ABSTRACT
Objectives
Graft failure (GF) is an intractable complication of transplantation, which can severely affect the efficacy of the graft; however, the characteristics, incidence, and risk factors of primary GF have not been well described. This study aimed to analyze the risk factors and outcomes of primary GF to swiftly identify high-risk patients for GF.
Methods
We performed a case-control study with a case-control ratio of 1:4 with 869 patients who underwent allogeneic hematopoietic stem cell transplantation (allo-HSCT) between January 2015 and December 2019 at our center.
Results
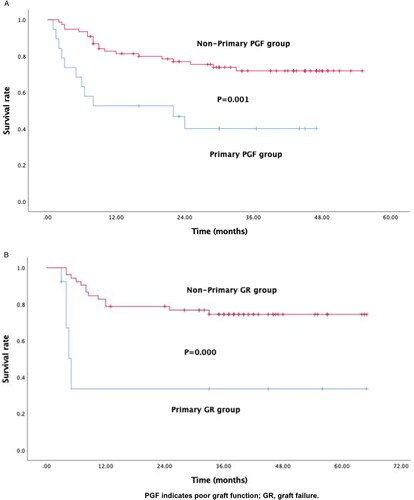
Nineteen (2.19%) patients experienced primary poor graft function (PGF), while eleven (1.27%) patients developed primary graft rejection (GR). Univariate and multivariate logistic analyses identified two independent risk factors for primary PGF: splenomegaly [P = 0.030; odds ratio (OR), 3.486; 95% confidence interval (CI), 1.139 to 13.109], and donor type [non-matched sibling donor (non-MSD)] (P = 0.018; OR, 4.475; 95% CI, 1.289 to 15.537). However, only donor type (non-MSD) was statistically significant (P = 0.020; OR, 19.432; 95% CI, 1.595 to 236.691) for primary GR. The overall survival was significantly lower in the primary PGF (P = 0.001) and GR group (P = 0.000), respectively, compared to the control group.
Conclusion
GF can significantly affect the overall survival of patients who underwent allo-HSCT, despite its considerably low incidence. A human leukocyte antigen-matched sibling donor should be the first choice for patients undergoing allo-HSCT for the prevention of GF. Moreover, splenomegaly is an independent risk factor for PGF, and caution must be exercised while treating such patients.
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a curative treatment for patients with malignant or nonmalignant hematopoietic diseases. However, several intractable complications occurring after transplantation, including graft failure (GF), can severely affect the efficacy of the graft. The failure to achieve a threshold absolute neutrophil count (ANC) of 0.5 × 109/L for three consecutive days by day 28 after HSCT is the relatively well-established criterion for GF. However, the relationship between poor graft function and GF varies among different sudies [Citation1–5].
Recent studies [Citation6–8] defined poor graft function (PGF) as at least bilinear severe cytopenia, which is characterized by the following: (1) ANC⩽0.5 × 109/L; (2) platelet count ⩽20 × 109/L; and (3) hemoglobin ⩽70 g/L for at least 3 consecutive days beyond day +28 after HSCT or the requirement of transfusion with full chimerism and a hypoplastic-aplastic bone marrow, while severe graft versus host disease (GVHD) and relapse were excluded. In this study, we classified GF according to the chimerism status: PGF referred to full chimerism, whereas graft rejection (GR) represented mixed chimerism or complete recipient chimerism [Citation4,Citation6,Citation9,Citation10]. GF is a life-threatening complication of HSCT, and the survival rate of patients with GF is considerably lower than that in patients with complete engraftment [Citation3,Citation11]. Moreover, it is also important to distinguish between primary GF and secondary GF, as the prognosis of primary GF is more dismal. Primary GF is defined as incomplete engraftment, whereas secondary GF indicates loss of initial engraftment. However, the pathogenesis of primary GF has yet to be elucidated, while the treatment options are very limited. Recent studies suggested that cell dose, human leukocyte antigen (HLA) disparity, blood-type mismatch, high serum ferritin level, splenomegaly, GVHD, and cytomegalovirus (CMV) infection may be related to primary GF [Citation7,Citation9,Citation10,Citation12,Citation13].
Therefore, we performed a case–control study to analyze the risk factors and outcomes in patients with primary GF in order to identify those with a high risk of developing GF as early as possible and consequently ensure better management.
Patients and methods
Patients and definitions
A total of 869 patients who received allo-HSCT at the Hematopoietic Stem Cell Transplantation Center of Blood Diseases Hospital, Chinese Academy of Medical Sciences, between January 2015 and December 2019 were reviewed retrospectively. Nineteen of the 869 patients met the criteria for primary PGF and eleven were diagnosed with primary GR. A matched control group was selected using the case-pair method to analyze the risk factors based on the following criteria: (1) sex, (2) month in which the transplantation was received (±2 months), (3) age (± 5 years), and (4) a case-control ratio of 1:4. The final date of follow-up was June 30, 2020 for patients who did not experience any untoward events. All patients or their legal representatives provided written informed consent before transplantation. This study adhered to the Declaration of Helsinki and was approved by the Ethics Review Committee of our center.
GF was defined as the failure to achieve a threshold absolute neutrophil count (ANC) of 0.5 × 109/L for three consecutive days by day 28 after HSCT. And GF was classified into PGF and GR according to the chimerism status: PGF referred to full chimerism, whereas graft rejection (GR) represented mixed chimerism or complete recipient chimerism.
The Mount Sinai Acute GVHD International Consortium (MAGIC) criteria was used to diagnose and grade acute GVHD (aGVHD) [Citation14]. CMV (cytomegalovirus) -DNA was detected by plasma sample using real-time PCR and CMV infection was defined as >1000 copies/mL.
In this study, splenomegaly was defined as splenic thickness >4 cm or craniocaudal length >12 cm, as identified by ultrasonography [Citation7].
Transplantation regimen
All patients undergoing allo-HSCT underwent myeloablative pre-conditioning. However, the pre-conditioning regimen was heterogenous for patients with different underlying diseases.
The principal regimen for patients diagnosed with acute myeloid leukemia (AML)/myelodysplastic syndrome (MDS)/myeloproliferative neoplasms (MPN) included busulfan and cyclophosphamide (Bu + Cy), in addition to optional fludarabine (Flu), cytarabine (Ara-c), or antithymocyte globulin (ATG). The regimen for patients with aplastic anemia (AA) included Flu + ATG + Cy, with optional administration of Bu. Patients with lymphoma/acute lymphocytic leukemia (ALL) received total body irradiation (TBI)/melphalan (Mel) + Cy, with the optional administration of Flu, Ara-c, and ATG.
All transplant recipients received cyclosporine A or FK506 and short-term methotrexate, in addition to the optional administration of mycophenolate mofetil, for the prophylaxis of GVHD.
Statistical analysis
Data were analyzed using the IBM software, SPSS statistics 25 and GraphPad Prism 8. The chi-squared test and Fisher’s exact test were used for categorical variables and descriptive statistics were used for continuous variables to compare the incidence of GF using univariate analysis. Parameters with P-values < 0.10 were regarded as potential risk factors and further analyzed using multivariate logistic regression. The Kaplan-Meier method was used to estimate the survival curves and differences were compared using the log-rank test. Two-sided P-values < 0.05 were considered statistically significant.
Results
Incidence and characteristics
A total of 869 patients who underwent allo-HSCT between January 1, 2015 and December 31, 2019 were enrolled in the incidence cohort. Nineteen (2.19%) of the 869 patients experienced primary PGF, while eleven (1.27%) developed primary GR.
The characteristics of patients with primary PGF and primary GR are listed in . Fourteen of the 19 patients with primary PGF were men and 5 were women, whereas 6 of the 11 patients with primary GR were men and 5 were women. The median ages of the patients with primary PGF and GR were 37 years (13–56) and 27 years (8–59), respectively. Thirteen patients in the primary PGF group were diagnosed with acute leukemia, 4 with MDS, 1 with lymphoma and 1 patient was diagnosed with MPN. Three patients in the primary GR group were diagnosed with acute leukemia, 3 with MDS, 3 with aplastic anemia, and 2 patients were diagnosed with MPN.
Table 1. Characteristics of primary PGF (A) and GR (B) patients.
Risk factors
A control group with a 4:1 ratio for primary PGF and GR was used to identify the risk factors for PGF and GR, respectively. Univariate analysis revealed that the time from diagnosis to transplantation (>6 months), splenomegaly, SF (serum ferritin) level (>1000 ng/mL), donor type [non-matched sibling donor (non-MSD)], and blood-type-mismatch were potential risk factors (P < 0.10) for primary PGF (A). Univariate analysis for primary GR revealed different potential risk factors, including acute GVHD occurring in 30 days after transplantation, disease, and donor type (non-MSD) (B). Multivariate logistic analysis identified two independent risk factors for primary PGF, i.e. splenomegaly [P = 0.030; odds ratio (OR), 3.486; 95% confidence interval (CI), 1.139 to 13.109], and donor type (non-MSD) (P = 0.018; OR, 4.475; 95% CI, 1.289 to 15.537) (A). However, only donor type (non-MSD) was a statistically significant factor (P = 0.020; OR, 19.432; 95% CI, 1.595 to 236.691) associated with primary GR (B).
Table 2. Univariate analysis of risk factors for primary PGF (A) and GR (B).
Table 3. Multivariate logistic analysis of risk factors for primary PGF (A) and GR (B).
Treatment and outcomes
All patients with decreased blood cell counts received supportive treatment, including transfusion and administration of growth factors. Moreover, 2 patients from the primary PGF group received donor stem cell infusion and 1 patient received mesenchymal stromal cell infusion. The median follow-up period was 18.6 months (1–47 months), during which 11 patients (58%) died (1 died of relapse, while the others died of infection or severe GVHD). Eight patients in the primary GR group received donor stem cell infusion and 3 patients underwent a second transplantation; the donor was changed in 2 of these 3 patients because of complete rejection of the former graft. The median follow-up period was 16.1 months (3–65 months), and 7 patients (63.6%) died (1 died of relapse, while the others died of infection or severe GVHD). The overall survival was significantly lower in the primary PGF (P = 0.001) and GR group (P = 0.000), respectively (A and B), compared to the control group.
Discussion
GF is a rare complication of allo-HSCT, which is characterized by high mortality. Previous studies [Citation3,Citation6,Citation13] reported that the incidence rate of primary GR and poor graft function ranged from 3.8% to 5.6%, and 5% to 27%, respectively. The incidence rate of primary PGF and GR in this study was 2.19% and 1.27%, respectively. As all patients treated in our center received a myeloablative pre-conditioning regimen, the prevalence of GF cannot be compared with that of previous studies.
It is imperative to identify the possible risk factors and take measures to prevent PGF and GR, since the overall survival of patients with primary PGF and GR was significantly lower than that in the control group (P = 0.001 and P = 0.000, respectively).
GR is thought to result from the recipient’s immune response against donor hematopoietic cells, which is mediated by the residual host immunity. Underlying disease, HLA disparity, low dose of infused CD34+ cells, conditioning regimen, major ABO blood group incompatibility, stem cell source, etc. have been reported to be amongst the risk factors associated with GR [Citation15–18]. Mattsson J et al. revealed that the incidence rate of GR was 0.1% in patients who received grafts from HLA-identical siblings, whereas it rose to 5% in those who received HLA-mismatched grafts [Citation16]. In our study, non-MSD was an independent risk factor, consistent with the findings of previous studies.
According to previous studies, the risk factors for PGF include low dose of infused CD34+ cells, CMV infection, acute GVHD, donor-specific antibody, iron overload, splenomegaly etc [Citation6,Citation7,Citation19,Citation20]. The current study identified donor type (non-MSD) and splenomegaly as independent risk factors for PGF.
Splenomegaly was an independent risk factor for primary PGF in our study, concurrent with recent studies [Citation5,Citation7,Citation21]. The entrapment and destruction of hematopoietic cells by the enlarged spleen could be a possible mechanism underlying the development of PGF. Shimomura et al. [Citation21] suggested that splenic enlargement (>320 cm3) was linked to low neutrophil and platelet engraftment after allo-HSCT in patients with AML and MDS. Moreover, the persistence of significant splenomegaly (⩾10 cm under the costal margin) on day +30 following HSCT was associated with a higher incidence of PGF (33% versus 12%; P = 0.05) in patients with myelofibrosis [Citation5]. Akpek et al. [Citation22] suggested that splenomegaly would not delay engraftment if a CD34+ cell dose >5.7 × 106/kg was transplanted, indicating that higher doses of CD34+ cells should be considered for patients with an enlarged spleen. However, in our study, the CD34+ cell dose did not differ significantly between the PGF and good graft function group, possibly because the majority of CD34+ cell doses administered in our center were lower than 5.7 × 106/kg and few patients can attain this dose. Another independent risk factor was donor type (non-MSD), which was also an independent risk factor for GR, suggesting that an HLA-matched sibling donor should be the first choice for the prevention of GF.
Treatment for patients with GF traditionally primarily consists of supportive treatment, donor cell infusion, and second allo-HSCT. New strategies include CD34+ stem cell boosts, mesenchymal stem cells, thrombopoietin receptor agonists, and drugs that can improve the impaired bone marrow microenvironment [Citation6,Citation7,Citation23–27]. Although the prognosis of GF has improved owing to the advancement in treatment methods, it is still a lethal complication of allo-HSCT.
In conclusion, this study demonstrated that primary GF was a rare but severe complication of allo-HSCT. Our study provided some clinically relevant indications, despite limitations such as its retrospective design and small sample size (due to the low incidence of primary GF). Selecting an HLA-matched sibling donor is a better option for patients undergoing allo-HSCT in order to prevent GF, although other types of donor may confer other benefits. Moreover, splenomegaly is a risk factor for PGF, and clinicians should take measures to manage splenomegaly before transplantation. It is vital to explore effective treatment strategies since the prognosis of primary GF is still dismal.
Supplemental Material
Download MS Word (17.8 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ciurea SO, Cao K, Fernandez-Vina M, et al. The European Society for Blood and Marrow Transplantation (EBMT) consensus guidelines for the detection and treatment of donor-specific anti-HLA Antibodies (DSA) in Haploidentical Hematopoietic Cell Transplantation. Bone Marrow Transplant. 2018;53:521–534. doi:10.1038/s41409-017-0062-8.
- Bacigalupo A, Socie G, Lanino E, et al. Fludarabine, cyclophosphamide, antithymocyte globulin, with or without low dose total body irradiation, for alternative donor transplants, in acquired severe aplastic anemia: a retrospective study from the EBMT-SAA Working Party. Haematologica. 2010;95:976–982. doi:10.3324/haematol.2009.018267.
- Ozdemir ZN, Civriz Bozdağ S. Graft failure after allogeneic hematopoietic stem cell transplantation. Transfus Apher Sci. 2018;57:163–167. doi:10.1016/j.transci.2018.04.014.
- Forman SJ, Negrin RS, Appelbaum FR. Thomas’ hematopoietic cell transplantation: stem cell transplantation. 5th ed. 2016. John Wiley & Sons, Ltd.
- Alchalby H, Yunus DR, Zabelina T, et al. Incidence and risk factors of poor graft function after allogeneic stem cell transplantation for myelofibrosis. Bone Marrow Transplant. 2016;51:1223–1227. doi:10.1038/bmt.2016.98.
- Kong Y. Poor graft function after allogeneic hematopoietic stem cell transplantation—an old complication with new insights⋆. Semin Hematol. 2019;56:215–220. doi:10.1053/j.seminhematol.2018.08.004.
- Zhao Y, Gao F, Shi J, et al. Incidence, risk factors, and outcomes of primary poor graft function after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2019;25:1898–1907. doi:10.1016/j.bbmt.2019.05.036.
- Shi MM, Kong Y, Song Y, et al. Atorvastatin enhances endothelial cell function in posttransplant poor graft function. Blood. 2016;128:2988–2999. doi:10.1182/blood-2016-03-702803.
- Sun Y-Q, He G-L, Chang Y-J, et al. The incidence, risk factors, and outcomes of primary poor graft function after unmanipulated haploidentical stem cell transplantation. Ann Hematol. 2015;94:1699–1705. doi:10.1007/s00277-015-2440-x.
- Chang Y-J, Zhao X-Y, Xu L-P, et al. Donor-specific anti-human leukocyte antigen antibodies were associated with primary graft failure after unmanipulated haploidentical blood and marrow transplantation: a prospective study with randomly assigned training and validation sets. J Hematol Oncol. 2015;8. doi:10.1186/s13045-015-0182-9.
- Ferra C, Sanz J, Diaz-Perez MA, et al. Outcome of graft failure after allogeneic stem cell transplant: study of 89 patients. Leuk Lymphoma. 2015;56:656–662. doi:10.3109/10428194.2014.930849.
- Doring M, Cabanillas Stanchi KM, Feucht J, et al. Ferritin as an early marker of graft rejection after allogeneic hematopoietic stem cell transplantation in pediatric patients. Ann Hematol. 2016;95:311–323. doi:10.1007/s00277-015-2560-3.
- Xiao Y, Song J, Jiang Z, et al. Risk-factor analysis of poor graft function after allogeneic hematopoietic stem cell transplantation. Int J Med Sci. 2014;11:652–657. doi:10.7150/ijms.6337.
- Harris AC, Young R, Devine S, et al. International, multicenter standardization of acute graft-versus-host disease clinical data collection: a report from the Mount Sinai acute GVHD International Consortium. Biol Blood Marrow Transplant. 2016;22:4–10. doi:10.1016/j.bbmt.2015.09.001.
- Olsson R, Remberger M, Schaffer M, et al. Graft failure in the modern era of allogeneic hematopoietic SCT. Bone Marrow Transplant. 2012;48:537–543.
- Cluzeau T, Lambert J, Raus N, et al. Risk factors and outcome of graft failure after HLA matched and mismatched unrelated donor hematopoietic stem cell transplantation: a study on behalf of SFGM-TC and SFHI. Bone Marrow Transplant. 2016;51:687–691. doi:10.1038/bmt.2015.351.
- Mattsson J, Ringdén O, Storb R. Graft failure after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2008;14:165–170. doi:10.1016/j.bbmt.2007.10.025.
- Nakasone H, Shigeo F, Yakushijin K, et al. Impact of total body irradiation on successful neutrophil engraftment in unrelated bone marrow or cord blood transplantation. Am J Hematol. 2017;92. doi:10.1002/ajh.24613.
- Chen J, Wang H, Zhou J, et al. Advances in the understanding of poor graft function following allogeneic hematopoietic stem-cell transplantation. Ther Adv Hematol. 2020;11:2040620720948743. doi:10.1177/2040620720948743.
- Dominietto A, Raiola AM, van Lint MT, et al. Factors influencing haematological recovery after allogeneic haemopoietic stem cell transplants: graft-versus-host disease, donor type, cytomegalovirus infections and cell dose. Br J Haematol. 2001;112:219–227. doi:10.1046/j.1365-2141.2001.02468.x.
- Shimomura Y, Hara M, Katoh D, et al. Enlarged spleen is associated with low neutrophil and platelet engraftment rates and poor survival after allogeneic stem cell transplantation in patients with acute myeloid leukemia and myelodysplastic syndrome. Ann Hematol. 2018;97:1049–1056. doi:10.1007/s00277-018-3278-9.
- Akpek G, Pasquini MC, Logan B, et al. Effects of spleen status on early outcomes after hematopoietic cell transplantation. Bone Marrow Transplant. 2012;48:825–831. doi:10.1038/bmt.2012.249.
- Mainardi C, Ebinger M, Enkel S, et al. CD34(+) selected stem cell boosts can improve poor graft function after paediatric allogeneic stem cell transplantation. Br J Haematol. 2018;180:90–99. doi:10.1111/bjh.15012.
- Liu X, Wu M, Peng Y, et al. Improvement in poor graft function after allogeneic hematopoietic stem cell transplantation upon administration of mesenchymal stem cells from third-party donors: a pilot prospective study. Cell Transplant. 2014;23:1087–1098. doi:10.3727/096368912X661319.
- Tang C, Chen F, Kong D, et al. Successful treatment of secondary poor graft function post allogeneic hematopoietic stem cell transplantation with eltrombopag. J Hematol Oncol. 2018;11. doi:10.1186/s13045-018-0649-6.
- Marotta S, Marano L, Ricci P, et al. Eltrombopag for post-transplant cytopenias due to poor graft function. Bone Marrow Transplant. 2019;54:1346–1353. doi:10.1038/s41409-019-0442-3.
- Kong Y, Wang Y, Zhang YY, et al. Prophylactic oral NAC reduced poor hematopoietic reconstitution by improving endothelial cells after haploidentical transplantation. Blood Adv. 2019;3:1303–1317. doi:10.1182/bloodadvances.2018029454.