performed the research and wrote the paper, Yuan Yang and Chen Yang helped collect the data, Miao Chen and Bing Han designed the research study and corrected the paper.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- de Latour RP, Mary JY, Salanoubat C, et al. Paroxysmal nocturnal hemoglobinuria: natural history of disease subcategories. Blood. 2008;112:3099–3106.
- Schrezenmeier H, Muus P, Socié G, et al. Baseline characteristics and disease burden in patients in the international paroxysmal nocturnal hemoglobinuria registry. Haematologica. 2014;99:922–929.
- Takeda J, Miyata T, Kawagoe K, et al. Deficiency of the GPI anchor caused by a somatic mutation of the PIG-A gene in paroxysmal nocturnal hemoglobinuria. Cell. 1993;73:703–711.
- Brodsky RA, Mukhina G, Li S, et al. Improved detection and characterization of paroxysmal nocturnal hemoglobinuria using fluorescent aerolysin. Am J Clin Pathol. 2000;114:459–466.
- Hansen DL, Moller S, Andersen K, et al. Increasing incidence and prevalence of acquired hemolytic anemias in Denmark, 1980–2016. Clin Epidemiol. 2020;12:497–508.
- Hillmen P, Lewis SM, Bessler M, et al. Naturnal history of paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1995;333:1253–1258.
- Rosse WF. Epidemiology of PNH. The Lancet. 1996;348:560.
- International PNH Interest Group (IPIG). International PNH interest group. Available from: https://www.pnhinterestgroup.org/
- Kim JS, Jang JH, Yoon S-S, et al. Distinct subgroups of paroxysmal nocturnal hemoglobinuria(PNH) with cytopenia: results from South Korean National PNH registry. Ann Hematol. 2016;95:125–133.
- Ueda Y, Obara N, Yonemura Y, et al. Correction to: effects of eculizumab treatment on quality of life in patients with paroxysmal nocturnal hemoglobinuria in Japan. Int J Hematol. 2018;108:233–235.
- Villegas A, Núñez R, Gaya A, et al. Presence of acute and chronic renal failure in patients with paroxysmal nocturnal hemoglobinuria results of a retrospective analysis from the Spanish PNH registry. Ann Hematol. 2017;96:1727–1733.
- Füreder W, Sperr WR, Heibl S, et al. Prognostic factors and follow-up parameters in patients with paroxysmal nocturnal hemoglobinuria (PNH) experience of the Austrian PNH network. Ann Hematol. 2020;99:2303–2313.
- Ge M, Li X, Shi J, et al. Clinical features and prognostic factors of Asian patients with paroxysmal nocturnal hemoglobinuria: results from a single center in China. Ann Hematol. 2012;91:1121–1128.
- Fu R, Li L, Liu H, et al. Analysis of clinical characteristics of 92 patients with paroxysmal nocturnal hemoglobinuria: A single institution experience in China. J Clin Lab Anal. 2020;34:e23008.
- Yu F, Du Y, Han B. A comparative analysis of clinical characteristics of patients with paroxysmal nocturnal hemoglobinuria between Asia and Europe/America. Int J Hematol. 2016;103:649–654.
- Brodsky RA. How I treat paroxysmal nocturnal hemoglobinuria. Blood. 2009;113:6522–6527.
- Hillmen P, Young NS, Schubert J, et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2006;355:1233–1243.
- Brodsky RA, Young NS, Antonioli E, et al. Multicenter phase 3 study of the complement inhibitor eculizumab for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Blood. 2008;111:1840–1847.
- Kelly RJ, Hill A, Arnold LM, et al. Long-term treatment with eculizumab in paroxysmal nocturnal hemoglobinuria: sustained efficacy and improved survival. Blood. 2011;117:6786–6792.
- Krauss JS. Laboratory diagnosis of paroxysmal nocturnal hemoglobinuria. Ann Clin Lab Sci. 2003;33:401–406.
- Lin G. Paroxysmal nocturnal hemoglobinuria. Chin J Postgraduates Med. 2006;29(31):10–12. doi:10.3760/cma.j.issn.1673-4904.2006.31.004.
- Zhao X, Yin X, Zhang L. The epidemiology of the paroxysmal nocturnal hemoglobinuria of Mudanjiang district surveied in ten years. J Mudanjiang Med College. 1997;3:5–7.
- Schrezenmeier H, Röth A, Araten DJ, et al. Baseline clinical characteristics and disease burden in patients with paroxysmal nocturnal hemoglobinuria (PNH): updated analysis from the International PNH registry. Ann Hematol. 2020;99:1505–1514.
- Urbano-Ispizua Á, Muus P, Schrezenmeier H, et al. Different clinical characteristics of paroxysmal nocturnal hemoglobinuria in pediatric and adult patients. Haematologica. 2017 Mar;102(3):e76–e79.
- Hill A, Kelly RJ, Hillmen P. Thrombosis in paroxysmal nocturnal hemoglobinuria. Blood. 2013;121:4985–4996. quiz 5105.
- Dragoni F, Iori AP, Pignoloni P, et al. Thrombophilic screening in patients with paroxysmal nocturnal haemoglobinuria: a pilot study. Br J Haematol. 2010;150:492–494.
- Munoz-Linares C, Ojeda E, Fores R, et al. Paroxysmal nocturnal hemoglobinuria a single Spanish center’s experience over the last 40 yr. Eur J Haematol. 2014;93:309–319.
- Mercier T, Devos T, Mukovnikova M, et al. Diagnosing nocturnal paroxysmal hemoglobinuria a single-center 4-year experience. Int J Lab Hematol. 2017;39:329–336.
- Kim JS, Jo D-Y, Jang JH. Distinct subgroups of paroxysmal nocturnal hemoglobinuria (PNH) with cytopenia results from South Korean National PNH registry. J Korean Med Sci. 2016;31:214–221.
- Ninomiya H, Obara N, Chiba S, et al. Interim analysis of post-marketing surveillance of eculizumab for paroxysmal nocturnal hemoglobinuria in Japan. Int J Hematology. 2016;104:548–558.
- Zhao X, Zhang L, Jing L, et al. The role of paroxysmal nocturnal hemoglobinuria clones in response to immunosuppressive therapy of patients with severe aplastic anemia. Ann Hematol. 2015;94:1105–1110.
- Lian Y, Shi J, Nie N, et al. Evolution patterns of paroxysmal nocturnal hemoglobinuria clone and clinical implications in acquired bone marrow failure. Exp Hematol. 2019;77:41–50.
- Pu JJ, Mukhina G, Wang H, et al. Natural history of paroxysmal nocturnal hemoglobinuria clones in patients presenting as aplastic anemia. Eur J Haematol. 2011;87:37–45.
- Du Y, Han B. Advances in hematopoietic stem cell transplantation for patients with paroxysmal nocturnal hemoglobinuria. Transplant Cell Ther. 2021;27:301–307.
- Kelly RJ, Hochsmann B, Szer J, et al. Eculizumab in pregnant patients with paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2015;373:1032–1039.
- Hallstensen RF, Bergseth G, Foss S, et al. Eculizumab treatment during pregnancy does not affect the complement system activity of the newborn. Immunobiology. 2015;220:452–459.
- Chen F, Hu S, Ruan J, et al. Mutational landscape and its clinical significance in paroxysmal nocturnal hemoglobinuria. Blood Cancer J. 2021;11:58.
- Sun L, Babushok DV. Secondary myelodysplastic syndrome and leukemia in acquired aplastic anemia and paroxysmal nocturnal hemoglobinuria. Blood. 2020;136:36–49.
- Malcovati L, Cazzola M. The shadowlands of MDS idiopathic cytopenias of undetermined significance (ICUS) and clonal hematopoiesis of indeterminate potential (CHIP). Hematology Am Soc Hematol Educ Program. 2015;2015:299–307.
- Fattizzo B, Ireland R, Dunlop A, et al. Clinical and prognostic significance of small paroxysmal nocturnal hemoglobinuria clones in myelodysplastic syndrome and aplastic anemia. Leukemia. 2021;35:3223–3231.
