ABSTRACT
Daratumumab injection was approved by China in 2019 for the treatment of recurrent or refractory multiple myeloma. However, the molecular weight of daratumumab, an immunoglobin G1 kappa human monoclonal antibody, was similar to that of M protein and could not be distinguished from IgG κ M protein in SPEP and SIFE. It might lead to false-positive detection resulting in misdiagnose and confusing evaluation of therapeutic response, especially for patients with IgG κ M proteins. Herein, we reported two cases encountered in our daily clinical work. These two case reports could serve as a reminder to global hematologists who have not yet started or just begun to use the drug of daratumumab.
Dear editor,
Daratumumab injection (generic name: daratumumab) was approved by Food and Drug Administration (FDA) in the United States in 2015 and China in 2019 for the treatment of recurrent or refractory multiple myeloma (RRMM). The approval of this new indication will further fill the gap in the treatment of RRMM in our country and bring more innovative options to patients, especially who are at an earlier stage of treatment for first relapse.
Serum protein electrophoresis (SPEP) and immunofixation (SIFE) analysis were routinely used to monitor the disease status and therapeutic effect of myeloma patients. However, the molecular weight of daratumumab, an immunoglobin G1 kappa human monoclonal antibody, was similar to that of M protein and could not be distinguished from IgG κ M protein in SPEP and SIFE. It might lead to false-positive detection resulting in misdiagnose and confusing evaluation of therapeutic response, especially for patients with IgG κ M proteins [Citation1]. If daratumumab was misreported as the M-protein band of the patient in the SPEP/SIFE analysis, it might affect clinical decision-making according to the unified response standard of the International Myeloma Working Group (IMWG) and the guidelines for Diagnosis and Treatment of Multiple Myeloma in China (2020 revised version) [Citation2,Citation3].
Here are two cases encountered in our daily clinical work.
Case one
The admission diagnoses of a 53-year-old man were: MM IgG κ, DS stage II, with deletion of RB1 and D13S319 sites (a probe on 13q14.2-14.3 that overlaps with DLEU1 and DLEU2 genes), amplification of 1q21 sites, high risk. On April 15, 2021, the tests of SPEP and SIFE were carried out as shown in . Daratumumab regimen chemotherapy was then given to patient since April 15, 2021. The second tests of SPEP and SIFE were carried out on May 27, 2021 as shown in . The Hydrashift 2/4 Daratumumab Assay demonstrated that the abnormal extra bands (arrow) were due to drug interference. Accordingly, this patient could finally be screened out relapse due to clonal evolution, rather than very good partial response (VGPR) initially misjudged due to false-positive M proteins according to the 2016 IMWG efficacy evaluation criteria [Citation2].
Figure 1. The results of the first serum protein electrophoresis and immunofixation electrophoresis (April 15, 2021). (A) shows that there was one M protein in the SPE. (B) shows that the immunotyping was IgG κ.
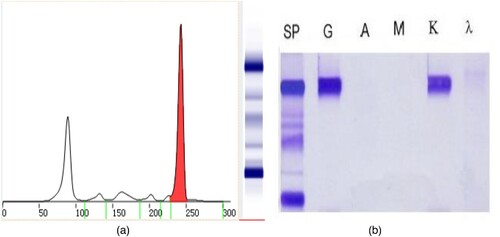
Figure 2. Results of the second serum protein electrophoresis, immunofixation electrophoresis, immunophenotyping and the Hydrashift 2/4 Daratumumab Assay (May 27, 2021). (A) (SPE) shows that there was one M proteins in the SPE, with an extra band (the arrow points). (B) (SIFE) shows that the immunotyping was IgG κ, with an extra band (the arrow points). (C) (immunophenotyping) also shows that the immunotyping was IgG κ, with an extra band (the arrow points). (D) (Hydrashift 2/4 Daratumumab Assay) shows the extra band was removed from the point of the arrow to the red box.
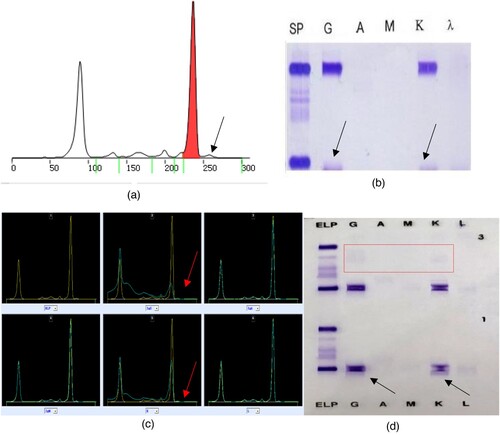
Case two
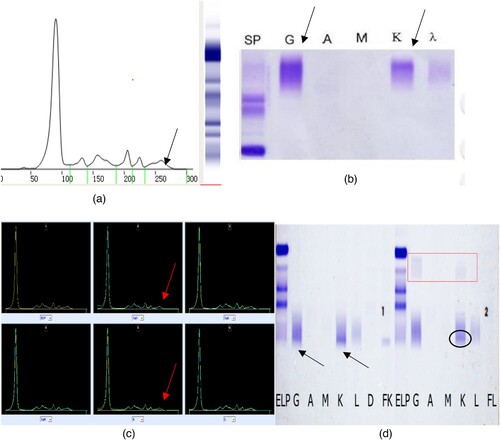
A 58 years old man, developed left chest pain beyond 1 year when admission. The admission diagnoses were: Multiple myeloma, Kappa light chain type, DS stage IIIA, ISS stage II, R-ISS stage III, high-risk group by Mayo stratification for myeloma and risk-adapted therapy (mSMART) [Citation4]. On January 8, 2021, the tests of SPEP and SIFE were carried out as shown in . Daratumumab regimen chemotherapy was then given to patient since February 10, 2021. The second tests of SPEP and SIFE were carried out on March 10, 2021 as shown in . The both reports of SPE and SIFE were M protein (+). It was not until May 27, 2021, after the patient completed the fourth course of daratumumab, that abnormal bands in SPE and SIFE were considered to be caused by drug interference and no positive results were reported (shown in ). Accordingly, this patient could finally be regarded as stable disease (SD), rather than progressive disease (PD) initially misjudged as clonal evolution [Citation5] due to emerging false-positive M proteins according to the 2016 IMWG efficacy evaluation criteria [Citation2].
Figure 3. The results of the first serum protein electrophoresis and immunofixation electrophoresis (January 8, 2021). The serum protein electrophoresis in our hospital showed no M protein. Immunofixation electrophoresis + immunoglobulin light chain quantification: no obvious clonal bands were observed.
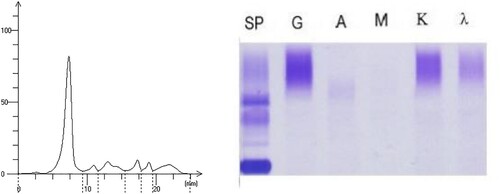
Figure 4. The results of the second serum protein electrophoresis and immunofixation electrophoresis (March 10, 2021). (A) shows that there was one M protein in the SPE. (B) shows that the report of the SIFE was IgG κ immunotyping.
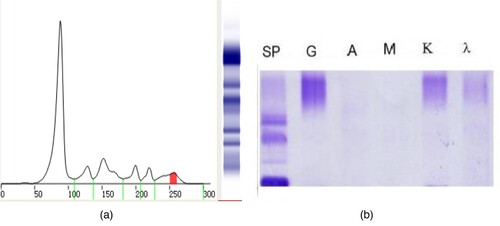
Figure 5. The results of serum protein electrophoresis, immunofixation electrophoresis, immunophenotyping and the Hydrashift 2/4 Daratumumab Assay (May 27, 2021). (A–C) shows that there maybe one M protein (the arrow points). (D) (Hydrashift 2/4 Daratumumab Assay) shows the ‘M’ protein was removed from the point of the arrow to the red box with an M protein (circled by an oval, with the immunophenotyping of κ light chain).
If the new M-protein band is detected during the treatment (in a different position than before), if it is judged to be a new pathological M protein — it means clonal evolution and the current treatment is not effective and needs to be changed; if it is a DARA interference and other indicators are stable or improving — it means that the current DARA treatment should be maintained and the protocol does not need to be changed. If it is DARA interference and other indicators are stable or improving — it means that the current DARA treatment should be adhered to and the program does not need to be changed. Therefore, even if the new M protein is determined, it is important for the decision of treatment direction.
When MM patients are treated with t-mAb combination therapy, the response assessment of electrophoresis M-protein diagnosis will become more and more difficult. Incorrect response assessment due to interference in SPEP/IFE analysis is usually a misclassification between very good partial response (VGPR) and complete response (CR), which may lead to underestimation of the prognosis and possible overestimation of individual treatment of patients. According to the research from Ravi et al. [Citation6], the frequency of detecting interference bands after DARA treatment is not affected by the type of myeloma (i.e. IgG and IgA), but by the size of the M-peak. This may reflect the larger size of the disease in the bone marrow and less M protein detected in the peripheral blood. During the weekly dosing period, the appearance of side bands associated with daratumumab treatment may be a surrogate early marker for predicting disease response.
In summary, there was an urgent need for laboratory tools that could distinguish between the false-positive interference and disease (M protein). At present, the Hydrashift 2/4 Daratumumab Assay was the only commercial method to solve the false-positive interference caused by daratumab on SIFE. Hydrashift analysis was a commercial product of daratumab interference reflection analysis, which could be carried out with existing instruments and standards [Citation7,Citation8]. This method, approved by the US FDA in January 2018, could distinguish daratumab from disease-related M proteins on IF, and found the source of immunoglobulin κ bands, which would not interfere with the judgment of patients’ condition changes. This monoclonal murine anti-idiotypic antibody against daratumumab was used to shift the migration of daratumumab on the gel, which allowed the presence of the drug to be confirmed and the patient's M protein to be viewed without interference. The analysis may be most useful when the M-protein level is approximately equal to or less than the daratumumab concentration (approximately 1 g/l) [Citation7–9]. However, hydrashift was only valid for daratumumab. With the introduction of new t-mAbs (combinations), a new Hydrashift test must be developed and approved by the FDA every time to use these new t-mAb-specific antisera. Currently, the daratumumab Hydrashift test (Sebia) is the only commercially available IFE test approved by the FDA that can be used to reduce antibody interference [Citation10]. Another limitation of hydrashift analysis is that it is a qualitative analysis. However, the limitations of this approach may not be so important for some organizations. For example, the use of Hydrashift analysis in reference laboratories may be particularly important because of their limited knowledge of medical history and previous laboratory results [Citation11]. Moreover, there were other monoclonal drugs that might cause false-positive interference with SIFE [Citation12], and new therapeutic monoclonal antibodies (mAbs) were under development, so a more general method was needed to distinguish therapeutic monoclonal from M proteins. Moore et al. [Citation13] found that MALDI-TOF MS method might be used to differentiate drug effects from patients’ own M proteins. Previous studies which used liquid chromatography-quadrupole time-of-flight mass spectrometry (LC-QTOF-MS) had a 100% success rate in differentiating mAbs from M proteins [Citation14].
However, limited by actual economic conditions and operating levels, not all hospitals could use the above-mentioned reagents or detection methods. At the same time, we should also note that the Hydrashift 2/4 Daratumumab Assay was suitable for the judgment of drug interference in non-free light chain type of MM. For the light chain type of MM, it was necessary to combine the detection of serum free light chain and clinical manifestations for judgment.
Whether the drug-induced monoclonal band can be observed on IFE was affected by many factors: the concentration of the patient's infusion of the drug; the patient's immunoglobulin level, high immunoglobulin content will cover the drug band; endogenous M protein, the position of the strip will overlap with the drug-based monoclonal strip; the individual differences in drug metabolism in patients; the interval time between patient infusions of drugs [Citation9].
Maybe the 4-step plan from the laboratory of Lynch et al. [Citation15] for patients who are known to be treated with daratumumab should be tried. First, system alarms during registration of daratumumab patient samples, including informing senior scientists of protein testing. Second, use Sebia Hydrashift 2/4 Daratumab a reflection test (DIRA) to confirm that the new IgG κ band is daratumumab. Third, the patient's report, with appropriate comments on the presence of daratumumab. Finally, due to the impact of new clones, non-daratumumab bands will be directly notified to clinicians. Currently, daratumumab screening for every new IgG κ paraprotein is impractical, so the laboratory is highly dependent on close communication with oncologists.
And most importantly, in order to predict the possible interference of t-mAbs treatment on M-protein monitoring, laboratory experts must understand the use of monoclonal antibodies in MM treatment. A daratumumab patient registry would be useful. Also, these two case reports could serve as a reminder to hematologists by worldwide who have not yet started or just begun to use the drug of daratumumab.
Author contributions
All authors contributed to the writing and editing of the manuscript and approved the final version of the manuscript.
Ethics approval and consent to participate
The study was approved by the Institutional Ethics Committee of West China Hospital of Sichuan University and complied with Declaration of Helsinki. Written informed consent was obtained from all subjects.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Murata K, McCash SI, Carroll B, et al. Treatment of multiple myeloma with monoclonal antibodies and the dilemma of false positive M-spikes in peripheral blood. Clin Biochem. 2018;51:66–71.
- Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17:e328–e346.
- Guidelines for diagnosis and treatment of multiple Myeloma in China (revised in 2020). Chin J Int Med. 2020;59:341–346.
- Dingli D, Ailawadhi S, Bergsagel PL, et al. Therapy for relapsed multiple myeloma: guidelines from the Mayo stratification for myeloma and risk-adapted therapy. Mayo Clin Proc. 2017;92(4):578–598.
- Tang M, Zhao R, van de Velde H, et al. Myeloma cell dynamics in response to treatment supports a model of hierarchical differentiation and clonal evolution. Clin Cancer Res. 2016;22(16):4206–4214.
- Ravi G, Andrade L, Vuyyala S, et al. Host and disease factors impacting presence of accessory band during therapy with daratumumab in multiple myeloma patients. Biol Blood Marrow Transplant. 2020;26(3):s390.
- McCudden C, Axel AE, Slaets D, et al. Monitoring multiple myeloma patients treated with daratumumab: teasing out monoclonal antibody interference. Clin Chem Lab Med. 2016;54:1095–1104.
- van de Donk NW, Otten HG, El Haddad O, et al. Interference of daratumumab in monitoring multiple myeloma patients using serum immunofixation electrophoresis can be abrogated using the daratumumab IFE reflex assay (DIRA). Clin Chem Lab Med. 2016;54:1105–1109.
- McCudden CR, Jacobs JFM, Keren D, et al. Recognition and management of common, rare, and novel serum protein electrophoresis and immunofixation interferences. Clin Biochem. 2018;51:72–79.
- Shah N, Aiello J, Avigan DE, et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of multiple myeloma. J Immunother Canc. 2020;8:e000734.
- Thoren KL, Pianko MJ, Maakaroun Y, et al. Distinguishing drug from disease by use of the Hydrashift 2/4 Daratumumab Assay. J Appl Lab Med. 2019;3(5):857–863.
- Willrich MAV, Ladwig PM, Andreguetto BD, et al. Monoclonal antibody therapeutics as potential interferences on protein electrophoresis and immunofixation. Clin Chem Lab Med. 2016;54:1085–1093.
- Moore LM, Cho S, Thoren KL. MALDI-TOF mass spectrometry distinguishes daratumumab from M-proteins. Clin Chim Acta. 2019;492:91–94.
- Mills JR, Kohlhagen MC, Willrich MAV, et al. A universal solution for eliminating false positives in myeloma due to therapeutic monoclonal antibody interference. Blood. 2018;132:670–672.
- Lynch C, Yen T, Trambas CM, et al. New laboratory protocol to identify iatrogenic IgG kappa monoclonal bands due to daratumumab therapy. Pathology. 2019;51:S113.
