ABSTRACT
Background
Heparin-induced thrombocytopenia (HIT) is an immune-mediated adverse drug reaction associated with thrombosis. Clinical scoring systems and the presence of anti-platelet factor 4 (anti-PF4)/heparin antibodies determine the diagnosis.
Case presentation
A 57-year-old man who was treated with acenocoumarol due to a chronic left ventricular thrombus was admitted to the hospital for severe SARS-CoV-2 pneumonia and pulmonary embolism. The patient was started on bemiparin and discharged. Left lower limb acute arterial ischemia and thrombocytopenia were diagnosed 18 days later. Computed tomography angiography revealed a large left ventricular thrombus and multiple arterial thrombi. Left femoral-popliteal thromboembolectomy was performed. Anti-PF4/heparin antibodies confirmed an HIT diagnosis. Fondaparinux (7.5 mg/24 h) was initiated, but cardiac surgery was necessary. Bivalirudin was used during surgery, with an initial load (1.25 mg/kg) and maintenance infusion (2.5 mg/kg/h). The cardiac thrombus was extracted, but the patient experienced a postsurgical myocardial infarction. Percutaneous cardiovascular intervention (PCI) required a bivalirudin load (0.75 mg/kg) and maintenance infusion (1.75 mg/kg/h). No coronary lesions were detected, and argatroban was started afterwards (0.5 µg/kg/min). When the platelet count exceeded 100 × 109/L, acenocoumarol was initiated. Thereupon, acetylsalicylic acid (100 mg/24 h) was added. No other complications have been reported to date.
Conclusion
The clinical presentation of intraventricular and multiple arterial thrombi is remarkable. SARS-CoV-2 infection likely contributed to a hypercoagulable state. The management of patients with HIT undergoing cardiac surgery is challenging. If surgery cannot be delayed, then treatment with bivalirudin is recommended. Additionally, this drug is recommended for PCI. Bivalirudin is safe and well-tolerated in both procedures.
Background
Heparin-induced thrombocytopenia (HIT) is an immune-mediated adverse reaction to heparin associated with potentially lethal venous and arterial thrombosis. HIT is estimated to occur in 0.1%–5% of patients receiving heparin. A higher risk is associated with the use of unfractionated heparin rather than with low-molecular-weight heparin. Additionally, women and patients undergoing major surgery are at a greater risk of developing HIT than other patients [Citation1].
HIT induces a highly prothrombotic state due to an atypical immune response that causes the synthesis of anti-platelet factor 4 (PF4) IgG antibodies [Citation2]. HIT diagnosis is based on a clinical probability estimation that generates risk scores, e.g. the 4 Ts clinical scoring system, and the presence of anti-PF4/heparin antibodies [Citation1].
Here, we report the case of a patient with a recent history of severe SARS-CoV-2 bilateral pneumonia who developed HIT with a large ventricular thrombus. We describe the challenging case management, which include administering multiple anticoagulation therapies and performing cardiac surgery.
Case presentation
A 57-year-old Caucasian man was admitted to the intensive care unit (ICU) because of severe SARS-CoV-2 bilateral pneumonia with low-risk pulmonary embolism (PE). He had a previous medical history of an apical acute myocardial infarction (AMI) 9 years ago, which was treated with surgical revascularization. Consequently, he developed an apical aneurysm that was covered by a 4-mm chronic thrombus and was treated with acenocoumarol. A mildly reduced left ventricular ejection fraction (LVEF) (46%) was observed during the patient’s most recent cardiac magnetic resonance imaging examination.
The patient required non-invasive mechanical ventilation. The acenocoumarol treatment was discontinued, and a therapeutic dose of bemiparin was initiated (10,000 IU/24 h, considering the patient’s weight [81 kg] and a normal renal and hepatic function). The patient’s progress was favourable, and he was discharged 10 days later and prescribed at-home subcutaneous injections of bemiparin. No other drugs were added to his usual medications.
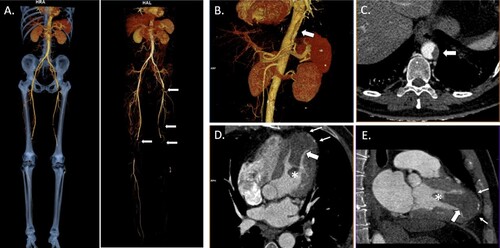
The patient was admitted to the emergency department 18 days later complaining of acute and intense pain in his left leg. On examination, the extremity was pale and cold, with no peripheral pulse. Blood tests showed thrombocytopenia (70 × 109/L; basal value 350 × 109/L), elevated D-dimer (20,714 µg/L fibrinogen equivalent units [FEU]; normal value [NV] <494 µg/L) and fibrinogen levels (616 mg/dL; NV 200–400 mg/dL) with slightly prolonged prothrombin time (14.7 s; NV 10–14 s), and normal activated partial thromboplastin time (27.7 s; NV 22.5–36 s). Acute arterial ischemia was suspected; therefore, computed tomography angiography was requested. Multiple thrombi were observed in different locations, including the left ventricle (). Subsequently, urgent left femoral and popliteal thromboembolectomy were performed. After the procedure, the patient achieved his lowest platelet count (51 × 109/L).
Figure 1. Three-dimensional reconstruction and computed tomography angiography (CTA) images of post-COVID-19 heparin-induced thrombocytopenia. (A) CTA three-dimensional image of multiple acute bilateral arterial thrombi (arrows) in the lower extremities suggesting thromboembolic origin with occlusion of the left popliteal artery. It is remarkable that there is no significant pre-existing aortic atherosclerosis. Three-dimensional reconstruction (B) and axial CTA (C) images showing a non-occlusive mural thrombus in the thoracoabdominal aortic hiatus. Four-chamber (D) and two-chamber views (E) revealing the presence of a large thrombus (65×32×34 mm) with a mobile distal pedicle (*) in the peripherally calcified apical left ventricular aneurysm (small arrows).
The presence of multiple thrombi in conjunction with thrombocytopenia and previous heparin treatment suggested a case of HIT. The clinical probability of HIT was high (4 Ts score = 6 points), and screening for anti-PF4 antibodies was requested. The result was positive (2.28 U/mL; NV <1 U/mL) in an automated chemiluminescent assay (HemosIL AcuStar HIT IgG [PF4-H], Instrumentation Laboratory, Bedford, MA, USA), supporting the diagnosis. Additionally, SARS-CoV-2 nasopharyngeal swab reverse transcription–polymerase chain reaction and screening for IgG/IgM antibodies were positive.
Heparin administration was discontinued, and platelet transfusion was avoided. Anticoagulation therapy with fondaparinux (7.5 mg/24 h) was initiated. The high embolic risk and massive size of the ventricular thrombus supported a surgical approach. Surgery was delayed by 48 h until the patient achieved a stable platelet count of >50 × 109/L.
The anticoagulant agent used during surgery was bivalirudin. An initial load was administered (1.25 mg/kg over 5 min and 50 mg in pump prime), followed by a maintenance infusion (2.5 mg/kg/h) [Citation1]. Left atriotomy allowed access to a large and well-organised thrombus that adhered to the ventricular apex. It was extracted through the mitral valve using videothoracoscopy.
Because of persistent venous bleeding at the end of the procedure, one unit of fresh frozen plasma was transfused. Fortunately, bleeding reduction started before transfusion was initiated. The absence of residual thrombus was confirmed using a transoesophageal echocardiogram.
After 1 h in the ICU, the patient had an inferior and posterior ST-elevation AMI. A transthoracic echocardiogram detected recurrence of the left ventricular thrombus (18 × 15 mm) with akinetic inferior and posterior areas (LVEF 35%–40%). Urgent coronariography was required. Bivalirudin (load of 0.75 mg/kg) was then administered; this was followed by a maintenance infusion during the procedure (1.75 mg/kg/h) [Citation1]. Coronariography did not show new coronary lesions. Afterwards, a reduced maintenance infusion of bivalirudin was continued (0.25 mg/kg/h). A few hours later, bivalirudin was discontinued, and argatroban (0.5 µg/kg/min) was started. Blood tests showed a progressive increase in the levels of cardiac biomarkers, achieving the troponin I peak 24 h later (32.4 ng/mL). No haemorrhagic complications were observed; therefore, argatroban infusion speed was increased.
This extraordinary clinical presentation made us consider the presence of an underlying condition, such as a catastrophic antiphospholipid syndrome, vasculitis, or other autoimmune disease. Consequently, anti-beta-2 glycoprotein I IgG/IgM/IgA, anti-cardiolipin IgG/IgM/IgA, anti-phosphatidylserine/prothrombin IgG/IgM, anti-nuclear antibodies, and lupus anticoagulant testing were requested. All test results were negative.
Additional anti-PF4 antibody assays were performed on the 4th and 9th day after diagnosis. Anti-PF4 antibody levels decreased (1.49 and 1.2 U/mL, respectively), accompanied by an improvement of the thrombocytopenia. A gradual reduction in fibrinogen and D-dimer levels was observed. The patient reached a platelet count of >100 × 109/L 10 days after the last heparin administration. Once this threshold was fulfilled, acenocoumarol was started, overlapping with argatroban administration for 4 days. As soon as the patient achieved stable international normalised ratio (INR) control to a value between 2 and 3, acetylsalicylic acid (100 mg/24 h) was added. Close monitoring of the patient was continued with no evidence of thrombotic or haemorrhagic complications.
Discussion and conclusions
We have detailed a demanding case of a patient with recent bilateral SARS-CoV-2 pneumonia associated with PE who had multiple thrombi in the convalescence period due to HIT. Several non-heparin anticoagulant therapies including cardiac surgery and coronariography were carried out at different stages of care.
It is well known that HIT is associated with increased thrombotic risk. Venous thromboses are more frequent than arterial thromboses in patients with HIT [Citation1]. However, our patient presented exclusively with multiple arterial thrombi. Furthermore, only few cases of HIT with intraventricular thrombus have been reported [Citation3,Citation4]. Some of these cases occurred in patients without cardiac injuries. By contrast, we presumed that the chronic mural thrombus enabled intraventricular thrombus formation.
Recent SARS-CoV-2 infection could have contributed to a prothrombotic environment. Moreover, several cases of COVID-19 and HIT have been described recently [Citation5–12]. Very remarkable is the case of an asymptomatic patient with COVID-19 who suffered from acute limb ischaemia complicated by HIT [Citation13]. Warkentin et al. suggested that COVID-19 could confer a higher risk of HIT [Citation14]. Some authors have reported increasing incidence of HIT in COVID-19 patients, likely due to the higher doses of heparin used for the treatment of these patients, as well as exacerbated immune reactions [Citation5].
The diagnosis of HIT requires a clinical suspicion and a laboratory confirmation. In this case, a high clinical probability and a positive result in a chemiluminescent assay supported the diagnosis. However, a consistent functional assay (heparin-induced platelet activation assay or serotonin release assay) could have enhanced the diagnostic approach [Citation1].
Discontinuing heparin treatment and starting non-heparin anticoagulants are the initial steps in the treatment of HIT. Previous studies have provided incomplete evidence to determine the optimal anticoagulation therapy. Danaparoid, argatroban, bivalirudin, and fondaparinux are possible alternatives. Additionally, some studies have reported positive results with direct oral anticoagulants [Citation15].
Management of acute HIT patients undergoing cardiac surgery with cardiopulmonary bypass is challenging, as the available evidence is limited. Delaying the procedure until the acute phase is complete is recommended. If this is not possible, then, as mentioned in the American College of Chest Physicians (ACCP) guidelines, treatment with bivalirudin is recommended [Citation16]. However, the American Society of Hematology (ASH) guidelines propose other options: use of intraoperative heparin after treatment with plasma exchange, or intraoperative heparin in combination with a potent antiplatelet agent [Citation15]. Nevertheless, there is less supportive evidence of non-bivalirudin protocols, and treatment preference depends on availability, experience of the centre, and features of each patient. In addition, new strategies with argatroban and high-dose intravenous immunoglobulins (both before and after surgery) and cangrelor and heparin (both during cardiopulmonary bypass) are increasingly being used [Citation17].
Alternative anticoagulants have been suggested for percutaneous cardiovascular intervention (PCI). The ASH and ACCP guidelines recommend the use of bivalirudin for this procedure, although argatroban could be considered [Citation15,Citation16].
In our experience, bivalirudin has demonstrated to be a safe and well-tolerated drug in cardiac surgery and PCI. However, continuous bleeding without hemodynamic instability was observed in the immediate postsurgical period. This could be explained by a reduction in bivalirudin proteolysis due to hypothermia during cardiac surgery [Citation1].
Rapid recurrence of intraventricular thrombus should be noted. The residual hypokinetic area added to the postsurgical AMI and bivalirudin infusion discontinuation after surgery might have contributed to this complication. Additionally, the aetiology of the postsurgical AMI remains unclear. The possible explanation is the release of a small embolus originating in the ventricular thrombus or a coronary vasospasm.
In summary, we report a case of post-COVID-19 HIT with numerous arterial thrombi and a large intraventricular thrombus requiring surgical extraction and resulting in a postsurgical AMI. The patient required multiple non-heparin anticoagulant therapies according to the procedures executed and the clinical status.
Acknowledgements
The authors wish to thank the Cardiac and Vascular Surgery, Anesthesiology, Cardiology, and Intensive Care Medicine Departments of our centre for their intervention in this case.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Salter BS, Weiner MM, Trinh MA, et al. Heparin-induced thrombocytopenia. J Am Coll Cardiol. 2016;67(21):2519–2532. doi:10.1016/j.jacc.2016.02.073
- Staibano P, Arnold DM, Bowdish DME, et al. The unique immunological features of heparin-induced thrombocytopenia. Br J Haematol. 2017;177(2):198–207. doi:10.1111/bjh.14603
- Nippert M, de Maistre E, Rodermann M, et al. [Treatment with lepirudin in heparin-induced thrombocytopenia. A case report]. Arch Mal Coeur Vaiss. 2002;95(12):1234–1238.
- Vignon P, Guéret P, François B, et al. Acute limb ischemia and heparin-induced thrombocytopenia: the value of echocardiography in eliminating a cardiac source of arterial emboli. J Am Soc Echocardiogr. 1996;9(3):344–347. doi:10.1016/S0894-7317(96)90150-9
- Daviet F, Guervilly C, Baldesi O, et al. Heparin-induced thrombocytopenia in severe COVID-19. Circulation. 2020;142(19):1875–1877. doi:10.1161/CIRCULATIONAHA.120.049015
- Riker RR, May TL, Fraser GL, et al. Heparin-Induced thrombocytopenia with thrombosis in COVID-19 adult respiratory distress syndrome. Res Pract Thromb Haemost. 2020;4(5):936–941. doi:10.1002/rth2.12390
- Ogawa Y, Nagata T, Akiyama T, et al. Argatroban therapy for heparin-induced thrombocytopenia in a patient with coronavirus disease 2019. J Thromb Thrombolysis. 2020;50(4):1012–1014. doi:10.1007/s11239-020-02248-8
- Lozano R, Franco ME. Incidence of heparin-induced thrombocytopenia in patients with 2019 coronavirus disease. Med Clin (Engl Ed). 2020;155(9):409–410. doi:10.1016/j.medcle.2020.05.023
- Huang CT, Hsu SY, Chang KW, et al. Heparin-induced thrombocytopenia and thrombosis in a patient with COVID-19. Thromb Res. 2020;196:11–14. doi:10.1016/j.thromres.2020.07.056
- Phan XT, Nguyen TH, Tran TT, et al. Suspected heparin-induced thrombocytopenia in a COVID-19 patient on extracorporeal membrane oxygenation support: a case report. Thromb J. 2020;18(1):37), doi:10.1186/s12959-020-00252-9
- Sartori M, Cosmi B. Heparin-induced thrombocytopenia and COVID-19. Hematol Rep. 2021;13(1):8857. doi:10.4081/hr.2021.8857
- Julian K, Bucher D, Jain R. Autoimmune heparin-induced thrombocytopenia: a rare manifestation of COVID-19. BMJ Case Rep. 2021;14(5):e243315. doi:10.1136/bcr-2021-243315
- Siddiqui NA, Luvsannyam E, Jain MS, et al. Acute limb ischemia complicated by heparin-induced thrombocytopenia in an asymptomatic COVID-19 patient. Cureus. 2021;13(7):e16162. doi:10.7759/cureus.16162
- Warkentin TE, Kaatz S. COVID-19 versus HIT hypercoagulability. Thromb Res. 2020;196:38–51. doi:10.1016/j.thromres.2020.08.017
- Cuker A, Arepally GM, Chong BH, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv. 2018;2(22):3360–3392. doi:10.1182/bloodadvances.2018024489
- Linkins LA, Dans AL, Moores LK, et al. Treatment and prevention of heparin-induced thrombocytopenia. Chest. 2012;141(2 Suppl.):e495S–e530S. doi:10.1378/chest.11-2303
- Koster A, Erdoes G, Nagler M, et al. How would we treat our own heparin-induced thrombocytopenia during cardiac surgery? J Cardiothorac Vasc Anesth. 2021;35(6):1585–1593. doi:10.1053/j.jvca.2020.11.002
