ABSTRACT
Background
Allogeneic haematopoietic stem cell transplantation (allo-HSCT) is currently the only curative treatment for thalassaemia major (TM). Cord blood (CB) from a sibling has different characteristics from marrow and has potential advantages and disadvantages as a stem cell source.
Methods
We retrospectively analyzed 68 children with beta-thalassaemia major (β-TM) who underwent fresh cord blood transplantation (F-CBT) from an HLA-matched sibling donor (MSD) between June 2010 and July 2018 in the Department of Pediatrics, Nanfang Hospital and Haematology-Oncology, Shenzhen Children’s Hospital.
Results
The median infused doses of total nucleated cells (TNCs) and CD34 + cells were 8.51×107/kg and 3.16×105/kg, respectively. The median time to neutrophil and platelet engraftment were, respectively, 27 and 31 days. The cumulative probabilities of acute and chronic graft-versus-host disease (GVHD) were very low after F-CBT (7.8% and 0.0%, respectively). Of the 68 paediatric patients, 67 patients survived during a median follow-up period of 61 months. The estimated 5-year probability of overall survival (OS) and disease-free survival (DFS) were 98.5% and 87.9%, respectively. Three patients experienced graft rejection (GR) (4.5%), and we identified CD34 + cell dose as a significant risk factor for graft failure (p = 0.036) in stratify analysis.
Conclusions
The above results indicate that patients with β-TM have excellent outcomes after F-CBT from an HLA-MSD.
Abbreviations
| allo-HSCT | = | allogeneic haematopoietic stem cell transplantation |
| TM | = | thalassaemia major |
| CB | = | cord blood |
| F-CBT | = | fresh cord blood transplantation |
| MSD | = | matched sibling donor |
| TNCs | = | total nucleated cells |
| GVHD | = | graft-versus-host disease |
| TRM | = | transplantation-related mortality |
Introduction
Allogeneic haematopoietic stem cell transplantation (allo-HSCT) has achieved a long-term cure for thalassaemia major (TM) without dependence upon daily drugs and/or erythrocyte transfusion. A significant improvement in the long-term survival of patients with TM who underwent allo-HSCT from an HLA-matched sibling donor (MSD) has been achieved, and disease cure rate was about 80–87% according to risk classes [Citation1]. However, graft-versus-host disease (GVHD) is the major barrier to a successful result of allo-HSCT. It has reported a 15–20% probability of acute GVHD (aGVHD) grade III-IV after allo-HSCT from an HLA-MSD for TM [Citation2,Citation3]. Low rates of GVHD make cord blood transplantation (CBT) attractive to patients with haemoglobinopathy disease [Citation1,Citation4,Citation5]. The occurrence of graft rejection (GR) remains a principal limiting factor of CBT, and it occurs more frequently among patients with TM [Citation1,Citation6]. The cell dose of progenitor cells [total nucleated cells (TNCs) or CD34 + cells] infused per kilogram of recipient body weight was considered to be the most significant factor affecting the engraftment result [Citation7–9]. Various measures have been evaluated for their ability to overcome the constraints of cell dose, including simultaneous transfusion of double unit cord blood (CB) from different donors [Citation10,Citation11], CB stem cells ex vivo expanded [Citation12], and CB stem cell mobilization in vivo stimulated. Generally, CB is cryopreserved in liquid nitrogen and can be used if necessary. However, viability decrease and content loss of CB stem cells were reported during long-term storage and during the process of cryopreservation and thawing [Citation13]. Unnecessary cell loss of 20%–25% during the processing of washing techniques [Citation14] reduces the infused cell dose further. Therefore, the use of fresh CB allows the achievement of a higher TNC dose.
Transplantation during the early periods of TM before significant tissue and organ injury, and exposure to multiple transfusions result in less transplant failure, lower transplantation-related mortality (TRM), and better survival [Citation15,Citation16]. CB is collected much earlier from the same donor for the reason that in infancy the donor is preferred to be at least six months old in order to donate bone marrow (BM) even though there are younger ones. CB from an HLA-MSD may offer an additional choice to some families, and utilization of CB in younger recipients may also obtain a higher TNC dose, owing to the lower weight of younger kids. To maintain the content and viability of CB and to shorten the waiting time in patients who are planned to have allo-HSCT, we considered using fresh sibling CB as a haematopoietic cell source. Here, we report excellent outcomes of F-CBT from an HLA-MSD in patients with β-TM.
Patients and methods
Patients
Inclusion categories for the study were as follows: diagnosis of β-TM in patients with weight less than 30 kg, patients with TM who are planned to have allo-HSCT and their families who expect to shorten the waiting time for transplantation as well reduce the donor discomfort and risk; mother carrying a foetus, TM was excluded by prenatal diagnosis and donor–recipient full HLA compatibility was determined; mother who had a history of caesarean section or the indication of caesarean section in this pregnancy. According to statistics, 858 mothers–patients were enrolled in the Department of Pediatrics, Southern Medical University Nanfang Hospital and Hematology-Oncology, Shenzhen Children’s Hospital, 161 patients had a foetus HLA compatibility and 68 patients of them met the criteria. The 68 patients with β-TM underwent F-CBT from an HLA-MSD between June 2010 and July 2018. This research was conducted in compliance with ethical principles based on the Declaration of Helsinki. All participants or guardians gave written informed consent and the research was approved by the institutional ethical review board of the Nanfang Hospital and Shenzhen Children’s Hospital Center. Our patients with β-TM were not accurately stratified according to the Pesaro risk classification without a routine liver biopsy in pretransplant evaluation. On the basis of patient’s age, liver size, and serum ferritin concentrations, patients were classified into three risk groups of low–medium–high risk similar to the Pesaro risk I, II, and III [Citation17]. Details of patient characteristics are described in .
Table 1. Patient characteristics of MSD-F-CBT for β-TM.
Collection and infusion of fresh CB
The amniotic fluid was drawn between 18 and 22 weeks of gestation from foetuses with a great risk of TM for prenatal diagnosis and HLA typing. The donor–recipient histocompatibility was determined by medium- or high-resolution HLA typing of HLA-A and HLA-B as well as high-resolution typing of the DRB1 loci. Pregnant mothers underwent caesarean delivery at 37–39 weeks of gestation , and the CB was collected at the time of birth. All CB collections were performed before placental delivery (in utero) by trained midwives or the obstetric staff. The umbilical cord was clamped immediately using two haemostatic clamps and the newborn was separated after delivery. The cord was cleaned with 75% alcohol and an iodine swab. CB was collected from the umbilical vein by gravity in a closed sterile collection 300 mL double bag (Gaowei, China) containing 28 mL CPDA anticoagulant. CB collection was stored at 4 °C until processing and a routine examination of bacterium, viruses, and pathological agents was conducted, and the numbers of CD34 + cells and nucleated cells were measured by flow cytometry. Fresh CB within 24 h of collection was infused through a central vein injection on the next day after preconditioning, which was called the zero-day. The premedication regimen that includes hydrocortisone and benazepril was given 15–30 min before infusing fresh CB.
Conditioning regimen
We used 3 mg/kg/day azathioprine and 30 mg/kg/day hydroxyurea daily to all patients with β-TM at the beginning of 45 days before transplantation in order to depress the high proliferation of patient’s BM, lighten the burden of the conditioning regimen, and enhance the probability of engraftment [Citation18]. NF-08-TM transplant protocol [Citation17] as a myeloablative conditioning regimen on the basis of intravenous cyclophosphamide (Cy), busulfan (Bu), thiotepa (TT), and fludarabine (Flu) was given to patients with β-TM. Twenty-eight patients did not receive TT because of prohibition sale in China during 2015–2018, and they received the same daily dose of busulfan for one more day on day –3. Detailed information of the drugs of NF-08-TM conditioning regimen are shown in .
Table 2. NF-08-TM conditioning regimens.
GVHD prophylaxis
With respect to GVHD prophylaxis, none of the 68 patients received anti-thymocyte globulin (ATG). GVHD prophylaxis on the basis of cyclosporine A (CsA) was given to all 68 patients. Initial dose of CsA was 1.5 mg/kg/day i.v. from day –10 to –2, added up to 3 mg/kg/day i.v. from day –1 through about day 25, and subsequently i.v. CsA was switched to oral. The concentration of CsA was monitored once or twice per week, and the dosage was regulated to achieve a targeted concentration level of 200 ± 50 ng/mL. The duration of CsA depended on the occurrence of GVHD, and the dosage of CsA was gradually reduced from day 90 until discontinuation by the end of 1 year. Mycophenolate mofetil was given on day 1 at 30 mg/kg/day and was disabled on day 30 if the patient had no signs of grade ≥ II aGVHD. Methotrexate (MTX)was given to patients without skin and mucous membrane damage. Fifty-six patients in this study received MTX, and the MTX doses were 15, 10, and 10 mg/m2 on days 1, 3, and 6,respectively.
Definitions and endpoints
OS was defined as the duration from the date of transplantation until death or last follow-up for surviving patients. DFS was defined as the duration from the date of transplantation to the first event, which referred to graft failure, death, or transfusion dependence recurrence. TRM was defined as death after HSCT for any reason related to the procedure of transplantation. GR was defined as recurrence of thalassaemia at any time after engraftment. Neutrophil engraftment was defined as the first day when the absolute neutrophil count (ANC) was >0.5 × 109/L for 3 consecutive days. Platelet engraftment was defined as the first day when the platelet count was >20× 109/L for 7 consecutive days. The diagnosis and grade of aGVHD and chronic GVHD (cGVHD) were reported in accordance with the Seattle criteria [Citation19]. Patients who survived for more than 14 days and 100 days after transplantation were assessed for aGVHD and cGVHD presence, respectively. The Baltimore criteria were used to diagnose veno-occlusive disease [Citation20].
Statistical methods
Continuous variables are presented as median and range, and categorical variables are presented as percentage (%). OS, DFS, and TRM were estimated using the Kaplan–Meier analysis and are expressed as percentages. The probabilities of grade GVHD were estimated using cumulative incidence estimator. Univariate and multivariate prognostic analyses were performed to evaluate the impact of TNC dose, CD34 dose, Css of busulfan and GVHD prophylaxis on engraftment, GR, and DFS. The primary end point used the log-rank test to examine the influence of transplant-related factors on each end point. The SPSS version 13. soft was used for statistical analyses. A p value of <0.05 was considered to be statistically significant.
Results
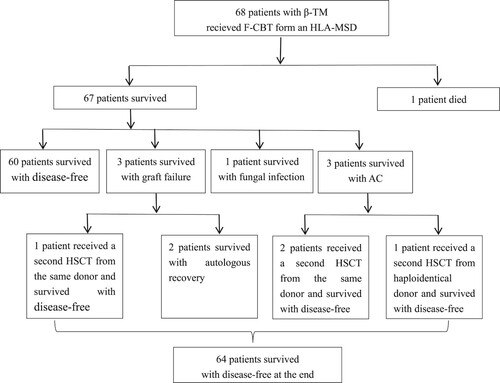
Up to 30 June 2020, the median follow-up after F-CBT from an HLA-MSD was 67 months (range 24–120 months) among surviving patients. The clinical outcomes of patients who were given F-CBT are shown in and .
Table 3. Clinical outcomes of patients who were given F-CBT.
Graft failure and engraftment
The median infused doses of TNCs and CD34 + cells were 8.51 × 107/kg (range, 3.0–20.3 × 107/kg) and 3.16 × 105/kg (range, 0.78–15.6 × 105/kg), respectively. Primary GR was observed in three (4.5%) patients who underwent F-CBT, the infused doses of TNCs and CD34 + cells were 5.16 × 107/kg and 0.79 × 105/kg, 6.80 × 107/kg and 0.87 × 105/kg, 5.28 × 107/kg and 1.62 × 105/kg, respectively. None of the patients experienced secondary GR. One patient who suffered graft failure was alive and received a second HSCT in March 2019 using peripheral blood stem cells (PBSCs) from the same sibling donor, and other two patients had an autologous recovery. The 68 patients were assigned to five groups according to the infused CD34 + cell dose: CD34 < 1 × 105/kg(n = 8), 1–2 × 105/kg(n = 14), 2–3 × 105/kg(n = 11), 3–4 × 105/kg(n = 16), >4 × 105/kg(n = 19), and GR were 25%, 7.1%, 0%, 0%, and 0%, respectively. In the stratify analysis, we identified CD34 + cell dose as a significant risk factor for graft failure (p = 0.036), and when infused CD34 cell counts were less than 1 × 105/kg, the risk of graft failure would be higher. Nineteen patients were infused with sibling donor peripheral blood (range 10–20 mL) as stem cells on the day +1 when the infused doses of CD34 + cells were less than 1.5 × 105/k. Most patients have 100% donor chimerism, while eight patients (12.3%) have steady mixed chimerism (range: 72–92% donor cells) in the early (i.e. the first 3–4 months) post-transplantation period. Five patients converted to full donor chimerism, two within 6 months and three within 1 year after transplantation, while the remaining three children have stable mixed chimerism after discontinuance of immunosuppressive agents for 1 year or more. None of the 8 patients experienced primary or secondary graft failure. For 65 patients who were engrafted, the median times of neutrophil and platelet recovery were 27 days (range, 14–47 days) and 31 days (range, 9–132 days), respectively. In univariate analysis, the use of MTX did not have a significant influence on neutrophil recovery (p = 0.697)and platelet recovery (p = 0.627).
Acute and chronic GVHD
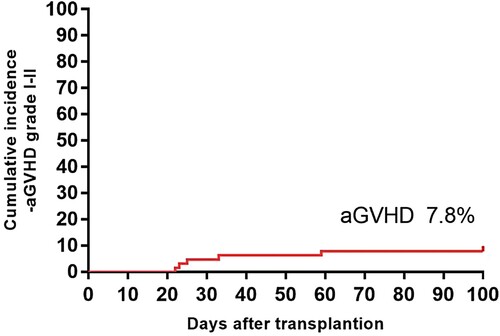
Of the 65 patients who had successful engraftment, 5 patients developed aGVHD and was scored as grade Ⅰ (n = 3) and grade Ⅱ (n = 2) disease. There were no grade III-IV aGVHD and no cGVHD after long-term follow-up. The cumulative incidences of aGVHD () and cGVHD after F-CBT were very low (7.8% and 0.0%, respectively). The skin appears to be particularly affected in most patients with mild to moderate disease.
Transplantation-related complications
Nine patients were diagnosed with cytomegalovirus (CMV) reactivation and all patients were successfully cured by preemptive treatment with i.v. ganciclovir. Bacteraemia was documented in 17 patients, 13 of them had developed septicaemia. Six cases had Gram-positive bacterial infection, and half of them were infected with Staphylococcus aureus. Gram-negative bacteria infection occurred in 7 patients, of which 4 cases were infected with Escherichia coli. However, no patients died of bacteraemia in our study. Invasive fungal disease occurred in 6 patients, including 5 patients with fungal pulmonary infection, and 1 patient with fungal septicaemia and fungal endocarditis who is still taking oral voriconazole antifungal treatment at the follow-up date. Mucositis was the most common toxic side effects of the conditioning regimen, which was observed in 31 of the 68 registered patients. Haemorrhagic cystitis (HC) occurred in 15 patients, and one patient was diagnosed with VOD with an elevated serum bilirubin(>2 mg/dL). Autoimmune haemolytic anaemia (AHA) occurred in 3 patients and was confirmed through a positive direct Coombs test, of which 2 patients were from ABO major incompatible donors and 1 patient from an ABO compatible donor. The onset time of them were 1 month, 3 months, and 6 months after HSCT, respectively. They all received initial therapies including corticosteroids, Cs-A, and IVIG. The patient from an ABO compatible donor recovered quickly over 1 week with the initial therapies. While 2 patients from ABO major incompatible donors were subsequently treated with anti-CD20 MoAb (rituximab) once a week. One was cured after 3 weeks, whereas the other had poor responses after 4 weeks of treatment with rituximab and developed multilineage cytopenias in 8 months after HSCT and AIHA onset was observed within 2 months after HSCT. In addition, there were two other patients who developed autoimmune cytopenias (AC) and the time of onset was respectively 3 months and 8 months after HSCT. Therapies for the three patients included corticosteroids, IVIG, rituximab, sirolimus, and prior donor lymphocyte infusion(DLI), but all of them had a little response to these treatments. Three patients received a second allo-HSCT, 2 had 100% donor chimerism and one had 92% donor chimerism before the second allo-HSCT. Two patients underwent a second transplantation from the same sibling donor after 1 year, and one patient received a haploidentical HSCT the next year. Three patients had complete resolution.
Overall survival, disease-free survival and transplantation-related mortality
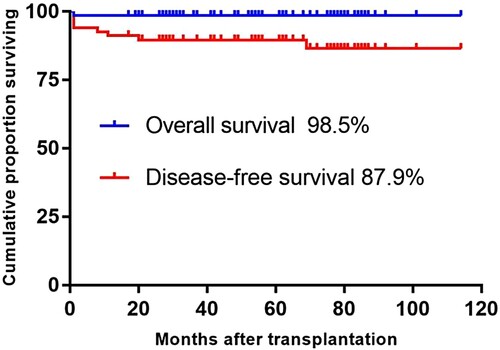
Of all the 68 recipients enrolled in this study, 67 were alive and 60 were disease-free in the duration from the date of transplantation to the first event. The estimated 5-year Kaplan–Meier OS and DFS after F-CBT were, respectively, 98.5% and 87.9% (). One patient died of respiratory and heart failure due to severe pneumonia 1 month after transplantation. The cumulative incidence proportion of TRM at 1 year was 1.5%.
Discussion
Sibling donor CB serves as a potential alternative source of haematopoietic stem cells for transplantation in TM. The outcome of CBT from MSD for the treatment of TM is now approaching be used for bone marrow transplantation (BMT) [Citation1,Citation5]. However, compared with patients who underwent BMT from an HLA-MSD, CBT recipients were at an increased risk of GR and it has already been reported [Citation4,Citation21]. All previous publications on CBT have shown that one of the most significant reasons affecting the engraftment result was the cell dose of progenitor cells infused per kilogram of the recipient body weight, indicated either as TNC or CD34 + cell dose [Citation7–9,Citation22,Citation23]. Some transplant centres [Citation23–25] considered that the doses of infused TNC and CD34 + cell were respectively 7.6–7.9 × 107/kg and 3.3–4.0 × 105/kg for patients with nonmalignant disorders, and a higher cell dose produced a higher level of donor cell engraftment. Patients with TM, especially in China, usually display relatively high incidences of GR due to repeated irregular blood transfusions without leukodepletion for long-term resulting in higher probability of being sensitized to allo-HLA [Citation7,Citation17]. Attempts to decrease the GR rate have usually included more intensive conditioning regimens and higher cell dose infusions. Considering the decrease of TNC and CD34 + cell activity in frozen CB during the cryopreservation and thawing processes, we used fresh CB to achieve a higher TNC dose. The median TNC dose infused in F-CBT recipients was 8.51×107/kg in our study, reaching the recommended minimum number of available cells. In our data, the cumulative incidence of GR in patients who received F-CBT from an HLA-MSD was 4.5%, which showed decreasing tendency compared to the outcome reported in the literature for the largest series from a multicentre study representing the Eurocord group [Citation1]. It is possible that the high TNC dose in fresh CB overcame the increased incidence of GR. The results of OS, DFS, and TRM were comparable to our previously published data [Citation17], where in 30 patients having β-TM with an MSD-BMT, the 3-year probabilities of OS, DFS, and TRM were 90.0%, 83.3%, and 10%, respectively. Our study suggests that the outcomes of F-CBT were better than the MSD-HSCT for paediatric patients with TM at the same institution, with significant differences in OS and TRM.
The best outcomes in patients who underwent CBT can be acquired through optimizing GVHD prophylaxis and preconditioning regimen. Our research suggests that there was a trend towards a lower incidence of GR in patients who received TT containing regimen. However, this result needs to be further verified by randomization. In addition, we found that MTX for GVHD prophylaxis after F-CBT had no significant detrimental effect on engraftment kinetics, unlike the drawback of MTX reported after CBT [Citation1,Citation26]. One disadvantage of CBT includes delayed time to engraftment and immune reconstitution, during which there is an increased risk for infection [Citation3,Citation4,Citation27]. When compared with a previously published study of TM with an HLA-MSD-BMT at the same institution [Citation17], neutrophil and platelet engraftment were slower with F-CBT than with MSD-BMT (27 days vs. 17.5 and 31 days vs. 17 days), but this characteristic was not associated with an increased risk of severe infection and haemorrhagic complications.
The rates and severity of aGVHD and cGVHD with CBT are reduced in most series when compared with BMT or peripheral blood stem cell transplantation (PBSCT), even where donors/recipients are not fully HLA-matched [Citation1,Citation28]. In this current analysis, we found that the rate of grade ≥ II acute GVHD (3.1%) was lower in the patients transplanted with CBT than those in our previous research, where 30.7% of the 26 MSD-BMT patients suffered grade ≥ II GVHD [Citation6]. There was no grade III-IV acute GVHD and no chronic GVHD in our transplanted patients. It needs to be emphasized that the quality of life of patients with extensive cGVHD may be definitely worse than that of patients with TM treated by conventional/supportive treatment, and the risk of cGVHD is generally considered as a cause not to pursue transplantation in paediatric patients who inherit these diseases [Citation28,Citation29].
Mixed chimerism was defined as the presence of more than 5% of recipient cells. Longer follow-up time is needed to determine whether the mixed chimerism is stable and able to cure these patients and eliminate the end-organ of underlying disease. A previous study has shown that sustained donor/recipient mixed chimerism of circulating leukocytes could be found in a considerable proportion of patients among 27 TM recipients who underwent CBT from an HLA-matched sibling [Citation30]. In this study, it also found that the mixed chimerism was not associated with an increased risk of GR, suggesting that CBT may help advance development of a reciprocal tolerance state between donor and recipient cells.
Four mothers in this study received in vitro fertilization (IVF) with preimplantantion genetic diagnosis (PGD) with an HLA tissue typing in order to reproduce a ‘saviour child’ who may provide stem cells for transplantation to his/her ill sibling. A number of cord blood transplantations have successfully been performed using ‘saviour child’ as the donor. There is a growing literature with comments and ethical considerations associated with this procedure [Citation31–35]. Also, it is clear from the preceding review of the literature that there may well be situations where IVF and PGD for selecting HLA-compatible embryos to produce a MSD should be presented to parents as a therapeutic option for their sick child. When no suitable MSD is available, when transplantation using an unrelated donor carries additional risks as compared with a sibling donor transplantation such as thalassaemia and sickle cell disease, when no suitable unrelated donor is available, when transplantation is non-urgent, when the mother is of reproductive age, and when an expansion of the family is sought, IVF and PGD for an HLA typing to select HLA-compatible embryos may be considered. As there are no indications that donor children will be harmed by the procedure and some sick people can be saved, it would be unethical not to allow this technology and not to explore its further potentialities.
Despite encouraging results of CBT, a number of medical, scientific, technical, and ethical challenges remain. There is minimal harm to the mother or newborn provided that priority is given to maternal/newborn safety during childbirth management. CB collection must not adversely affect the health of the mother or newborn. In our centres, the umbilical cord was clamped immediately using two haemostatic clamps and none of newborn babies developed anaemia and hypoxia due to delayed clamping of the cord. There have been discussions about whether early clamping of the umbilical cord can negatively affect the neonate but this has been disproved [Citation36,Citation37]. Also, pregnant women and their partners should be informed about the benefits of delayed cord clamping and of its impact on cord blood collection and banking [Citation38]. Health care professionals must be trained in standardized procedures of CB collection to ensure the sterility and quality of the collected CB. The risk of insufficient cells from CB countered successfully by the administration of the immunosuppressive therapy before CBT and by the supplementation with sibling donor peripheral blood as stem cells when the cellular content of the CBU is judged insufficient to guarantee engraftment. This strategy has proved successful as demonstrated by the excellent outcomes after CBT reported here.
To overcome the cell dose from a single CB sample, our strategy is to use fresh CB that avoids cell loss during the process of cryopreservation and thawing to enhance the TNC dose. The encouraging outcomes of this retrospective study demonstrate that F-CBT from an HLA-MSD offers a comparable probability of a long-term cure of β-TM.
Acknowledgements
The authors thank Dr Chi-Kong Li (Department of Pediatrics, Hong Kong Children’s Hospital, The Chinese University of Hong Kong, Hong Kong SAR, China) and Dr. Wing Leung (KK Women’s and Children’s Hospital, Duke-NUS, Singapore) for the contributions of designing the NF-08-TM HSCT protocol. The authors thank the many agencies that provided grant support, including the Guangdong Province Science and Technology Plan projects (grant number 2016A020215102 and 2014A020212186) and the Clinical Research Startup Program of Southern Medical University by High-level University Construction Funding of Guangdong Provincial Department of Education (project code LC2016ZD017). The authors also thank American Journal Experts (www.aje.com) for their assistance in polishing the language and correcting grammatical errors that were paid for by the authors.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Locatelli F, Kabbara N, Ruggeri A, et al. Outcome of patients with hemoglobinopathies given either cord blood or bone marrow transplantation from an HLA-identical sibling. Blood. 2013;122(6):1072–1078.
- Gaziev J, Lucarelli G. Hematopoietic stem cell transplantation for thalassemia. Curr Stem Cell Res Ther. 2011;6(2):162–169.
- Soni S, Boulad F, Cowan MJ, et al. Combined umbilical cord blood and bone marrow from HLA-identical sibling donors for hematopoietic stem cell transplantation in children with hemoglobinopathies. Pediatr Blood Cancer. 2014;61(9):1690–1694.
- Rocha V, Wagner JE, Sobocinski KA, et al. Graft-versus-host disease in children who have received a cord-blood or bone marrow transplant from an HLA-identical sibling.Eurocord and international bone marrow transplant registry working committee on alternative donor and stem cell sources. N Engl J Med. 2000;342(25):1846–1854.
- Pinto FO, Roberts I. Cord blood stem cell transplantation for haemoglobinopathies. Br J Haematol. 2008;141(3):309–324.
- Wen J, Haque Q, Pei F, et al. Transplant outcomes in beta-thalassemia major patients receiving combined granulocyte colony-stimulating factor-primed bone marrow and cord blood graft compared to granulocyte colony-stimulating factor-primed bone marrow alone. Acta Haematol. 2018;140(1):20–29.
- Tang X, Fang J, Yu J, et al. Clinical outcomes of unrelated cord blood transplantation in children with malignant and non-malignant diseases: multicenter experience in China. Pediatr Transplant. 2018;22(1):e13090.
- Gluckman E, Ruggeri A, Volt F, et al. Milestones in umbilical cord blood transplantation. Br J Haematol. 2011;154(4):441–447.
- Rocha V, Gluckman E. Improving outcomes of cord blood transplantation: HLA matching, cell dose and other graft- and transplantation-related factors. Br J Haematol. 2009;147(2):262–274.
- Barker JN, Weisdorf DJ, DeFor TE, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105(3):1343–1347.
- Jaing TH, Hung IJ, Yang CP, et al. Second transplant with two unrelated cord blood units for early graft failure after cord blood transplantation for thalassemia. Pediatr Transplant. 2009;13(6):766–768.
- Wilkinson AC, Ishida R, Kikuchi M, et al. Long-term ex vivo haematopoietic-stem-cell expansion allows nonconditioned transplantation. Nature. 2019;571(7763):117–121.
- Chen G, Yue A, Ruan Z, et al. Comparison of the effects of different cryoprotectants on stem cells from umbilical cord blood. Stem Cells Int. 2016;2016:1396783.
- McManus MP, Wang L, Calder C, et al. Comparison of pre-cryopreserved and post-thaw-and-wash-nucleated cell count on major outcomes following unrelated cord blood transplant in children. Pediatr Transplant. 2012;16(5):438–442.
- Locatelli F, De Stefano P. Innovative approaches to hematopoietic stem cell transplantation for patients with thalassemia. Haematologica. 2005;90(12):1592–1594.
- Galambrun C, Pondarré C, Bertrand Y, et al. French multicenter 22-year experience in stem cell transplantation for beta-thalassemia major: lessons and future directions. Biol Blood Marrow Transplant. 2013;19(1):62–68.
- Li C, Wu X, Feng X, et al. A novel conditioning regimen improves outcomes in β-thalassemia major patients using unrelated donor peripheral blood stem cell transplantation. Blood. 2012;120(19):3875–3881.
- Sodani P, Gaziev D, Polchi P, et al. New approach for bone marrow transplantation in patients with class 3 thalassemia aged younger than 17 years. Blood. 2004;104(4):1201–1203.
- Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15(6):825–828.
- Johnson DB, Savani BN. How can we reduce hepatic veno-occlusive disease-related deaths after allogeneic stem cell transplantation? Exp Hematol. 2012;40(7):513–517.
- Bizzetto R, Bonfim C, Rocha V, et al. Outcomes after related and unrelated umbilical cord blood transplantation for hereditary bone marrow failure syndromes other than fanconi anemia. Haematologica. 2011;96(1):134–141.
- Wagner JE, Barker JN, DeFor TE, et al. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002;100(5):1611–1618.
- Ruggeri A, Eapen M, Scaravadou A, et al. Umbilical cord blood transplantation for children with thalassemia and sickle cell disease. Biol Blood Marrow Transplant. 2011;17(9):1375–1382.
- Martin PL, Carter SL, Kernan NA, et al. Results of the cord blood transplantation study (COBLT): outcomes of unrelated donor umbilical cord blood transplantation in pediatric patients with lysosomal and peroxisomal storage diseases. Biol Blood Marrow Transplant. 2006;12(2):184–194.
- Jaing TH, Chen SH, Tsai MH, et al. Transplantation of unrelated donor umbilical cord blood for nonmalignant diseases: a single institution's experience with 45 patients. Biol Blood Marrow Transplant. 2010;16(1):102–107.
- Herr AL, Kabbara N, Bonfim CM, et al. Long-term follow-up and factors influencing outcomes after related HLA-identical cord blood transplantation for patients with malignancies: an analysis on behalf of Eurocord-EBMT. Blood. 2010;116(11):1849–1856.
- Tan W, He Y, Feng X, et al. Thiotepa-based conditioning regimen compared to non-thiotepa conditioning regimen prior to allogeneic stem cell transplantation in β thalassemia major: impact on survival. Blood. 2018;132:2082–2082.
- Boncimino A, Bertaina A, Locatelli F. Cord blood transplantation in patients with hemoglobinopathies. Transfus Apher Sci. 2010;42(3):277–281.
- Ferrara JLM, Levine JE, Reddy P, et al. Graft-versus-host disease. Lancet (London, England). 2009;373(9674):1550–1561.
- Lisini D, Zecca M, Giorgiani G, et al. Donor/recipient mixed chimerism does not predict graft failure in children with beta-thalassemia given an allogeneic cord blood transplant from an HLA-identical sibling. Haematologica. 2008;93(12):1859–1867.
- Devolder K. Preimplantation HLA typing: having children to save our loved ones. J Med Ethics. 2005;31(10):582–586.
- Samuel GN, Strong KA, Kerridge I, et al. Establishing the role of pre-implantation genetic diagnosis with human leucocyte antigen typing: what place do “saviour siblings” have in paediatric transplantation? Arch Dis Child. 2009;94(4):317–320.
- Burgio GR, Nespoli L, Maccario R, et al. Conceiving a hematopoietic stem cell donor: twenty-five years after our decision to save a child. Haematologica. 2012;97(4):479–481.
- Taylor-Sands M. Summary of saviour siblings. J Med Ethics. 2015;41(12):926.
- Eyricha M, Schulzeb H. HLA matching in pediatric stem cell transplantation. Transfus Med Hemother. 2019;46:348–354.
- Burgio GR, Locatelli F. Transplant of bone marrow and cord blood hematopoietic stem cells in pediatric practice, revisited according to the fundamental principles of bioethics. Bone Marrow Transplant. 1997;19:1163–1168.
- Impact of delayed umbilical cord clamping on public cord blood donations: can we help future patients and benefit infant donors? Transfusion. 2018;58:1427–1433.
- Anthony Armson B, Allan DS, Casper RF. Umbilical cord blood: counselling,collection,and banking. J Obstet Gynaecol Can. 2015;37(9):832–844.