ABSTRACT
Objectives: Venetoclax combinations are a new standard for patients with acute myeloid leukemia (AML). We aimed to evaluate the safety and efficacy of these combinations in a period of accelerated approval in Latin-America.
Methods: This observational study evaluated adults with acute myeloid leukemia who received venetoclax-based therapy in 11 public or private centers in Mexico and Peru for both newly diagnosed or relapsed and refractory AML.
Results: Fifty patients were included; 28 with newly diagnosed (ND) AML and 22 with relapsed/refractory (RR) disease. ND patients were older (64 vs. 40 years; p < 0.001) with a lower functional capacity (ECOG ≥2 64.3% vs 9%; p < 0.001). Venetoclax was frequently combined with azacytidine (60%) and prophylactic azoles (82%) with a median maximum dose of 200 mg (range, 100–600 mg). Hematologic toxicities were common. Complete response rates including patients with incomplete hematopoietic recovery were 78.6% in ND and 45.5% in RR patients, with a median overall survival of 9.6 (95% CI 3.7–15.5) and 8 months (95% CI 4.8–11.2).
Discussion: Our study showed a preferred use of venetoclax plus azacytidine over cyatrabine. Patients in the first-line setting were similar to those in the landmark studies, while most patients with relapsed disease had received prior intensive therapies. Responses were favorable, with a median survival in agreement to other reports, albeit shorter than that observed in the randomized phase-3 trials.
Conclusion: Venetoclax-based therapy in AML was effective despite dose reductions and prophylactic antifungals in two middle-income countries outside of a clinical trial setting.
1. Introduction
Acute myeloid leukemia (AML) has a dismal prognosis in adults who are not candidates for intensive induction chemotherapy due to coexisting comorbidities or a decreased functional capacity, frequently in the context of advanced age. Low-dose cytarabine (LDAC) and hypomethylating agents are reduced-intensity treatment options for this patient population; however, both have a low probability of complete response (CR) with an overall survival (OS) of 6–12 months [Citation1,Citation2]. Similarly, patients who are refractory or relapse after intensive chemotherapy also have dismal outcomes with long-term survival conditioned by achieving remission followed by allogeneic hematopoietic stem cell transplant (HSCT), which remains the only potentially curative therapy in this context [Citation3,Citation4].
Venetoclax, a selective Bcl-2 inhibitor, limits the stimulation of cell proliferation by sequestering pro-apoptotic proteins promoting cell death and acts synergistically with both hypomethylating agents and cytarabine [Citation5,Citation6]. These drug combinations were evaluated in phase I/II studies in a first-line setting, achieving responses in more than 60% of patients. These studies were followed by early regulatory approval in several countries worldwide, including Mexico and Peru [Citation7,Citation8]. Recently, two randomized Phase 3 clinical trials in a front-line treatment setting showed an OS benefit for venetoclax/azacytidine (Ven/Aza) and venetoclax/low-dose cytarabine (Ven/LDAC) [Citation9,Citation10] in comparison to Aza or LDAC plus placebo, becoming a new standard of care. Experience in patients with relapsed/refractory (RR) AML has also been positive, with CR achieved in approximately 30% of cases without data from randomized clinical trials available in this population [Citation11].
Less is known about the efficacy of Ven-based combinations outside of a clinical trial setting in low- and middle-income countries (LMICs). This multicenter study aimed to determine the patterns of use and practice variations with Ven-based combinations in patients with AML in two Latin American countries and describe the results and outcomes of a patient cohort treated outside of a clinical trial setting in both first line and RR settings.
2. Patients and methods
This study was a multicenter observational registry conducted in 11 public and private centers in Mexico and Peru who are part of the Grupo de Trabajo de Leucemias Agudas (GTLA), a working group of the Mexican national hematology association (Agrupación Mexicana para el Estudio de la Hematología, AMEH). The study was performed from August 2019 to August 2020. Each center contributed data to a prospectively maintained database with information from electronic medical records across institutions with centralized data quality management. Our study was reviewed and approved by local Institutional Review Boards and conducted in accordance with the Declaration of Helsinki.
2.1. Patients
We included patients older than 16 years with a diagnosis of non-promyelocytic AML, either de novo or secondary to treatment or a myelodysplastic/myeloproliferative neoplasm in a first-line or RR setting who received Ven in combination with either hypomethylating agents or LDAC. Patients with newly diagnosed (ND) AML who received Ven-based treatment were not considered candidates for intensive therapy according to the criteria of each center and/or treating physician. Patients with RR disease were included regardless of prior treatment and whether they had received intensive chemotherapy and/or allogeneic or autologous HSCT independently of cell source and conditioning regimen. We excluded patients with incomplete clinical information, alternatively diagnosed, or who did not receive Ven-based therapy. The Hematopoietic Cell Transplantation Comorbidity Index (HCT-CI) [Citation12] at the time of Ven-based treatment was also documented, and the relapse risk was defined according to European Leukemia Net (ELN) 2017 criteria [Citation13]. Given the lack of widespread access to genetic risk assessment in our region, patients with an incomplete evaluation were also documented.
2.2. Treatment and response
The patterns of Ven use were assessed, including a starting dosing ramp, the maximum dose reached, the event of a dose-reduction during treatment, and whether patients received the recommended target dose of Ven (400 mg for Aza and 600 mg for LDAC) in the context of concomitant azole use. Due to the retrospective nature of the study, time dependent Ven exposure measures were not analyzed. Insurance access vs. out-of-pocket treatment coverage was documented. Hematologic toxicities, particularly the incidence of severe or febrile neutropenia, and thrombocytopenia were recorded; packed red blood cell and platelet transfusion events and other Common Terminology Criteria for Adverse Events (CTCAE) Grade 3 or higher toxicities [Citation14].
Treatment response was assessed according to ELN criteria at the end of the first cycle (Day 28) as it was the most consistently studied timepoint across centers. Response assessments were performed locally and were not centrally evaluated. Measurable residual disease was not included in the analysis due to variations in the methods between centers. Relapse or progression and subsequent treatments were noted. Event-free and overall survival probabilities were defined according to ELN 2017 recommendations [Citation13].
2.3. Statistical analysis
Central tendency measures were used to describe the data. The chi-square test was performed to compare categorical variables. For quantitative variables, Student’s t-test or the Mann Whitney U test were performed according to the Kolmogorov–Smirnov test. Survival analysis was determined using the Kaplan-Meier method without censoring for transplantation. The log-rank test was used to compare groups. A univariate Cox proportional hazards regression analysis was performed to assess the influence of several variables known to be associated with EFS and OS. A multivariate analysis was not attempted, given the small sample size and number of events. Statistical analysis was performed with SPSS version 25 for Mac.
3. Results
3.1. Baseline characteristics
Fifty patients were included in the study: 28 with ND AML and 22 with RR disease. Baseline characteristics are shown in . Patients who were treated in the first-line setting were older (median age 64 vs. 40 years; p < 0.001), had a lower functional capacity (Eastern Cooperative Group Score [ECOG] score ≥2 in 64.3 vs. 9%; p < 0.001), and a higher proportion of secondary AML (39.2% vs. 9%; p = 0.04) with non-significant differences in comorbidities (HCT-CI score ≥2 28.6% vs. 9%; p = 0.087). Most patients treated for RR disease had received prior intensive therapy (n = 21; 75%), five had received prior HMA with a median of three prior treatment lines (range, 1–4), and only three had undergone HSCT with a median time from diagnosis to treatment of 5.5 months (range, 0.7–69).
Table 1. Baseline characteristics of 50 patients with acute myeloid leukemia treated with venetoclax-based combinations in Latin America.
3.2 Treatment variations
Ven was more frequently combined with Aza (n = 30; 60%) than LDAC (n = 20; 40%) in both first line and RR patient groups (). Ven was started with an initial dosing ramp in 72% and in combination with prophylactic azoles in 82% of patients. Consequently, the median maximum Ven dose reached overall was 200 mg, with 30% of patients receiving a maximum dose of only 100 mg. Patients in a front-line treatment setting received a lower maximum dose than RR patients (200 mg vs. 400 mg; p = 0.02) while patients who received Ven/LDAC received a similar median maximum dose of 200 mg (range, 100–600 mg) vs. 400 mg (range, 100–600 mg) in the Ven/Aza group (p = 0.092). A reduced dose was more frequently prescribed in patients who received prophylactic azoles than not (82.9 vs. 22.2%; p < 0.001), with the maximum dose being 200 mg (range, 100–600 mg) vs. 400 mg (range, 400–600 mg) (p < 0.01). Further dose reductions during follow-up were frequent, occurring in 54% of cases. No dose reduction recommendations were documented for either LDAC or Aza. Over half of patients covered the costs of treatment out-of-pocket (55%, n = 28). Remarkably, patients without insurance coverage were more likely to receive Ven/LDAC than Ven/Aza (80% vs. 40%; p = 0.008), and a maximum Ven dose lower than 400 mg (81.5% vs. 26.1%; p < 0.001), with no significant difference in the frequency of concomitant azole use (61% vs. 39%; p = 0.16).
Table 2. Patterns of treatment, adverse events, and response with venetoclax-based combinations in 50 patients with acute myeloid leukemia.
3.3 Adverse events
Hematologic adverse events were common (), with 80% and 74% of patients requiring red blood cell and platelet transfusions, respectively. Neutropenic fever occurred in 62% of patients during treatment. Notable non-hematologic severe adverse events reported were mostly gastrointestinal, occurring in 12% of cases, including nausea, diarrhea, colitis, and peptic ulcer disease; a case of deep venous thrombosis and a patient who developed hemophagocytic lymphohistiocytosis in the context of infection were also observed. A single early 30-day treatment-related mortality event occurred in a patient with RR disease due to sepsis (2%) with an additional death during the second cycle due to an exacerbation of chronic obstructive pulmonary disease and infection, with a 60-day mortality rate of 4%. Three more patients died during follow-up in remission, one due to sepsis and two cases of invasive aspergillosis; two were still receiving Ven-based therapy.
3.4 Treatment response
All patients treated with Ven as a first line were evaluable for response with a CR/CRi rate of 78.6% (n = 22) and a CR rate of 39.3% (n = 11) after 1 cycle of treatment (). Regarding treatment combinations, 13/18 patients treated with Ven/Aza achieved CR/CRi (72.2%), similarly to patients who received Ven/LDAC 9/10 (90%) (p = 0.4). The proportion of patients who achieved CR/CRi was similar in patients who received a lower Ven dose or prophylactic azoles than those who did not (79.2 vs. 75% in both; p = 0.85). The median maximum dose of Ven in responders was 200 mg (range, 100–400) vs. 100 mg in those who did achieve CR/CRi (range, 100–400) (p = 0.24). Two patients received a lower Ven dose without prophylactic azoles due to cost constraints, only one responded. Two patients achieved a morphologic leukemia-free state after the first cycle, and two were refractory. All continued to receive Ven-based treatment for at least a second cycle, with a median number of 2.5 cycles received (range, 1–5), but only one gained CR after cycle 2. All others experienced disease progression during treatment and had a fatal outcome. Responding patients (n = 23) who received a median of 3 cycles (range, 1–17), 17.4% relapsed during treatment after a median of 5 months of remission (range, 3.4–6.2). Two patients underwent haploidentical transplantation while n = 19 continued in remission and under treatment at the last evaluation.
Regarding RR patients, CR/CRi was achieved in 45.5% (n = 10), with 36.4% CR (n = 8). Most patients who achieved CR/CRi had received Ven/LDAC (n = 7; 70%), and conversely, only 25% of patients who received Ven/Aza achieved CR/CRi (p = 0.035). Similarly, in patients treated in the first line, the CR/CRi proportion was comparable regardless of receiving a lower Ven dose (50% vs. 40%; p = 0.85). The maximum Ven dose was 300 mg in patients who achieved CR/CRi (range, 100–600) vs. 400 mg in those who did not (range, 100–400) (p = 0.55). One patient achieved a partial response, while 11 did not respond (50%) regardless of continued treatment with a median of 2 cycles administered (range, 1–3). Five of 12 patients (41.7%) who did not achieve CR/CRi went on to receive palliative care, while n = 7 received further chemotherapy including allogeneic transplantation (n = 5), and three remained in remission. On the other hand, patients who achieved CR/CRi (n = 10) received a median of 2 cycles (range, 1–6), 60% received allo-HSCT and continue to be in CR, while 20% continued in remission with Ven; 20% relapsed during follow-up in a median of 4.6 months (2.8–6.4) with fatal outcomes shortly after the events.
3.5 Survival outcomes
Overall, ND patients had a median OS of 9.6 months (95% CI 3.7–15.5) and an EFS of 6.2 months (95% CI 2.5–9.9). Patients with front-line treatment with Ven/Aza had a median OS of 9.6 months (95% CI 4.2–15) and an EFS of 5.6 months (95% CI 0.3–10.8), while patients treated with Ven/LDAC had not-reached a median OS and a 6.2-month EFS (95% CI 6.1–6.3) without a significant difference between the groups (). Patients who achieved CR/CRi did not reach the median OS vs. 6.3 months (95% CI 2.1–10.5) for those who did not. Patients with RR disease had a median OS of 8 months (95% CI 4.8–11.2) and an EFS of only 2 months (95% CI 0.3–3.6). According to the Ven combination in RR patients, no differences were observed in EFS or OS (). In patients who CR/CRi was achieved, the median OS was not reached vs. 6 months (95% CI 4–8) in those who did not respond. Furthermore, no differences in outcomes were observed in patients with secondary AML or across ELN risk groups (data not shown). Remarkably, patients who received a maximum dose ≤ 200 mg had a similar OS () and EFS in both ND and RR patient groups. Patients with higher HCT-CI scores or who received multiple treatment lines after relapse had worse outcomes. However, interestingly patients who received Ven combinations a first-line therapy had worse OS than those with 1 or 2 prior lines of treatment (). The most common cause of death during Ven treatment was sepsis (n = 8), followed by cerebral hemorrhage in the context of progressive disease (n = 2). Univariate analysis for EFS and OS revealed prior treatment lines as a predictor for a worse OS with an HCT-CI score ≥2 having a trend towards significance; conversely, a diagnosis of secondary AML, RR disease, ECOG score, out-of-pocket payment, and a Ven dose ≤200 mg did not ().
Figure 1. Outcomes of patients with newly diagnosed AML treated with venetoclax-based combinations. Panel A: Overall survival. Panel B: Event-free survival.
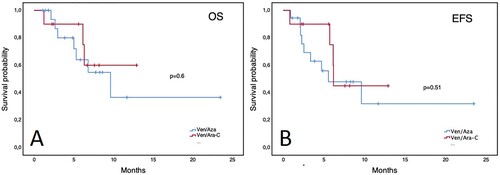
Figure 2. Outcomes of patients with relapsed/refractory AML treated with venetoclax-based combinations. Panel A: Overall survival. Panel B: Event-free survival.
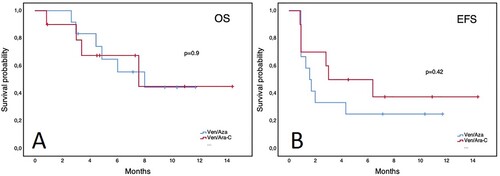
Figure 3. Overall survival of patients with AML according to maximum venetoclax dose reached. Panel A: newly diagnosed patients. Panel B: relapsed/refractory patients.
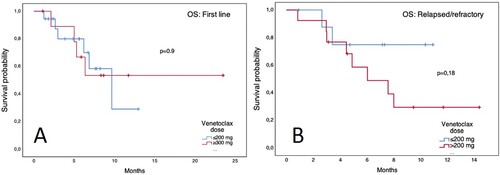
Figure 4. Overall survival of patients with AML treated with venetoclax-based combinations. Panel A. Prior treatment lines. Panel B. Hematopoietic cell transplantation comorbidity index in newly diagnosed (ND) and relapsed/refractory (RR) patients.
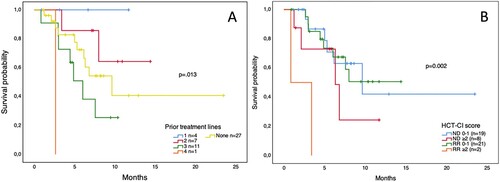
Table 3. Univariate analysis for event-free survival and overall survival in 50 patients with acute myeloid leukemia who received venetoclax-based treatment.
4. Discussion
Our study shows that in two middle-income countries in Latin America, outside of a clinical trial setting, the combinations Ven/Aza and Ven/LDAC are both useful in ND and RR AML. This study was developed due to concerns that the purported benefit of Ven-based therapy based on promising but uncontrolled phase II data supported by early regulatory approval [Citation7,Citation8] would not translate to our reality. VIALE-A and VIALE-C randomized controlled trials in ND patients judged unfit for intensive induction chemotherapy have proven that adding Ven offers a survival advantage over Aza and LDAC plus placebo [Citation9,Citation10]. As observed in these landmark trials, patients in our study who received Ven-based first-line treatment were older, had more comorbidities, and lower functional capacity. We observed a higher incidence of grade ≥3 hematologic adverse events than those reported in both randomized trials with almost universal neutropenia (vs. 46% and 42% in VIALE-C and A, respectively), anemia in 80% (vs. 22% and 26%), and thrombocytopenia of 76% (vs. 45% in both), with a similar proportion of AEs in both ND and RR patients [Citation9,Citation10,Citation15]. Similar observations have been reported outside of a clinical trial setting [Citation16,Citation17]. We also show that 82% of patients treated in our multicenter cohort ultimately received low doses of Ven plus HMA, with a median maximum dose of 200 mg overall, usually due to its combination with prophylactic azoles. A previous retrospective experience in MD Anderson reported a similar duration of neutropenia regardless of azole exposure with a 6-day delay in platelet recovery [Citation12]. Similar results were reported in the Mayo Clinic, where the median dose of Ven was 150 mg without a compromise in responses [Citation18]. In VIALE-A, of 431 patients enrolled, only 41 and 19 patients in the treatment and placebo arms received CYP3A inhibitors, respectively, without observing a difference in response rates or outcomes [Citation19]. These experiences are like ours since responses in front-line treatment were remarkable with a 78.6% CR/CRi rate. We observed a median OS of 9.6 months compared to 14.7 months in the VIALE-A and 8.4 months in VIALE-C. Other single-center experiences in high-income countries report comparable survival rates ranging from 10–12 months [Citation16,Citation18,Citation20,Citation21]. Interestingly, Ven-Aza was slightly preferred over LDAC in 60% of cases even in our limited-resource context, perhaps due to the impression of the superiority of Aza over LDAC; however, Ven-based regimens have not been compared head-to-head. Considering the caveat of publication bias, reports of ‘real world’ use highlight the preferred use of Aza over LDAC in general [Citation15–18,Citation20–30]. We did not observe large differences in outcomes between combinations in contrast to other reports [Citation27], and importantly, LDAC is significantly less expensive than Aza in Peru (100 vs. 880 USD, per cycle, respectively), but even more so in Mexico (300 vs. 6,500 USD per cycle) where no Aza generics are available. Thus, Ven/LDAC was the preferred combination in 80% of patients without insurance coverage. Consequently, it is important to establish whether Ven/Aza is truly superior to Ven/LDAC in randomized trials.
Subgroup analysis was limited by our small sample size and lack of genetic risk assessment, as 42.9% of our patients could not be stratified according to ELN 2017 criteria (). Others have described NPM1, IDH, SRSF2 mutations as favorable and TP53, FLT3, PTPN11 mutations, a monocytic morphology, and the presence of measurable residual disease as unfavorable outcome predictors [Citation31–34]. Patients with RR disease were younger, most having been failed by prior 7 + 3 and high dose cytarabine containing regimens. Interestingly, there is no randomized trial for Ven use in RR AML. A metanalysis of 6 observational studies and 1 phase I/II clinical trial reported an ORR of 38.7% with Ven/Aza or LDAC similar to our 45.5% and median OS of 3–6 months which have, in comparison to 8 months, possibly been associated with our younger population with increased access to HSCT [Citation11]. Comparable outcomes have been obtained by more recent reports in this population [Citation16–18,Citation22,Citation23,Citation25,Citation28,Citation30]. In aggregate, these studies show that while Ven-based combinations are not in themselves curative, some patients can achieve long-term responses despite being refractory to other treatments. Others can eventually receive transplantation and have favorable outcomes obtaining a second chance for cure [Citation29]. On the other hand, patients who relapse after Ven combinations either in front-line treatment or in RR AML have dismal outcomes [Citation24]. In our setting, most patients with RR disease had already received multiple treatment lines, mostly intensive chemotherapy. Whether the use of Ven-based combinations can substitute (or add to) high-dose cytarabine-containing regimens in a second-line setting should also be assessed in a randomized fashion.
This study is limited by its retrospective nature, the lack of a control group, heterogeneity in patient evaluation across centers and regions, the lack of a centralized and blinded response assessment, and the limited availability of dosing data. It is important to note a lack of information from LMICs, currently limited to case reports and a single multicenter experience in China in 48 patients with RR disease [Citation15]. In our context, 54% of the population covered the cost of treatment out of pocket, with Ven priced at 7,250 USD in Mexico and 8,650 USD in Peru per month at 400 mg per day dosing. In a single treatment cycle, these prices surpass the average yearly household income of the population in both Mexico (2,755 USD) and Peru (4,660 USD) and are, therefore, unaffordable for most [Citation35,Citation36]. Remarkably, even in the United States, the use of Ven/Aza has been reported as not cost-effective, with an incremental cost-effectiveness ratio of 260,343 USD per quality-adjusted-life-years gained, exceeding the local willingness-to-pay the threshold of 150,000 USD, where generic Aza is available but Ven costs 12,000 USD per month [Citation37].
Consequently, the use of lower doses plus azoles regardless of the neutrophil count in combination with LDAC or generic Aza as a less expensive regimen could be explored. The cost of Ven can be reduced by 75% with a dose of 100 mg per day in combination with an azole antifungal. Notably, no pharmacokinetic data for azoles other than posaconazole have been published [Citation38] and is an area of opportunity for further research. An alternative to dose reduction could be the use of shorter treatment cycles, as 69% of responding patients in the VIALE-A study ultimately received ≤21-day cycles without an apparent impact on outcomes [Citation39].
In conclusion, Ven-based therapy was effective in patients with AML in the ND and RR setting in a real-world multicenter experience of Latin American countries, with similar response rates, increased toxicity, and a decreased OS in comparison to patients enrolled in the landmark VIALE-A clinical trial. This finding is in agreement with other reports in high-income countries, despite the frequent use of azoles and a lower Ven dose.
Acknowledgment
The authors would like to thank Sergio Lozano for his kind review of our manuscript
Disclosure statement
David Gómez-Almaguer: honoraria, advisory support for attending meetings and/or travel, from AbbVie, Bristol Myers Squibb. Andrés Gómez-De León: honoraria, advisory board from AbbVie. Roberta Demichelis-Gómez: honoraria, advisory board from AbbVie and Bristol Myers Squibb. Yvette Neme-Yunes: honoraria, support for attending meetings and/or travel AbbVie and Bristol Myers Squibb. Margarita Rodríguez-Mejorada: honoraria, support for attending meetings and/or travel from AbbVie, Bristol Myers Squibb, Adrian Ceballos-López: honoraria, support for attending meetings and/or travel from Abbvie, Bristol Myers Squibb.
References
- Dombret H, Seymour JF, Butrym A, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015;126(3):291–299.
- Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30(21):2670–2677.
- DeWolf S, Tallman MS. How I treat relapsed or refractory AML. Blood. 2020;136(9):1023–1032.
- Ganzel C, Sun Z, Cripe LD, et al. Very poor long-term survival in past and more recent studies for relapsed AML patients: The ECOG-ACRIN experience. Am J Hematol. 2018;93(8):1074–1081.
- Bogenberger JM, Delman D, Hansen N, et al. Ex vivo activity of BCL-2 family inhibitors ABT-199 and ABT-737 combined with 5-azacytidine in myeloid malignancies. Leuk Lymphoma. 2015;56(1):226–229.
- Niu X, Zhao J, Ma J, et al. Binding of released Bim to Mcl-1 is a mechanism of intrinsic Resistance to ABT-199 which can be overcome by combination with daunorubicin or cytarabine in AML cells. Clin Cancer Res. 2016;22(17):4440–4451.
- DiNardo CD, Pratz KW, Letai A, et al. Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: a non-randomised, open-label, phase 1b study. Lancet Oncol. 2018;19(2):216–228.
- Wei AH, Strickland SA, Jr., Hou JZ, et al. Venetoclax combined with low-dose cytarabine for previously untreated patients with acute myeloid leukemia: results from a phase Ib/II study. J Clin Oncol. 2019;37(15):1277–1284.
- DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617–629.
- Wei AH, Montesinos P, Ivanov V, et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: a phase 3 randomized placebo-controlled trial. Blood. 2020;135(24):2137–2145.
- Bewersdorf JP, Giri S, Wang R, et al. Venetoclax as monotherapy and in combination with hypomethylating agents or low dose cytarabine in relapsed and treatment refractory acute myeloid leukemia: a systematic review and meta-analysis. Haematologica. 2020;105(11):2659–2663.
- Savic A, Kvrgic V, Rajic N, et al. The hematopoietic cell transplantation comorbidity index is a predictor of early death and survival in adult acute myeloid leukemia patients. Leuk Res. 2012;36(4):479–482.
- Dohner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–447.
- Institute NC. Common terminology criteria for adverse events: (CTCAE) Version 5.0. US Department of Health and Human Services; 2017.
- Lou Y, Shao L, Mao L, et al. Efficacy and predictive factors of venetoclax combined with azacitidine as salvage therapy in advanced acute myeloid leukemia patients: a multicenter retrospective study. Leuk Res. 2020;91:106317.
- Feld J, Tremblay D, Dougherty M, et al. Safety and efficacy: clinical experience of venetoclax in combination with hypomethylating agents in both newly diagnosed and relapsed/refractory advanced myeloid malignancies. Hemasphere. 2021;5(4):e549.
- Gaut D, Burkenroad A, Duong T, et al. Venetoclax combination therapy in relapsed/refractory acute myeloid leukemia: A single institution experience. Leuk Res. 2020;90:106314.
- Morsia E, McCullough K, Joshi M, et al. Venetoclax and hypomethylating agents in acute myeloid leukemia: Mayo Clinic series on 86 patients. Am J Hematol. 2020;95(12):1511–1521.
- Jonas B, Dinardo CD, Fracchiolla N, et al. CYP3A inhibitors and impact of these agents on outcomes in patients with acute myeloid leukemia treated with venetoclax plus azacitidin on the VIALE-A study. ASH Annual Meeting and Exposition: Blood. 2020;136(Suppl. 1):50–52.
- Winters AC, Gutman JA, Purev E, et al. Real-world experience of venetoclax with azacitidine for untreated patients with acute myeloid leukemia. Blood Adv. 2019;3(20):2911–2919.
- Apel A, Moshe Y, Ofran Y, et al. Venetoclax combinations induce high response rates in newly diagnosed acute myeloid leukemia patients ineligible for intensive chemotherapy in routine practice. Am J Hematol. 2021;96(7):790–795.
- Byrne M, Danielson N, Sengsayadeth S, et al. The use of venetoclax-based salvage therapy for post-hematopoietic cell transplantation relapse of acute myeloid leukemia. Am J Hematol. 2020;95(9):1006–1014.
- Ganzel C, Ram R, Gural A, et al. Venetoclax is safe and efficacious in relapsed/refractory AML. Leuk Lymphoma. 2020;61(9):2221–2225.
- Maiti A, Rausch CR, Cortes JE, et al. Outcomes of relapsed or refractory acute myeloid leukemia after frontline hypomethylating agent and venetoclax regimens. Haematologica. 2021;106(3):894–898.
- Piccini M, Pilerci S, Merlini M, et al. Venetoclax-based regimens for relapsed/refractory acute myeloid leukemia in a real-life setting: a retrospective single-center experience. J Clin Med. 2021;10(8):1684.
- Rausch CR, DiNardo CD, Maiti A, et al. Duration of cytopenias with concomitant venetoclax and azole antifungals in acute myeloid leukemia. Cancer. 2021;127(14):2489–2499.
- Stahl M, Menghrajani K, Derkach A, et al. Clinical and molecular predictors of response and survival following venetoclax therapy in relapsed/refractory AML. Blood Adv. 2021;5(5):1552–1564.
- Tenold ME, Moskoff BN, Benjamin DJ, et al. Outcomes of adults with relapsed/refractory acute myeloid leukemia treated with venetoclax plus hypomethylating agents at a comprehensive cancer center. Front Oncol. 2021;11:649209.
- Zappasodi P, Brociner M, Merati G, et al. Venetoclax and azacytidine combination is an effective bridge to transplant strategy in relapsed/refractory acute myeloid leukemia patients. Ann Hematol. 2021;100(4):1111–1113.
- Zucenka A, Pileckyte R, Trociukas I, et al. Outcomes of relapsed or refractory acute myeloid leukemia patients failing venetoclax-based salvage therapies. Eur J Haematol. 2021;106(1):105–113.
- Pei S, Pollyea DA, Gustafson A, et al. Monocytic subclones confer resistance to venetoclax-based therapy in patients with acute myeloid leukemia. Cancer Discov. 2020;10(4):536–551.
- Maiti A, DiNardo CD, Wang SA, et al. Prognostic value of measurable residual disease after venetoclax and decitabine in acute myeloid leukemia. Blood Adv. 2021;5(7):1876–1883.
- Chyla B, Daver N, Doyle K, et al. Genetic biomarkers of sensitivity and resistance to venetoclax monotherapy in patients with relapsed acute myeloid leukemia. Am J Hematol. 2018;93(8):E202–E205.
- Lachowiez CA, Loghavi S, Kadia TM, et al. Outcomes of older patients with NPM1-mutated AML: current treatments and the promise of venetoclax-based regimens. Blood Adv. 2020;4(7):1311–1320.
- (INEI) INdEeI. Principales Indicadores Nacionales Lima, Perú2019. Available from: https://www.inei.gob.pe/.
- (OECD) OfEC-oaD. Housold disposable income (Indicator) 2021. Available from: https://data.oecd.org/hha/household-disposable-income.htm.
- Patel KK, Zeidan AM, Shallis RM, et al. Cost-effectiveness of azacitidine and venetoclax in unfit patients with previously untreated acute myeloid leukemia. Blood Adv. 2021;5(4):994–1002.
- Agarwal SK, DiNardo CD, Potluri J, et al. Management of venetoclax-posaconazole interaction in acute myeloid leukemia patients: evaluation of dose adjustments. Clin Ther. 2017;39(2):359–367.
- Pratz KW DC, Selleslag D, Li J, et al. Cytopenia management in patients with newly diagnosed acute myeloid leukemia treated with venetoclax plus azacitidine in the VIALE-A study. Blood. 2020;136:51–53.
