ABSTRACT
Objectives:
We conducted a phase II trial to prospectively evaluate the efficacy and safety of bortezomib-cyclophosphamide-dexamethasone (VCD) induction, autologous stem cell transplantation (ASCT), VCD consolidation, and bortezomib maintenance in transplant-eligible newly diagnosed multiple myeloma (NDMM) patients in Japan (UMIN000010542).
Methods:
From 2013 to 2016, 42 patients with a median age of 58 (range 42–65) years with NDMM were enrolled in 15 centers. The primary endpoint was the complete response (CR) /stringent CR (sCR) rate after transplantation, and overall/progression-free survival rates were also evaluated.
Results:
Following induction therapy, the overall response rate was obtained in 71% of patients, including a CR/sCR of 10% and a very good partial response (VGPR) of 26%. Twenty-six of the 42 patients completed ASCT following the protocol and CR/sCR and VGPR rate 100 days after ASCT was 26% and 17%, respectively. During consolidation therapy, 3 of the 24 patients achieved deeper responses. Eight of the 18 patients completed 2-year bortezomib maintenance without disease progression and grade 3/4 toxicities. Five patients were VGPR or partial response after ASCT but maintained response with 2-year bortezomib maintenance. Two-year overall and progression-free survival rates were 92.5% (95% confidence interval [CI]: 78.5%−97.5%) and 62.6% (95% CI: 45.8%−75.5%), respectively. Grade 3/4 toxicities (≥ 10%) included neutropenia (19%) and anemia (17%) in induction, and thrombocytopenia (29%) in consolidation.
Conclusion:
VCD induction/consolidation and bortezomib maintenance with ASCT for NDMM resulted in a high CR/sCR rate and provided good overall/progression-free survival in Japan.
Introduction
Multiple myeloma is a neoplasm of plasma cells characterized by the presence of monoclonal immunoglobulins in the blood and urine. Autologous stem cell transplantation (ASCT) after induction chemotherapy was performed in newly diagnosed multiple myeloma (NDMM) patients younger than 65 years in the 1990s, and improved outcomes were observed [Citation1–3]. A meta-analysis showed significantly better progression-free survival (PFS) for ASCT [Citation4], which has become the standard of care in NDMM patients younger than 65 years. However, overall survival (OS) after ASCT did not differ from conventional chemotherapy [Citation4], and a further improvement in treatment outcomes is desired.
Recently, in Europe and the United States, new drugs such as thalidomide, lenalidomide, and bortezomib have shown high efficacy and are used for induction therapy. In 2013, when we planned to start the present study, thalidomide and lenalidomide were not approved for NDMM patients in Japan. Bortezomib-cyclophosphamide-dexamethasone (VCD) therapy was approved for NDMM in Japan at that time, and this therapy was reported with good efficacy and low toxicity in the United States and Europe [Citation5–10]. However, few clinical trials evaluated induction/consolidation therapies for transplant-eligible NDMM patients in Japan. Multiple induction therapies were recommended, and it was unclear which one was appropriate to choose.
Thalidomide and lenalidomide have been used for maintenance therapy after ASCT in the United States and Europe [Citation11,Citation12]. However, thalidomide was ineffective for patients with high-risk chromosomal abnormalities [Citation11], and lenalidomide was associated with secondary malignancy [Citation12]. Maintenance therapy with bortezomib was less toxic compared with thalidomide [Citation13], and some reports demonstrated that bortezomib was effective for patients with high-risk chromosomal abnormalities [Citation14].
Recently, polymerase chain reaction (PCR) tests for case-specific immunoglobulin heavy chain (IGH) VDJ sequences have been used to search for minimal residual disease (MRD), and the concept of molecular complete response (mCR) has been proposed [Citation15]. A low relapse rate has been reported in patients who have reached mCR after ASCT, pointing out the importance of reaching mCR [Citation16].
Taking these backgrounds into consideration, we conducted a phase II trial to prospectively evaluate the efficacy and safety of VCD induction, ASCT, VCD consolidation, and bortezomib maintenance therapy. Additionally, we assessed MRD status using an allele-specific oligonucleotide real-time quantitative PCR (RQ-PCR).
Methods
Eligibility
The present study prospectively recruited NDMM patients who were eligible for ASCT between 2013 and 2016. Patients were aged 20–65 years, had symptomatic secretory MM confirmed by the criteria of the International Myeloma Working Group (IMWG), had adequate organ function, had estimated life expectancy of more than 3 months, and showed an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–2. As for renal function, the inclusion criterion was a serum creatinine less than three times the upper limit of the institutional standard. Key exclusion criteria were non-secretary multiple myeloma, plasma cell leukemia, human immunodeficiency virus antibody positive, hepatitis B surface antigen-positive, hepatitis B virus deoxyribonucleic acid positive, hepatitis C virus antibody-positive, known hypersensitivity to mannitol or boron, pregnant or breast-feeding women, active malignancy within last 5 years, serious psychiatric disorders or illness that could potentially interfere with the completion of treatment according to the protocol, active infection or serious comorbid medical condition, interstitial pneumonitis or lung fibrosis by the clinical findings, the abnormal shadow of chest computed tomography, and history of severe hypersensitivity to drugs.
Study design
Patients received 4 cycles of VCD induction therapy with bortezomib 1.3 mg/m2 and cyclophosphamide 300 mg/m2 on days 1, 8, 15, and 22 and dexamethasone 40 mg on days 1–3, 8–10, 15–17, and 22–24 for the first two cycles and day 1, 8, 15, and 22 for the last two cycles of four 28-day cycles. A new cycle was initiated if the neutrophil count was 1000/μL or more, platelet count was 5 × 106/μL or more, and non-hematological adverse events were grade 2 or less. Treatment was discontinued if there was a 3-week delay in the schedule. Bortezomib-associated peripheral neuropathy was managed with the established dose modifications [Citation17]. Acyclovir was recommended during bortezomib treatment. Stem cell harvest was scheduled for patients with PS scores of 0–2 and acceptable organ functions. Patients who had stable disease (SD) or progressive disease (PD) discontinued the protocol. Peripheral blood stem cells were collected after cyclophosphamide 2 g/m2 for 2 days. The target number of CD34-positive cells in peripheral blood stem cell harvest (PBSCH) was 1.0×106 cells/kg or more. After PBSCH, the patients received ASCT with the conditioning of melphalan 200 mg/m2. One hundred days after ASCT, patients with PS scores of 0–2, acceptable organ functions, and without the stable disease (SD) or progressive disease (PD) proceeded to consolidation therapy. Patients received consolidation treatment with 3 cycles of VCD identical to the last 2 cycles of the induction therapy. After consolidation, patients with PS scores of 0–2, acceptable organ functions, and without the stable disease (SD) or progressive disease (PD) proceeded to maintenance therapy with bortezomib 1.3 mg/m2 every 2 weeks for 24 months. Responses were assessed according to IMWG criteria. Briefly, complete response (CR) was defined as negative immunofixation, very good partial response (VGPR) was defined as M-component detectable by immunofixation-negative but not on electrophoresis or greater than 90% reduction of M-protein, and partial response (PR) was defined as a reduction of M-protein ranging between 50 and 90%. Adverse events were reported according to Common Terminology Criteria for Adverse Events (CTCAE) ver. 4.0.
Based on the previous reports [Citation5,Citation7], the expected response rate and the threshold response rate for the present study were set at 30% and 15%, respectively. Assuming a one-tailed test with a significance level of 0.05 and a power of at least 80%, the required number of eligible cases based on the binomial distribution was 43. Dropout after enrollment was estimated at 10%, and the target number of enrolled cases was set at 48.
Evaluation of minimal residual disease
MRD was assessed using an allele-specific oligonucleotide RQ-PCR. Bone marrow specimens at the time of diagnosis are used for the primer settings. For the primer settings, first, cancer-specific IGH was amplified by PCR using the synthesized cDNA as a template. Next, the sequence of the IGH was determined. The reverse primer was set to span the V-D-J junction based on the results of detailed sequence analysis by IgBLAST [Citation18]. Finally, a forward primer for the reverse primer was designed at the Primer3 website [Citation19] and an appropriate one was selected. The probe method described in the Euro-MRD guidelines [Citation20] was used for RQ-PCR. For the quantification of the genomic copy number, the Ribonuclease P RNA Component H1 gene was used, and the plasmid DNA cloned from the gene was used for calibration curve preparation. The copy number of the plasmid DNA used ranged from 1.0 × 102 to –1.0 × 106 copies. The results of quantification, qPCR melting curve, and nested-PCR were used to determine the positivity or negativity of MRD.
Definition of endpoints
OS was defined as the duration from registration to death, and the patients who remained alive at the final follow-up were censored. PFS was defined as the duration from registration to death or progression/relapse, and the patients who remained alive without progression/relapse at the final follow-up were censored. Relapse included clinical relapse and paraprotein relapse. Time to next treatment (TTNT) was defined as the duration from registration to death or starting the next treatment, and the patients who remained alive without starting the next treatment at the final follow-up were censored.
The primary endpoint was CR/sCR rate 100 days after ASCT because this rate was reported to be associated with OS prolongation after ASCT [Citation21]. The secondary endpoints included response rates after induction therapy, PBSCH, ASCT, consolidation therapy, maintenance therapy. OS, PFS, TTNT, and incidence of adverse events were also included in the secondary endpoints.
Statistical analysis
Descriptive statistics were used to summarize variables related to the demographics and clinical characteristics of the patients. Groups were compared using Fisher’s exact test as appropriate for categorical variables. The probabilities of OS, PFS, and TTNT were estimated according to the Kaplan–Meier method, and univariable comparisons among the groups were performed using the log-rank test. Results were expressed as hazard ratios (HR) and their 95% confidence intervals (CI). All tests were two-sided, and a p-value of less than 0.05 was considered to indicate statistical significance. All statistical analyses were performed using Stata (ver. 13.0, Stata corporation) and EZR, a graphical user interface for R (The R Foundation for Statistical Computing, version 2.3.0) [Citation22].
Ethics approval
All patients gave their written informed consents. All methods were performed following the Declaration of Helsinki. The present study was approved by the institutional ethic committee of the Graduate School of Medicine, Kyoto University, and registered in the University hospital Medical Information Network Clinical Trials Registry (UMIN000010542).
Results
Patients’ characteristics
From August 2013 to May 2016, 42 patients with a median age of 58 (range 42–65) years with NDMM were found eligible and enrolled in 15 centers in Japan. Eleven patients had moderate renal impairment, with estimated glomerular filtration rate (eGFR) lower than 60 (26%). The International Staging System (ISS) values were I in 17 (40%), II in 20 (48%), and III in 5 patients (12%). Adverse cytogenetics of del(17p), t(4;14), and t(14;16) in FISH were observed in 20%, 18%, and 3% of the evaluable patients, respectively. Twenty-three percent of the patients had high-risk chromosomal abnormalities (CA) (del(17p) and/or t(4;14) and/or t(14;16)), 62% had standard-risk CA, and 14% had no data on CA. Details of characteristics are listed in . The scheme of the protocol and the number of patients who received induction, stem cell harvest, ASCT, consolidation, and maintenance are shown in .
Figure 1. Scheme of the protocol. NDMM: newly diagnosed multiple myeloma, BM: bone marrow, VCD: bortezomib-cyclophosphamide-dexamethasone, s.c.: subcutaneous, p.o.: per os, SD: stable disease, G-CSF: granulocyte colony-stimulating factor, ASCT: autologous stem cell transplantation.
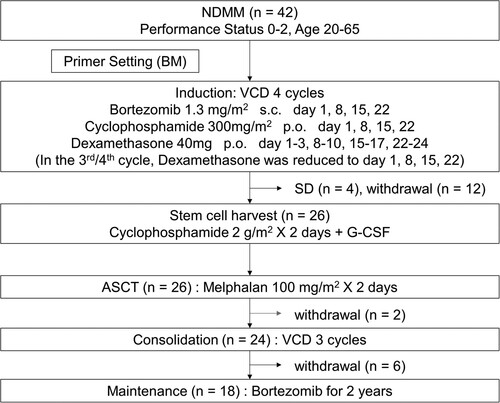
Table 1. Characteristics of the patients.
Induction therapy
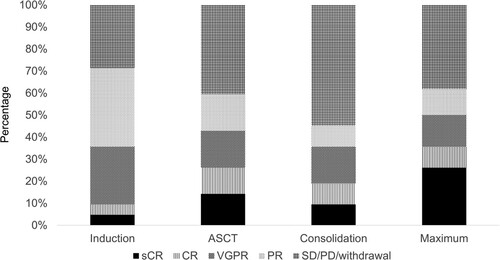
Following 4 induction cycles of VCD, the number of the patients who achieved stringent CR (sCR), CR, VGPR, and PR were 2, 2, 11, and 15, respectively. Four patients were evaluated to be SD after the induction therapy and did not proceed to PBSCH. After the induction therapy, MRD was evaluable in 11 patients, of which one was negative. Four patients discontinued the protocol during the induction therapy because of grade 4 interstitial pneumonia (n = 2), prolonged grade 3 drug eruption (n = 1), and grade 1 delirium (n = 1). Four patients discontinued the protocol due to doctor judgement (repetitive infection, n = 1; grade 3 alanine aminotransferase elevation, n = 1; requests from doctors for reasons other than adverse events, n = 2). Four patients did not proceed to PBSCH due to requests from patients for reasons other than adverse events (n = 2) and deviations from the protocol (n = 2). Among the 11 patients who had moderate renal impairment before induction, the number of the patients who achieved sCR, CR, VGPR and PR after induction were 0, 0, 4 and 3, respectively. The eGFR of these 11 patients improved or remained unchanged during the induction.
Stem cell harvest and autologous transplantation
Twenty-six patients proceeded to PBSCH. Enough CD34-positive cells were collected from all patients, and the number of days required for apheresis ranged from 1 to 3 days. The median number of CD34-positive cells collected was 4.7 × 106 cells/kg (range: 1.6–15.9). No patients required additional apheresis.
Twenty-six patients completed ASCT. The median number of CD34-positive cells infused was 2.5 × 106 cells/kg (range: 1.5–8.9). Engraftment was observed in all patients. One hundred days after ASCT, the number of patients who achieved sCR, CR, VGPR, and PR were 6, 5, 7, and 7, respectively. One patient was evaluated to be PD after ASCT and discontinued the protocol. CR/sCR rate 100 days after ASCT of all patients was 26%. There was no significant difference in CR/sCR rate between the patients with high-risk CA and standard-risk CA (40% vs 23%, p = 0.41). On the other hand, the CR/sCR rate of the patients with ISS I or II was significantly higher than that of the patients with ISS III (30% vs 0%, p = 0.005). Among the 6 patients who had moderate renal impairment before induction and received ASCT, the number of the patients who achieved sCR, CR, VGPR and PR after ASCT were 1, 1, 3 and 1, respectively. After ASCT, MRD was evaluable in 10 patients, of which three were negative. One patient discontinued the protocol after ASCT due to the doctor’s judgement (pre-existing spinal canal stenosis).
Consolidation and maintenance therapy
Twenty-four patients proceeded to consolidation therapy. During the consolidation therapy, 3 patients achieved a deeper response than before. The number of patients who achieved sCR, CR, VGPR, and PR after completing the consolidation was 4, 4, 7, and 4, respectively. After the consolidation therapy, MRD was evaluable in 9 patients, of which three were negative. Five patients discontinued the protocol during the consolidation due to repetitive grade 3 thrombocytopenia (n = 3), doctor’s judgement (prolonged grade 3 hematuria caused by BK virus, n = 1), and a request from a patient for a reason other than adverse events (n = 1). One patient did not proceed to the maintenance therapy due to grade 3 thrombocytopenia.
Eighteen patients received maintenance therapy. During the maintenance therapy, 4 patients achieved a deeper response than before. The number of patients who achieved sCR, CR, VGPR, and PR after completing the 2-year maintenance therapy was 4, 0, 2, and 2, respectively. Five patients were VGPR or PR after ASCT but completed maintenance therapy without progression. Seven patients were evaluated to be PD and discontinued the protocol during the maintenance therapy. After the maintenance therapy, MRD was evaluable in 3 patients, of which one was negative. Three patients discontinued the protocol during the maintenance because of grade 4 stroke (n = 1), doctor’s judgement (grade 2 gastrointestinal pain, n = 1), and a request from a patient for a reason other than adverse events (n = 1). The summary of grade 3/4 adverse events was shown in .
Table 2. Grade 3/4 adverse events.
Maximum response
The number of patients whose maximum response after ASCT was sCR, CR, VGPR, and PR was 11, 4, 6, and 5, respectively. The maximum response of the 16 patients was SD, PD, or withdrawal. Changes in the responses were shown in .
Figure 2. Changes in the responses after the treatments. Responses after induction, ASCT and consolidation, and maximum responses after transplantation. sCR: stringent complete response, CR: complete response, VGPR: very good partial response, PR: partial response, SD: stable disease, PD: progressive disease, ASCT: autologous stem cell transplantation.
Survival and time to next treatment
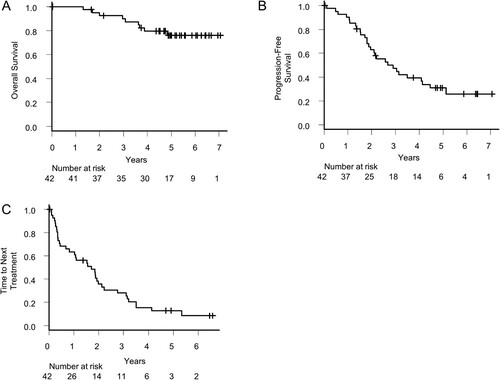
The median duration of observational follow-up among survivors was 5.0 years (range: 0.0–7.1). Median overall survival was not reached during the observation period (A). Median of PFS and TTNT were 2.68 (95% CI: 1.88–4.13) and 1.71 (95% CI: 0.68–1.99), respectively (B,C). Two-year OS, PFS and TTNT were 92.5% (95% CI: 78.5%−97.5%), 62.6% (95% CI: 45.8%−75.5%) and 35.4% (95% CI: 21.1–50.0%), respectively. Five-year OS, PFS and TTNT were 76.0% (95% CI: 58.6–86.9%), 31.0% (95% CI: 17.2–45.8%) and 12.8% (95% CI: 4.7–25.2%), respectively (A–C).
Figure 3. Survival and time to next treatment. (A) Overall survival, (B) Progression-free survival, and (C) Time to next treatment of all patients.
Firstly, we compared the OS and PFS of the patients who had high-risk CA with those who had standard risk CA. Patients with no data on CA were excluded. Five-year OS of the patients with high-risk CA and standard-risk CA was 58.3% (95% CI: 23.0–82.1) and 81.7% (95% CI: 57.9–92.8), respectively (p = 0.12, A). Five-year PFS of the patients with high-risk CA and standard-risk CA was 15.0% (95% CI: 1.0–45.7) and 36.3% (95% CI: 17.7–55.2), respectively (p = 0.43, B).
Figure 4. Comparison of survivals by chromosomal abnormalities and response after transplantation. (A) Overall survival and (B) Progression-free survival of the patients with or without high-risk chromosomal abnormalities. (C) Overall survival and (D) Progression-free survival of the patients whose stages in international staging systems were I/II or III. (E) Overall survival and (F) Progression-free survival of the patients who achieved complete response after transplantation or not.
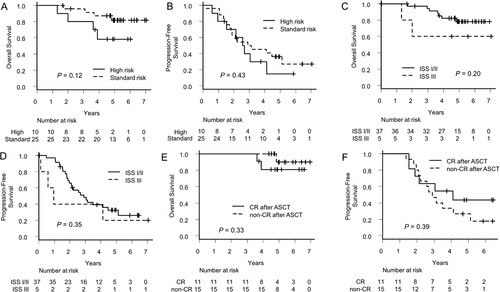
Secondly, we compared the OS and PFS of the patients with ISS I/II with those with ISS III. Five-year OS of the patients with ISS I/II and ISS III was 78.2% (95% CI: 59.3–89.1) and 60.0% (95% CI: 12.6–88.2), respectively (p = 0.20, C). Five-year PFS of the patients with ISS I/II and ISS III was 32.7% (95% CI: 17.7–48.5) and 20.0% (95% CI: 0.8–58.2), respectively (p = 0.35, D).
Thirdly, we compared the OS and PFS of the patients who achieved CR/sCR after ASCT (CR group) and those who did not (non-CR group). Five-year OS of CR group and non-CR group was 80.8% (95% CI: 42.3–94.9) and 90.0% (95% CI: 47.3–98.5%), respectively (p = 0.33, E). Five-year PFS of CR group and non-CR group was 43.6% (95% CI: 14.7–69.9) and 26.7% (95% CI: 8.3–49.6), respectively (p = 0.39, F).
Lastly, we compared the OS and PFS of the patients who showed MRD negativity after ASCT (MRD Negative group) and those who did not (MRD Positive group). There was no significant difference in OS and PFS of the two groups (Supplemental Figure 1A,B).
Discussion
In the present study, we investigated the efficacy and safety of VCD induction/consolidation and bortezomib maintenance therapy for transplant-eligible NDMM patients.
The present study is the first prospective study including bortezomib subcutaneous therapy in Japan because it was approved in December 2012. According to the previous reports, CR/sCR rate and ≥VGPR rate after VCD induction ranged from 3% to 12% and 13% to 61% () [Citation5–10,Citation23]. In the present study, CR/sCR rate and ≥VGPR rate after VCD induction were 10% and 36%, respectively. These results seemed equivalent to the previous studies. During the VCD induction therapy, no patients experienced peripheral neuropathy severer than grade 1, indicating that weekly subcutaneous bortezomib was feasible for Japanese patients. On the other hand, 5% of the patients developed grade 4 interstitial pneumonia, which may be an adverse event that we should be aware of in Japan. The incidences of the other toxicities were equivalent to the previous studies [Citation5,Citation7–10,Citation23].
Table 3. Previously reported VCD induction trials.
There were no prospective head-to-head studies comparing VCD induction with bortezomib-lenalidomide-dexamethasone (VRD) induction. Cost of novel drugs for myeloma is now an important issue, and cyclophosphamide is cheaper than lenalidomide. Moreover, cyclophosphamide can be used for patients with moderate renal failure without dose reduction, while it is difficult to use full dose lenalidomide for them. Prospective studies are needed to determine whether VCD or VRD is better for induction therapy on the aspects of efficacy, toxicity, and cost-effectiveness.
In the present study, 4 g/m2 cyclophosphamide was administered, and no patients withdrew from the study due to adverse events or poor mobilization. Plerixafor plus granulocyte colony-stimulating factor is now one of the standard strategies for stem cell harvest [Citation24], but high-dose cyclophosphamide should be retained because it is inexpensive and can deepen the response of myeloma. In the present study, CR/sCR rate improved from 10% to 26% after ASCT, and toxicity in ASCT was equivalent to the previous study in Japan [Citation25]. This result suggests that ASCT is recommended even in the era of bortezomib-based induction therapy.
During the VCD consolidation therapy, 13% of the patients achieved a deeper response than before but 21% of the patients discontinued the protocol, and 29% of the patients had grade 3 thrombocytopenia. In the report of VTD consolidation therapy in Japan, a 13% increase in CR/sCR rate was observed after consolidation compared to before consolidation, and 4 of the 47 patients dropped out during the consolidation [Citation23]. From these results, the efficacy of the VCD consolidation in the present study seemed equivalent but toxicity seemed worse compared with VTD consolidation. Agents that are less likely to cause thrombocytopenia, such as anti-CD38 antibodies, may be more suitable for consolidation therapy after ASCT.
There are few studies evaluating the benefit of maintenance therapy with bortezomib [Citation26]. The present study was the first study that evaluated the efficacy and toxicity of bortezomib maintenance after ASCT in Japan. Twenty-two percent of the patients achieved deeper responses than before and 44% of the patients completed 2-year maintenance without progression and grade 3/4 toxicities. Five patients were VGPR or PR after ASCT but completed maintenance therapy, suggesting the efficacy of maintenance therapy with bortezomib for high-risk patients. No neuropathy and interstitial pneumonia were observed during the maintenance therapy, indicating that administration of 1.3 mg/m2 bortezomib every 2 weeks could be continued without much concern about accumulated non-hematological toxicities. It was demonstrated that lenalidomide maintenance improved OS and PFS after ASCT [Citation27], but some reports showed that lenalidomide maintenance was not effective for patients with ISS III or high cytogenetic risk [Citation27,Citation28]. A recent report demonstrated that ixazomib maintenance therapy after ASCT improved PFS, and prolonged PFS was observed in patients with high cytogenetic risk [Citation29]. The present study showed some benefit of bortezomib maintenance, which should be further explored.
OS and PFS of the present study were almost equivalent to previous studies for transplant-eligible NDMM patients in Japan [Citation23,Citation25,Citation30]. The protocol of the present study was free from immunomodulatory drugs (IMiDs). Although the data of regimens and efficacies of the salvage therapies were not collected, it was speculated that many relapsed patients achieved deep response with salvage therapies including IMiDs.
It was suggested that bortezomib-based regimens might improve treatment outcomes in patients with t(4;14) [Citation31]. In the present study, the outcomes of the patients with high-risk cytogenetics, such as t(4;14), t(14;16), or del(17p) were not significantly inferior to those of the standard-risk patients. This result indicates that maintenance therapy with bortezomib may be beneficial for high-risk patients. A combination of proteasome inhibitors and anti-CD38 antibodies should be considered to improve efficacy [Citation32,Citation33].
Before the initiation of the induction therapy, MRD by PCR method was measurable in 21 cases. Because of the high number of withdrawals from the protocol, only 11 patients could be evaluated MRD after the induction therapy. Three of the 10 patients were MRD negative after ASCT, and the number was not enough to evaluate the efficacy of MRD negativity. One case was evaluated to be PR despite MRD negativity, and the early relapse was observed in this case. This result suggested that the clone different from the pre-treatment examination may have appeared and exacerbated the disease. In the present study, the value of MRD measurement by the PCR method could not be fully found. In the next study in our group, MRD measurement by multicolor flow cytometry and next-generation sequencing is being conducted (jRCTs051200043).
The present study has several limitations. First, the present study was planned in 2013. Two years after starting the study, the VRD induction regimen was approved for transplant-eligible patients and it has been one of the standard induction therapies in Japan [Citation34]. Some doctors and patients requested conversion to VRD induction and discontinued the protocol during VCD induction. In an era when new drugs appear one after another, it is difficult to conduct prospective trials within insurance coverage. Second, the present study was designed to primarily evaluate the CR/sCR rate after ASCT. A larger prospective study will be required to clarify the benefit of VCD consolidation and bortezomib maintenance.
In conclusion, VCD induction/consolidation therapy with ASCT for NDMM resulted in a high CR/sCR rate in a Japanese population and was feasible for patients with moderate renal impairment. Bortezomib maintenance was beneficial even if patients were VGPR or PR after ASCT. Further prospective studies are needed to determine the optimal treatment for NDMM in Japan.
Author contributions
H.M., J.K., M.K., T.Maeda., T.K., K.Y and A.T-K. designed and organized the project. H.M. and J.K. wrote the manuscript. M.K., T.Maeda, and Y.K. helped to write the manuscript. H.M., J.K., M.T., Y.U., T.I., M.N., M.W., K.I., T.Moriguchi., M.I., H.O., A.Y., H.H., N.Arima., K.A., N.Anzai, Y.K., H.K., and I.K., contributed to data gathering. K.N. performed polymerase chain reaction tests to evaluate minimum residual disease. K.K. and K.U. provided support for designing the research. H.M., S.T., and T.N. performed the statistical analysis. All the authors contributed to the final version of the manuscript and approved it for publication.
Supplemental Material
Download TIFF Image (573.3 KB)Acknowledgments
We thank the patients who participated in the present study. We also thank the physicians, medical staff, and data managers in the participating institutes who contributed to valuable data.
Disclosure statement
Junya Kanda received honoraria from Celgene, Bristol Myers Squibb, Takeda Pharmaceutical, Jansen Pharmaceutical, Ono Pharmaceutical, and Sanofi. Akifumi Takaori-Kondo received honoraria from Celgene, Bristol Myers Squibb, Novartis, MSD, Kyowa Kirin, Astellas and Nippon-Shinyaku, research funds from Celgene, Ono Pharmaceutical and COGNANO, and scholarships from Takeda Pharmaceutical, Chugai Pharmaceutical, Eisai, Nippon-Shinyaku, Astellas, Kyowa Kirin, Otsuka Pharmaceutical, Ohara Pharmaceutical, Sanofi, Shionogi, AbbVie, and Japanese Society of Hematology. The other authors have no competing interests to declare.
Data availability statement
The data that support the findings of the present study are available from the corresponding author on reasonable request.
References
- Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Français du Myélome. N Engl J Med. 1996;335(2):91–97.
- Barlogie B, Kyle RA, Anderson KC, et al. Standard chemotherapy compared with high-dose chemoradiotherapy for multiple myeloma: final results of phase III US Intergroup Trial S9321. J Clin Oncol. 2006;24(6):929–936.
- Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348(19):1875–1883.
- Koreth J, Cutler CS, Djulbegovic B, et al. High-dose therapy with single autologous transplantation versus chemotherapy for newly diagnosed multiple myeloma: A systematic review and meta-analysis of randomized controlled trials. Biol Blood Marrow Transplant. 2007;13(2):183–196.
- Reeder CB, Reece DE, Kukreti V, et al. Cyclophosphamide, bortezomib and dexamethasone induction for newly diagnosed multiple myeloma: high response rates in a phase II clinical trial. Leukemia. 2009;23(7):1337–1341.
- Reeder CB, Reece DE, Kukreti V, et al. Once- versus twice-weekly bortezomib induction therapy with CyBorD in newly diagnosed multiple myeloma. Blood. 2010;115(16):3416–3417.
- Kumar S, Flinn I, Richardson PG, et al. Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma. Blood. 2012;119(19):4375–4382.
- Einsele H, Engelhardt M, Tapprich C, et al. Phase II study of bortezomib, cyclophosphamide and dexamethasone as induction therapy in multiple myeloma: DSMM XI trial. Br J Haematol. 2017;179(4):586–597.
- Mai EK, Bertsch U, Dürig J, et al. Phase III trial of bortezomib, cyclophosphamide and dexamethasone (VCD) versus bortezomib, doxorubicin and dexamethasone (PAd) in newly diagnosed myeloma. Leukemia. 2015;29(8):1721–1729.
- Moreau P, Hulin C, Macro M, et al. VTD is superior to VCD prior to intensive therapy in multiple myeloma: results of the prospective IFM2013-04 trial. Blood. 2016;127(21):2569–2574.
- Neben K, Lokhorst HM, Jauch A, et al. Administration of bortezomib before and after autologous stem cell transplantation improves outcome in multiple myeloma patients with deletion 17p. Blood. 2012;119(4):940–948.
- Attal M, Lauwers-Cances V, Marit G, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366(19):1782–1791.
- Sonneveld P, Schmidt-Wolf IG, van der Holt B, et al. Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: results of the randomized phase III HOVON-65/ GMMG-HD4 trial. J Clin Oncol. 2012;30(24):2946–2955.
- Moreau P, Attal M, Pégourié B, et al. Achievement of VGPR to induction therapy is an important prognostic factor for longer PFS in the IFM 2005-01 trial. Blood. 2011;117(11):3041–3044.
- Rajkumar SV, Harousseau JL, Durie B, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117(18):4691–4695.
- Ladetto M, Pagliano G, Ferrero S, et al. Major tumor shrinking and persistent molecular remissions after consolidation with bortezomib, thalidomide, and dexamethasone in patients with autografted myeloma. J Clin Oncol. 2010;28(12):2077–2084.
- Richardson PG, Briemberg H, Jagannath S, et al. Frequency, characteristics, and reversibility of peripheral neuropathy during treatment of advanced multiple myeloma with bortezomib. J Clin Oncol. 2006;24(19):3113–3120.
- Ye J, Ma N, Madden TL, et al. IgBLAST: an immunoglobulin variable domain sequence analysis tool. Nucleic Acids Res. 2013;41(Web Server issue):W34–W40.
- Untergasser A, Cutcutache I, Koressaar T, et al. Primer3–new capabilities and interfaces. Nucleic Acids Res. 2012;40(15):e115.
- van der Velden VH, Cazzaniga G, Schrauder A, et al. Analysis of minimal residual disease by Ig/TCR gene rearrangements: guidelines for interpretation of real-time quantitative PCR data. Leukemia. 2007;21(4):604–611.
- Lahuerta JJ, Mateos MV, Martínez-López J, et al. Influence of pre- and post-transplantation responses on outcome of patients with multiple myeloma: sequential improvement of response and achievement of complete response are associated with longer survival. J Clin Oncol. 2008;26(35):5775–5782.
- Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458.
- Sunami K, Matsumoto M, Fuchida SI, et al. Bortezomib-based strategy with autologous stem cell transplantation for newly diagnosed multiple myeloma: a phase II study by the Japan Study Group for Cell Therapy and Transplantation (JSCT-MM12). Int J Clin Oncol. 2019;24(8):966–975.
- DiPersio JF, Stadtmauer EA, Nademanee A, et al. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood. 2009;113(23):5720–5726.
- Fuchida SI, Sunami K, Matsumoto M, et al. A phase II study of lenalidomide consolidation and maintenance therapy after autologous PBSCT in patients with multiple myeloma. Int J Hematol. 2019;109(1):107–114.
- Goldschmidt H, Lokhorst HM, Mai EK, et al. Bortezomib before and after high-dose therapy in myeloma: long-term results from the phase III HOVON-65/GMMG-HD4 trial. Leukemia. 2018;32(2):383–390.
- McCarthy PL, Holstein SA, Petrucci MT, et al. Lenalidomide maintenance after autologous stem-cell transplantation in newly diagnosed multiple myeloma: a meta-analysis. J Clin Oncol. 2017;35(29):3279–3289.
- Jackson GH, Davies FE, Pawlyn C, et al. Lenalidomide maintenance versus observation for patients with newly diagnosed multiple myeloma (Myeloma XI): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2019;20(1):57–73.
- Dimopoulos MA, Gay F, Schjesvold F, et al. Oral ixazomib maintenance following autologous stem cell transplantation (TOURMALINE-MM3): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2019;393(10168):253–264.
- Nakasone H, Terasako-Saito K, Hirano T, et al. Targeting complete response with upfront bortezomib consolidation versus observation after the achievement of complete response following autologous transplantation for multiple myeloma (TUBA study). Hematol Oncol. 2018;36(1):202–209.
- Avet-Loiseau H, Leleu X, Roussel M, et al. Bortezomib plus dexamethasone induction improves outcome of patients with t(4;14) myeloma but not outcome of patients with del(17p). J Clin Oncol. 2010;28(30):4630–4634.
- Voorhees PM, Kaufman JL, Laubach J, et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: the GRIFFIN trial. Blood. 2020;136(8):936–945.
- Moreau P, Attal M, Hulin C, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. Lancet. 2019;394(10192):29–38.
- Iida S, Ishida T, Murakami H, et al. JSH practical guidelines for hematological malignancies, 2018: III. Myeloma-1. Multiple myeloma (MM). Int J Hematol. 2019;109(5):509–538.
